Abstract
Introduction
Effective treatments for hormone-receptor-positive (HR+) breast cancer (BC) following relapse/progression on nonsteroidal aromatase inhibitor (NSAI) therapy are needed. Initial Breast Cancer Trials of OraL EveROlimus-2 (BOLERO-2) trial data demonstrated that everolimus and exemestane significantly prolonged progression-free survival (PFS) versus placebo plus exemestane alone in this patient population.
Methods
BOLERO-2 is a phase 3, double-blind, randomized, international trial comparing everolimus (10 mg/day) plus exemestane (25 mg/day) versus placebo plus exemestane in postmenopausal women with HR+ advanced BC with recurrence/progression during or after NSAIs. The primary endpoint was PFS by local investigator review, and was confirmed by independent central radiology review. Overall survival, response rate, and clinical benefit rate were secondary endpoints.
Results
Final study results with median 18-month follow-up show that median PFS remained significantly longer with everolimus plus exemestane versus placebo plus exemestane [investigator review: 7.8 versus 3.2 months, respectively; hazard ratio = 0.45 (95% confidence interval 0.38–0.54); log-rank P < 0.0001; central review: 11.0 versus 4.1 months, respectively; hazard ratio = 0.38 (95% confidence interval 0.31–0.48); log-rank P < 0.0001] in the overall population and in all prospectively defined subgroups, including patients with visceral metastases, patients with recurrence during or within 12 months of completion of adjuvant therapy, and irrespective of age. The incidence and severity of adverse events were consistent with those reported at the interim analysis and in other everolimus trials.
Conclusion
The addition of everolimus to exemestane markedly prolonged PFS in patients with HR+ advanced BC with disease recurrence/progression following prior NSAIs. These results further support the use of everolimus plus exemestane in this patient population. ClinicalTrials.gov #NCT00863655.
Electronic supplementary material
The online version of this article (doi:10.1007/s12325-013-0060-1) contains supplementary material, which is available to authorized users.
Keywords: Advanced breast cancer, Everolimus, Exemestane, Hormone receptor positive, Nonsteroidal aromatase inhibitors, Oncology, Postmenopausal, Progression-free survival
Introduction
The majority of women with breast cancer (BC; approximately 70% worldwide) have hormone-receptor-positive (HR+) tumors [1]. Almost all of these women will receive endocrine therapy as a standard part of their treatment for early and/or advanced-stage disease [2–4]. Currently, third-generation nonsteroidal aromatase inhibitors (NSAIs: anastrozole and letrozole) and steroidal exemestane (EXE) represent the preferred front-line therapy for postmenopausal women with HR+ advanced BC [4]. However, progressive disease ultimately develops in virtually all patients, either as early failure to respond to endocrine therapy (de novo resistance) or as relapse/progression after initial response (acquired resistance) [5].
A significant proportion of tumors in BC patients retain their sensitivity to endocrine-directed approaches even after disease progression on prior endocrine therapy, and may respond to another endocrine agent [6, 7]. In view of the favorable safety profile of endocrine-directed agents, extending the benefit of endocrine therapy at relapse/progression is an important clinical consideration. In particular, the low toxicity of endocrine agents compared with chemotherapy represents a major advantage in a population of patients with a high incidence of comorbidities. However, sequential lines of single-agent endocrine therapy are associated with modest clinical benefit [6, 7]. Accordingly, combination endocrine therapies [8–10] and co-targeting of downstream elements of the molecular pathways associated with BC progression and the development of endocrine resistance [e.g., histone deacetylase or mammalian target of rapamycin (mTOR)] have been investigated [11, 12].
Preclinical and clinical evidence shows that everolimus (EVE), a rapamycin derivative, has direct anticancer effects, and that mTOR inhibition can enhance the efficacy of endocrine therapy in breast tumors [13–15]. The strategy of dual inhibition with endocrine therapy and an mTOR inhibitor was investigated in the Breast Cancer Trials of OraL EveROlimus-2 (BOLERO-2) trial [16]. Data from the protocol-defined interim analysis at 7.6-month median follow-up of this randomized, placebo-controlled, phase 3 trial demonstrated that EVE+EXE significantly improved progression-free survival (PFS) compared with placebo (PBO) + EXE [hazard ratio (HR) 0.43; P < 0.001 based on local investigator assessment; HR 0.36; P < 0.001 based on the independent central radiology assessment] [16]. This led to the recent regulatory approval in the United States and Europe of EVE in combination with EXE for the treatment of postmenopausal women with HR+, human epidermal growth factor receptor 2-negative (HER2−) advanced BC recurring or progressing after prior NSAIs [9]. The final analysis of PFS, other efficacy endpoints, and updated safety are reported here.
Methods
Details of patient selection criteria and the clinical protocol of this study have been previously reported [16].
Patients
Enrolled patients were adult postmenopausal women with HR+ metastatic/locally advanced BC not amenable to surgery or radiotherapy and progressing after anastrozole or letrozole (defined as disease recurrence during or within 12 months of end of adjuvant treatment or progression during or within 1 month of end of treatment for advanced disease). Patients whose tumors showed HER2 overexpression (immunohistochemistry 3+) or gene amplification (in situ hybridization positive) or who had received prior therapy with EXE or mTOR inhibitors were excluded.
Written informed consent was obtained from all patients before enrollment. The institutional review board at each participating center approved the study, which was conducted in accordance with the principles of Good Clinical Practice, the provisions of the Declaration of Helsinki of 1975, as revised in 2000 and 2008, and other applicable local regulations. A steering committee supervised study conduct. An independent data and safety monitoring committee performed semiannual safety reviews and reviewed the interim efficacy results.
Study Design
BOLERO-2 was a multicenter, double-blind, randomized, placebo-controlled, international phase 3 study. Patients were randomly allocated in a 2:1 ratio to receive EVE 10 mg/day or matching PBO in a blinded manner; all patients received open-label EXE 25 mg/day (N = 724). Patients were stratified according to the presence of visceral metastasis (yes vs no) and sensitivity to previous hormonal therapy (yes vs no), as previously described [16]. The primary endpoint for this study was PFS as assessed by local investigator [based on Response Evaluation Criteria In Solid Tumors (RECIST) 1.0] and confirmed by central review. Secondary endpoints included overall response rate (complete response or partial response); clinical benefit rate (CBR; defined as complete response + partial response + stable disease for at least 24 weeks); overall survival (OS); quality of life (QOL), changes in bone marker levels, and patient safety.
Study Assessments
Tumor assessments were based on computed tomography (CT) scans or magnetic resonance imaging (MRI) of the chest, abdomen, and pelvis at baseline and every 6 weeks until disease progression. Patients who discontinued one or both study treatments for any reason other than progression were followed with the same assessment schedule until progression. A bone scan or skeletal survey using radiography, CT scanning, or MRI was required within 6 weeks before randomization. Abnormalities observed on bone scans were assessed using the same method every 6 weeks. After discontinuation of treatment, patients who progressed were followed every 3 months for survival.
Hematologic parameters, biochemical measures, and vital signs were assessed at baseline and at each visit, and the lipid profile was assessed every 6 weeks. Adverse events (AEs) were monitored continuously throughout the study and graded according to Common Terminology Criteria for Adverse Events, version 3.0 [17].
Statistical Analysis
The primary efficacy analysis of PFS by local investigator assessment required 528 PFS events to achieve 90% power to detect an HR of 0.74 (26% risk reduction) using a log-rank test and 2-look Lan-DeMets group [18] sequential design with O’Brien–Fleming-type boundary at a one-sided cumulative 2.5% significance level; one interim analysis was conducted after observing 60% of events (previously reported) [16]. Based on the magnitude and stability of the EVE treatment effect over time, as well as lower-than-expected event rates, final analysis after slightly fewer events than planned (i.e., 510 events) was considered appropriate.
Results
A total of 724 patients were randomized between June 2009 and January 2011 to receive EVE+EXE (n = 485) or PBO+EXE (n = 239). Baseline characteristics were similar between treatment groups (Table 1) [16]. At baseline, 77% of patients had bone lesions (21% had bone-only lesions), and of the approximately 59% with visceral disease, 84% had involvement at 2 or more sites. Approximately 48% of patients had been previously treated with tamoxifen (TAM), and approximately 17% had previously received fulvestrant (both in addition to the NSAI required per inclusion criteria). Approximately 80% of patients received prior therapy for metastatic disease, including chemotherapy (26%), whereas 20% of patients received study treatment as their first therapy for metastatic disease.
Table 1.
Patient demographic, baseline disease, and treatment characteristics
| Characteristic | EVE+EXE (n = 485), % | PBO+EXE (n = 239), % |
|---|---|---|
| Median age, years (range) | 62 (34–93) | 61 (28–90) |
| Race | ||
| White | 74 | 78 |
| Asian | 20 | 19 |
| Black | 3 | 1 |
| Other | 3 | 2 |
| ECOG performance status 0 | 60 | 59 |
| Visceral disease | 58 | 59 |
| Measurable diseasea | 70 | 68 |
| Metastatic site | ||
| Lung | 30 | 33 |
| Liver | 33 | 30 |
| Bone | 77 | 77 |
| Prior therapy | ||
| Setting of most recent treatment | ||
| Adjuvant | 21 | 16 |
| Advanced/metastatic disease | 79 | 84 |
| LET or ANA as most recent treatment | 74 | 75 |
| Tamoxifen | 47 | 50 |
| Fulvestrant | 17 | 16 |
| Chemotherapy (any setting) | 69 | 65 |
| Chemotherapy for metastatic BC | 26 | 26 |
| Radiotherapy | 70 | 69 |
| Number of prior therapiesb | ||
| 1 or 2 | 46 | 47 |
| ≥3 | 54 | 53 |
From Baselga et al. [16]. Copyright © 2012 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society. Any minor differences between this table and the original report by Baselga et al. [16] are a consequence of the investigator’s data correction at the subsequent analysis
ANA anastrozole, BC breast cancer, ECOG Eastern Cooperative Oncology Group, EVE everolimus, EXE exemestane, LET letrozole, PBO placebo
aAll other patients had ≥1 mainly lytic bone lesion
bPrior therapies include those used in the adjuvant setting or to treat advanced disease
At the cutoff date for the final PFS analysis, December 15, 2011, 510 PFS events had accrued based on local assessment and 320 per central radiology review. The median duration of follow-up at data cutoff was 17.7 months (range 10.9–28.6 months). Eighty-one patients (16.7%) in the EVE+EXE arm and 10 patients (4.2%) in the PBO+EXE arm continued to receive study treatment.
In the EVE+EXE arm, median duration of exposure to EVE was 23.9 weeks (range 1.0–123.3 weeks) and median exposure to EXE was 29.5 weeks (range 1.0–123.3 weeks). In the PBO+EXE arm, median exposure to EXE was 14.07 weeks (range 1.0–101.0 weeks). The median relative dose intensities for EVE and EXE were 86% and 100%, respectively, in the EVE+EXE arm. The median relative dose intensity for EXE was 100% in the PBO+EXE arm. This represents an increase in drug exposure of 117.5 patient-years (60%) in the EVE+EXE arm and 23.7 patient-years (32%) in the PBO+EXE arm compared with the protocol-specified interim analysis [16].
The main reason for treatment discontinuation in both study arms was disease progression (61.9% for EVE+EXE vs 88.7% for PBO+EXE). Among the patients who discontinued from treatment, the proportion receiving new anticancer therapy was numerically smaller in the EVE+EXE arm compared with PBO+EXE (81% in the EVE+EXE arm vs 91% in the PBO+EXE arm). The most common post-study systemic treatments in the EVE+EXE and PBO+EXE arms included cytotoxic chemotherapy (42% and 59% of patients, respectively), and hormonal therapy (35% and 40% of patients, respectively).
Efficacy
The addition of EVE to EXE significantly prolonged median PFS versus EXE alone per assessment by local investigators [7.8 vs 3.2 months, respectively; HR 0.45 (95% confidence interval (CI) 0.38–0.54); log-rank P < 0.0001] (Fig. 1a). Analysis by central assessment confirmed the PFS benefit [11.0 months for EVE+EXE vs 4.1 months for PBO+EXE; HR 0.38 (95% CI 0.31–0.48); log-rank P < 0.0001] (Fig. 1b). The effect of EVE+EXE treatment (assessed by local investigators) was consistent across patient subgroups defined by patient characteristics and prior therapy, with an estimated HR ranging between 0.25 and 0.62 (Fig. 2a). These analyses were concordant with similar subgroup analyses from data based on central review (Fig. 2b). In particular, EVE+EXE treatment substantially extended PFS benefits compared with PBO+EXE regardless of baseline disease or prior therapy characteristics (e.g., only prior adjuvant therapy, prior chemotherapy, and presence of visceral metastases or bone lesions).
Fig. 1.
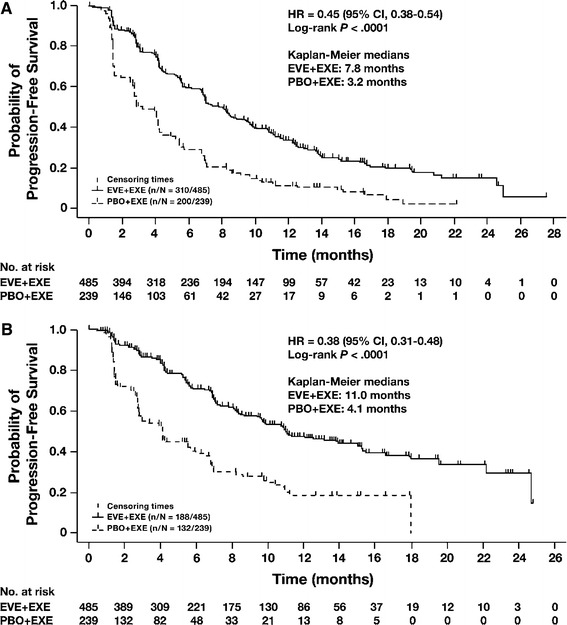
Kaplan–Meier estimates of progression-free survival of patients treated with everolimus plus exemestane versus exemestane alone based on assessment by a local investigator or b central review. CI confidence interval, HR hazard ratio, EVE everolimus, EXE exemestane, PBO placebo
Fig. 2.
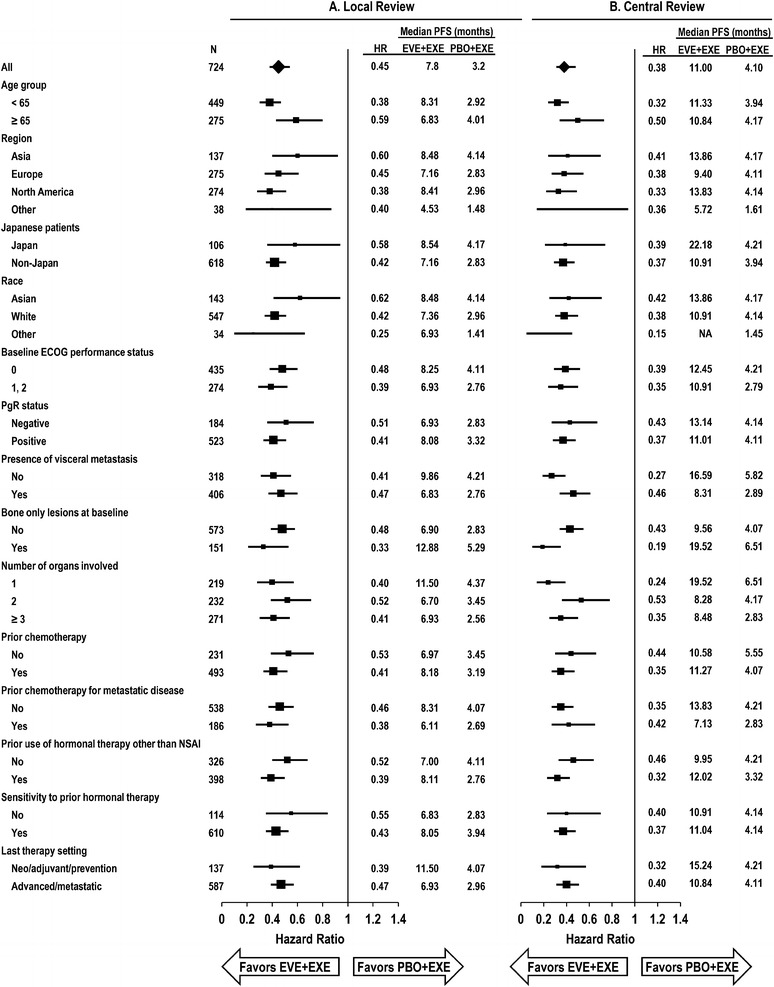
Subgroup analysis of progression-free survival by a local investigator review and b central review. ECOG Eastern Cooperative Oncology Group, EVE everolimus, EXE exemestane, HR hazard ratio, NSAI nonsteroidal aromatase inhibitor, PBO placebo, PFS progression-free survival, PgR progesterone receptor
At the time of analysis, fewer deaths were reported with EVE+EXE (25.4%) versus PBO+EXE (32.2%; Table 2). A final analysis of OS is planned after 398 events. Improvements were also observed with EVE+EXE versus PBO+EXE in overall response, objective response rate, and CBR according to both local and central assessments (Table 3).
Table 2.
Between-arm differences in overall survival over time
| PFS interim (7-month follow-up) | PFS update (12-month follow-up) | PFS final (18-month follow-up) | |
|---|---|---|---|
| Cutoff date | Feb 11, 2011 | Jul 8, 2011 | Dec 15, 2011 |
|
OS events, n EVE vs PBO, % of events |
83 10.6 vs 13.0 |
137 17.3 vs 22.7 |
200 25.4 vs 32.2 |
| Δ OS events, % of events | 2.4 | 5.4 | 6.8 |
EVE everolimus, OS overall survival, PBO placebo, PFS progression-free survival, Δ change
Table 3.
Summary of tumor response
| Response | Local assessment | Central assessment | ||
|---|---|---|---|---|
| EVE+EXE (n = 485) | PBO+EXE (n = 239) | EVE+EXE (n = 485) | PBO+EXE (n = 239) | |
| Best overall response (%) | ||||
| Complete response (CR) | 0.6 | 0 | 0 | 0 |
| Partial response (PR) | 12.0 | 1.7 | 12.6 | 2.1 |
| Stable disease (SD) | 71.3 | 59.0 | 73.4 | 62.8 |
| Progressive disease | 10.1 | 32.6 | 5.8 | 23.4 |
| Unknown | 6.0 | 6.7 | 8.2 | 11.7 |
| ORR (CR or PR), % | 12.6* | 1.7 | 12.6 | 2.1 |
| 95% CI for ORR | 9.8–15.9 | 0.5–4.2 | 9.8–15.9 | 0.7–4.8 |
| CBR (CR+PR+SD ≥ 24 weeks), % | 51.3* | 26.4 | 49.9 | 22.2 |
| 95% CI for CBR | 46.8–55.9 | 20.9–32.4 | 45.4–54.4 | 17.1–28.0 |
CI confidence interval, CBR clinical benefit rate, EVE everolimus, EXE exemestane, ORR objective response rate, PBO placebo
* Statistically significant difference, P < 0.0001
Safety
The most commonly reported AEs (affecting >25% of patients) in the EVE+EXE arm included stomatitis, rash, fatigue, diarrhea, nausea, decreased appetite, weight loss, and cough, versus nausea and fatigue in the PBO+EXE arm. The maximum grade of toxicity was 1/2 for approximately half the patients in the EVE+EXE arm. The most common grade 3/4 AEs with EVE+EXE included stomatitis, fatigue, dyspnea, anemia, hyperglycemia, and gamma-glutamyltransferase increase (Table 4). Gamma-glutamyltransferase increase was the most common grade 3/4 toxicity with PBO+EXE. Despite the increased toxicity observed with EVE+EXE versus PBO+EXE, health-related QOL was not worse with EVE+EXE [19].
Table 4.
Most common adverse events (reported in ≥10% of patients)
| AE (preferred term) | EVE+EXE (n = 482), % | PBO+EXE (n = 238), % | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grade | Grade | |||||||||
| All | 1 | 2 | 3 | 4 | All | 1 | 2 | 3 | 4 | |
| Stomatitis | 59 | 29 | 22 | 8 | 0 | 12 | 9 | 2 | <1 | 0 |
| Rash | 39 | 29 | 9 | 1 | 0 | 7 | 5 | 2 | 0 | 0 |
| Fatigue | 37 | 18 | 14 | 4 | <1 | 27 | 16 | 10 | 1 | 0 |
| Diarrhea | 34 | 26 | 6 | 2 | <1 | 19 | 14 | 4 | <1 | 0 |
| Nausea | 31 | 21 | 9 | <1 | <1 | 29 | 21 | 7 | 1 | 0 |
| Decreased appetite | 31 | 19 | 10 | 1 | 0 | 13 | 8 | 4 | <1 | 0 |
| Weight decreased | 28 | 10 | 16 | 1 | 0 | 7 | 3 | 5 | 0 | 0 |
| Cough | 26 | 21 | 4 | <1 | 0 | 12 | 8 | 3 | 0 | 0 |
| Dysgeusia | 22 | 18 | 4 | 0 | 0 | 6 | 6 | 0 | 0 | 0 |
| Dyspnea | 22 | 10 | 6 | 5 | <1 | 11 | 8 | 2 | <1 | <1 |
| Headache | 23 | 17 | 6 | <1 | 0 | 15 | 13 | 2 | 0 | 0 |
| Arthralgia | 21 | 15 | 5 | <1 | 0 | 17 | 11 | 5 | <1 | 0 |
| Peripheral edema | 21 | 14 | 6 | 1 | 0 | 6 | 5 | <1 | <1 | 0 |
| Anemia | 21 | 4 | 10 | 7 | <1 | 5 | 2 | 2 | <1 | <1 |
| Epistaxis | 17 | 16 | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| Vomiting | 17 | 11 | 6 | <1 | <1 | 13 | 9 | 3 | <1 | 0 |
| Pyrexia | 16 | 13 | 3 | <1 | 0 | 7 | 5 | <1 | <1 | 0 |
| Pneumonitis | 16 | 7 | 6 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Constipation | 15 | 11 | 2 | <1 | 0 | 13 | 8 | 5 | <1 | 0 |
| Back pain | 15 | 10 | 5 | <1 | 0 | 11 | 5 | 3 | 2 | 0 |
| Pruritus | 13 | 11 | 2 | <1 | 0 | 5 | 3 | 2 | 0 | 0 |
| Insomnia | 14 | 10 | 4 | <1 | 0 | 8 | 6 | 3 | 0 | 0 |
| Asthenia | 14 | 7 | 5 | 2 | <1 | 4 | 3 | <1 | <1 | 0 |
| AST increased | 14 | 6 | 5 | 3 | <1 | 5 | 2 | 2 | 1 | 0 |
| Hyperglycemia | 14 | 4 | 5 | 5 | <1 | 2 | <1 | <1 | <1 | 0 |
| ALT increased | 12 | 5 | 4 | 3 | <1 | 5 | <1 | 2 | 2 | 0 |
| Dry mouth | 11 | 10 | 1 | 0 | 0 | 7 | 7 | <1 | 0 | 0 |
| Alopecia | 10 | 9 | 1 | 0 | 0 | 5 | 5 | 0 | 0 | 0 |
| Nasopharyngitis | 10 | 9 | 1 | 0 | 0 | 9 | 7 | 2 | 0 | 0 |
| Pain in extremity | 10 | 6 | 3 | <1 | 0 | 12 | 5 | 5 | 2 | 0 |
| Urinary tract infection | 10 | 3 | 7 | <1 | 0 | 2 | <1 | 2 | 0 | 0 |
| GGT increase | 10 | 2 | 2 | 5 | 2 | 9 | <1 | <1 | 5 | 2 |
AE adverse event, ALT alanine aminotransferase, AST aspartate aminotransferase, EVE everolimus, EXE exemestane, GGT gamma-glutamyltransferase, PBO placebo
In the EVE+EXE arm, 66.8% of patients required dose interruptions or reductions for EVE and 23.9% of patients required dose interruptions or reductions for EXE. In the PBO+EXE arm, 11.8% of patients required dose modifications for EXE. The most common reasons for dose modification in both study arms were AEs (62.4% for EVE in the EVE+EXE arm vs 5.5% for EXE in the PBO+EXE arm). Stomatitis (23.7%), pneumonitis (7.5%), and thrombocytopenia (5.4%) were the most common AEs leading to dose modifications in the EVE+EXE arm (versus no single AE as a predominant cause in the PBO+EXE arm). Overall, the safety profile of EVE+EXE was consistent with that reported at the interim analysis [16].
Adverse events leading to discontinuation of at least 1 study drug were reported in 26.3% of patients in the EVE+EXE arm versus 5% of patients in the PBO+EXE arm. Rates of AEs leading to discontinuation that were suspected to be related to at least 1 study drug were 21.4% (EVE+EXE) versus 3.4% (PBO+EXE). The 2 most common AEs leading to treatment discontinuation in the EVE+EXE arm were pneumonitis (5.6%) and stomatitis (2.7%). The most common AEs leading to treatment discontinuation in the PBO+EXE arm were laboratory abnormalities [increased gamma-glutamyltransferase (1.7%) and increased aspartate aminotransferase (1.3%)]. Higher incidences of AEs, dose modifications, and treatment discontinuation among EVE-treated patients may, in part, be attributed to the longer treatment duration in the EVE+EXE arm. Details on dose modifications will be discussed in another manuscript.
The incidence of death because of AEs was 1.4% among patients receiving EVE+EXE versus 0.4% among patients receiving PBO+EXE. In the EVE+EXE arm, one death each was attributed to pneumonia, sepsis, staphylococcal sepsis, tumor hemorrhage, ischemic stroke, suicide, and renal failure. In the PBO+EXE arm, one death was attributed to pneumonia.
Discussion
The current protocol-defined final analysis of PFS from BOLERO-2 confirms the benefits of EVE+EXE on PFS, the primary endpoint of the trial, first reported at the interim analysis [16]. At 18-month median follow-up, EVE+EXE more than doubled median PFS (as assessed by the local investigator) versus PBO+EXE (an absolute difference in median PFS >4 months) in patients with HR+, HER2− advanced BC recurring/progressing on/after initial NSAI therapy. Moreover, subgroup analyses indicate that EVE+EXE is an effective therapeutic option in all patients, regardless of age, prior chemotherapy in the advanced setting, visceral disease, skeletal involvement, or setting of last prior therapy [adjuvant/neoadjuvant (i.e., those who recurred during or within 12 months of completion of adjuvant treatment and received study therapy as first-line treatment for metastatic disease) or therapy for advanced/metastatic disease]. No limitations of procedure or protocol were observed during the conduct of this study.
The central role of the mTOR pathway in BC progression and integrating proliferative signals provides a strong molecular rationale for combining endocrine therapy with mTOR inhibition. The results of the BOLERO-2 study are remarkably similar to those of the randomized phase 2 Tamoxifen Plus RAD-001 (TAMRAD) trial that compared EVE+TAM versus TAM alone in a population of metastatic BC patients who had progressed after prior aromatase inhibitor (AI) therapy [13]. In the TAMRAD study, EVE+TAM prolonged median PFS to 8.6 months (versus 4.5 months with TAM; HR 0.54), and demonstrated survival benefit (HR 0.45) [13]. Enhanced response in the neoadjuvant setting has also been reported with the combination of EVE with NSAI letrozole versus letrozole alone [14].
The principal treatment goal for HR+ advanced BC is disease control. In this context, the benefit of prolonging PFS is clinically relevant provided patient QOL is maintained. Analysis of patient-reported outcomes from BOLERO-2 demonstrated that, despite the higher incidence of AEs with EVE+EXE versus EXE alone, QOL was maintained [20]. These rates are slightly higher than the rates reported at the interim analysis [16], presumably because of increased drug exposure with longer follow-up. This suggests that the significantly improved clinical efficacy outcomes achieved by adding EVE to EXE may have outweighed the impact of toxicity [20].
Current guidelines recommend sequential administration of another line of endocrine therapy at relapse/progression after previous endocrine therapy, whereas chemotherapy is recommended for patients requiring rapid symptom control or who have exhausted three prior lines of endocrine treatment [2, 4]. Although endocrine therapy has a favorable toxicity profile, second- or third-line endocrine-directed approach has so far demonstrated modest efficacy, with CBR ranging from 25% to 35% [6, 8]. Randomized controlled comparisons of experimental single [6, 7] or combination endocrine agents [8, 10] showed minimal to no improvement in median PFS or time to progression versus EXE (3.7 months post-NSAI) [6] or fulvestrant (from 4.4 months post-NSAI to 6.5 months post-AI/antiestrogen) [7]. In contrast, the BOLERO-2 study data demonstrate that EVE+EXE significantly improved median PFS by more than twofold versus EXE alone.
Current guidelines also acknowledge that cytotoxic chemotherapy regimens (whether single agents or combinations) are generally effective in controlling rapidly progressing disease, but are associated with considerable toxicity [8, 21]. Moreover, the clinical benefits from sequential chemotherapy in patients previously exposed to cytotoxic agents in the adjuvant and/or metastatic setting may be limited because of treatment resistance, as well as the potential risk of cumulative toxicities such as cardiac, gastrointestinal, hematologic, and neurologic toxicities [21]. Given the palliative intent of treatment in the second or higher line of therapy and the toxicities associated with chemotherapy, postponing the initiation of cytotoxic therapy can be an important consideration for patients and physicians [22]. In this respect it is important to note that all patient subsets [including those with disease characteristics that might support the use of chemotherapy (e.g., visceral metastases and/or multiple metastatic sites)] in the BOLERO-2 study experienced clinical benefit similar to that of the overall population treated with EVE+EXE.
The AE profile of EVE+EXE in this analysis from BOLERO-2 after 18-month median follow-up is consistent with the established safety profile of EVE in other settings [23, 24]. Notably, these updated analyses show no substantial risk of cumulative toxicities or new safety signals despite a 60% increase in cumulative treatment exposure in the EVE+EXE arm. Adverse events of clinical interest associated with EVE treatment included stomatitis, rash, noninfectious pneumonitis, infections, and metabolic abnormalities, with the majority being grade 1/2. The majority of these events were effectively resolved using protocol-defined management strategies based on extensive prior experience and resulting clinical recommendations for the management of EVE-related AEs in medical oncology (e.g., renal cell carcinoma) [25–27]. Overall, vigilance and proactive monitoring for signs and symptoms of key AEs are key to facilitate prolonged treatment with EVE [26].
Conclusion
The BOLERO-2 trial is the first phase 3 study to demonstrate that dual blockade of the endocrine and mTOR pathways is a feasible and adequately tolerated strategy that provides significant clinical benefit. The final analysis of the primary endpoint from the BOLERO-2 study demonstrates that EVE+EXE is well tolerated and provides clinically meaningful PFS benefit versus EXE alone in the overall population of patients with HR+ advanced BC progressing during/after NSAI therapy, irrespective of age, and among clinically relevant subsets of patients including those receiving first-line treatment for advanced disease, and patients with visceral involvement. Overall, these data support the use of combination therapy with EVE+EXE to substantially improve PFS without compromising QOL, thereby achieving an important goal in the management of advanced BC.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Sponsorship and article processing charges for this study were funded by Novartis. We thank the patients who participated in the BOLERO-2 trial and the investigators, study nurses, and clinical research associates from the individual trial centers who provided ongoing support. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals. We thank Jerome F Sah, PhD, ProEd Communications, Inc., for his medical editorial assistance with this manuscript. Dr. Denise A. Yardley is the guarantor for this article, and takes responsibility for the integrity of the work as a whole.
Conflict of interest
Wentao Feng is an employee of Novartis.
Ayelet Cahana is an employee of Novartis.
Tetiana Taran is an employee of Novartis.
David Lebwohl is an employee of Novartis.
Francis P. Arena has been a consultant/advisor for Novartis and has received research funding from Novartis.
José Baselga has been a consultant/advisor for Novartis.
Mario Campone has been a consultant/advisor for Novartis, has received honoraria from Novartis, and has received research funding from Novartis.
Frans Erdkamp has been a consultant/advisor for Amgen, Roche, Novartis, and Sanofi-Aventis.
Michael Gnant has been a consultant/advisor for Novartis and Herrion, has received honoraria from Amgen, Pfizer, Novartis, GlaxoSmithKline, Bayer, Sandoz, AstraZeneca, and Genomic Health, and has received research funding from GlaxoSmithKline, Sanofi-Aventis, and Roche.
Gabriel N. Hortobagyi has been a consultant/advisor for Allergan, Amgen, Antigen Express, AstraZeneca, Galena, Genentech, Novartis, Rockpointe, and Taivex, and has received research funding from Novartis.
Bohuslav Melichar has been a consultant/advisor for Roche and Novartis, has received honoraria from Amgen, Pfizer, GlaxoSmithKline, and has received other forms of remuneration from Novartis and Roche.
Shinzaburo Noguchi has been a consultant/advisor for Novartis, has received honoraria from Novartis, and has received research funding from Novartis and Pfizer.
Kathleen I. Pritchard has been a consultant/advisor for, received honoraria from, and provided expert testimony for Novartis, GlaxoSmithKline, Boehringer-Ingelheim, Amgen, AstraZeneca, Roche, Bristol-Myers Squibb, Sanofi-Aventis, and Pfizer.
Denise A. Yardley has been a consultant/advisor for Novartis.
Martine Piccart has been a consultant/advisor for Novartis.
Barbara Pistilli has been a consultant/advisor for Novartis Oncology.
Wael A. Harb has received research funding from Horizon Oncology Research.
Hope S. Rugo has received research funding and other forms of remuneration from Novartis.
Howard A. Burris III and Katarina Petrakova declare that they have no conflicts of interest.
Compliance with ethical guidelines
This study was in accordance with the ethical standards of the institutional review board at each participating center and the study was conducted in accordance with the principles of Good Clinical Practice, the provisions of the Declaration of Helsinki of 1975, as revised in 2000 and 2008, and other applicable local regulations. Written informed consent was obtained from all patients before enrollment.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.Lim E, Metzger-Filho O, Winer EP. The natural history of hormone receptor-positive breast cancer. Oncology (Williston Park). 2012;26(8):688–94, 696. [PubMed]
- 2.National Comprehensive Cancer Network (NCCN) Clinical practice guidelines in oncology: breast cancer, Version 2.2011. http://www.nccn.org/patients/guidelines/breast/index.html (accessed October 7, 2013).
- 3.Burstein HJ, Prestrud AA, Seidenfeld J, et al. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28(23):3784–3796. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardoso F, Costa A, Norton L, et al. 1st International consensus guidelines for advanced breast cancer (ABC 1) Breast. 2012;21(3):242–252. doi: 10.1016/j.breast.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Ring A, Dowsett M. Mechanisms of tamoxifen resistance. Endocr Relat Cancer. 2004;11(4):643–658. doi: 10.1677/erc.1.00776. [DOI] [PubMed] [Google Scholar]
- 6.Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26(10):1664–1670. doi: 10.1200/JCO.2007.13.5822. [DOI] [PubMed] [Google Scholar]
- 7.Di Leo A, Jerusalem G, Petruzelka L, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2010;28(30):4594–4600. doi: 10.1200/JCO.2010.28.8415. [DOI] [PubMed] [Google Scholar]
- 8.Johnston SR, Kilburn LS, Ellis P, et al. Fulvestrant plus anastrozole or placebo versus exemestane alone after progression on non-steroidal aromatase inhibitors in postmenopausal patients with hormone-receptor-positive locally advanced or metastatic breast cancer (SoFEA): a composite, multicentre, phase 3 randomised trial. Lancet Oncol. 2013;14(10):989–998. doi: 10.1016/S1470-2045(13)70322-X. [DOI] [PubMed] [Google Scholar]
- 9.Mehta RS, Barlow WE, Albain KS, et al. Combination anastrozole and fulvestrant in metastatic breast cancer. N Engl J Med. 2012;367(5):435–444. doi: 10.1056/NEJMoa1201622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergh J, Jonsson PE, Lidbrink EK, et al. FACT: an open-label randomized phase III study of fulvestrant and anastrozole in combination compared with anastrozole alone as first-line therapy for patients with receptor-positive postmenopausal breast cancer. J Clin Oncol. 2012;30(16):1919–1925. doi: 10.1200/JCO.2011.38.1095. [DOI] [PubMed] [Google Scholar]
- 11.Miller TW, Hennessy BT, Gonzalez-Angulo AM, et al. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J Clin Invest. 2010;120(7):2406–2413. doi: 10.1172/JCI41680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein P, Ordentlich P, McCulloch W, Cruickshank S, Rees M, Yardley DA. Characterization of the overall survival benefit in ENCORE 301, a randomized placebo-controlled phase II study of exemestane with and without entinostat in ER+ postmenopausal women with metastatic breast cancer [abstract]. J Clin Oncol. 2012;30(Suppl):Abstract 567.
- 13.Bachelot T, Bourgier C, Cropet C, et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J Clin Oncol. 2012;30(22):2718–2724. doi: 10.1200/JCO.2011.39.0708. [DOI] [PubMed] [Google Scholar]
- 14.Baselga J, Semiglazov V, van Dam P, et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009;27(16):2630–2637. doi: 10.1200/JCO.2008.18.8391. [DOI] [PubMed] [Google Scholar]
- 15.Boulay A, Rudloff J, Ye J, et al. Dual inhibition of mTOR and estrogen receptor signaling in vitro induces cell death in models of breast cancer. Clin Cancer Res. 2005;11(14):5319–5328. doi: 10.1158/1078-0432.CCR-04-2402. [DOI] [PubMed] [Google Scholar]
- 16.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366(6):520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE), version 3.0 published August 9, 2006. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf (accessed October 7, 2013).
- 18.Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70(3):659–663. doi: 10.2307/2336502. [DOI] [Google Scholar]
- 19.Beck JT, Rugo HS, Burris HA, et al. BOLERO-2: Health-related quality-of-life in metastatic breast cancer patients treated with everolimus and exemestane versus exemestane [abstract]. J Clin Oncol. 2012;30(Suppl):Abstract 539.
- 20.Burris HA, 3rd, Lebrun F, Rugo HS, et al. Health-related quality of life of patients with advanced breast cancer treated with everolimus plus exemestane versus placebo plus exemestane in the phase 3, randomized, controlled, BOLERO-2 trial. Cancer. 2013;119(10):1908–1915. doi: 10.1002/cncr.28010. [DOI] [PubMed] [Google Scholar]
- 21.Cardoso F, Bedard PL, Winer EP, et al. International guidelines for management of metastatic breast cancer: combination vs sequential single-agent chemotherapy. J Natl Cancer Inst. 2009;101(17):1174–1181. doi: 10.1093/jnci/djp235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jerusalem G, Marinsek N, Ricci J-F, et al. Clinical management and resource utilisation for postmenopausal hormone-receptor-positive HER2-negative (HR+HER2-) advanced breast cancer (BC) in Europe [abstract]. Presented at the 2012 ESMO Congress, September 28–October 2, 2012, Vienna, Austria. Abstract 336P.
- 23.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372(9637):449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 24.Pavel ME, Hainsworth JD, Baudin E, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet. 2011;378(9808):2005–2012. doi: 10.1016/S0140-6736(11)61742-X. [DOI] [PubMed] [Google Scholar]
- 25.Porta C, Osanto S, Ravaud A, et al. Management of adverse events associated with the use of everolimus in patients with advanced renal cell carcinoma. Eur J Cancer. 2011;47(9):1287–1298. doi: 10.1016/j.ejca.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 26.Afinitor (everolimus) prescribing information. Revised 2012. Novartis Pharmaceuticals Corporation: East Hanover, NJ. http://www.pharma.us.novartis.com/product/pi/pdf/afinitor.pdf (accessed October 7, 2013).
- 27.White DA, Camus P, Endo M, et al. Noninfectious pneumonitis after everolimus therapy for advanced renal cell carcinoma. Am J Respir Crit Care Med. 2010;182(3):396–403. doi: 10.1164/rccm.200911-1720OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


