Graphical abstract
Highlights
► Determination of resistance status of Plasmodium vivaxin vivo requires a rigorous phenotyping procedure. ► Standard chloroquine therapy and measurement of blood level by HPLC was proposed as a means. ► The existence of asexual parasite at day 3–28 days post treatment represents resistant parasite.
Keywords: Plasmodium vivax, Phenotype, Papua, Indonesia
Abstract
Chloroquine (CQ)-resistant Plasmodium vivax was first documented in 1989 and threatens much of eastern Indonesia, with > 50% of therapeutic failure rates. We screened 2236 subjects for malaria infection through active case detection and identified 232 infected cases with 100 subjects carried P. vivax mono infection. We prospectively evaluated therapeutic responses to CQ in 73 subjects infected by P. vivax in northeastern Papua, Indonesia. We phenotyped these infections as susceptible or resistant to CQ using a 28-day in vivo test format. Eighteen subjects (25%) had persistent or recurrent parasitemia during the test and were provisionally classified as resistant. Among the remainder, 46 (63%) subjects had no persistent or recurrent parasitemia and were classified as having infections sensitive to CQ, 4 were lost to follow up, and 5 dropped out. Among the 18 provisionally resistant cases, 1 subject (6%) had persistent parasitemia at Day 3 and was considered as a direct treatment failure, 2 subjects (11%) had recurrent parasitemia by Day 7 and were considered early treatment failures, and 7 (39%) and 8 (44%) had recurrent parasitemia by Days 14 and 28, respectively. Analysis of blood for CQ+N-desethylchloroquine (DCQ) levels on day of recurrence from 15 of the 18 with treatment failures showed 11 subjects having CQ+DCQ blood levels ⩾ 100 ng/ml and 2 with CQ+DCQ blood levels < 100 ng/ml. The 28-day cumulative incidence of therapeutic failure likely due to parasite resistance was 17.5%. These findings affirm P. vivax resistance to CQ in eastern Indonesia, albeit at lower levels than reported elsewhere. This simple means of phenotyping P. vivax infections could be implemented in other malaria endemic areas of Indonesia.
1. Introduction
Plasmodium vivax threatens 2.8 billion people with a debilitating and potentially lethal infection (Guerra et al., 2010). Chloroquine (CQ) remains first-line therapy for acute vivax malaria after 65 years despite more than 20 years of mounting evidence of resistance (Baird, 2004, 2009). Resistance of Plasmodium falciparum to CQ appeared in the late 1950s in Southeast Asia and South America (Wellems and Plowe, 2001), and today it occurs globally (Price and Nosten, 2001). Resistance of P. vivax to CQ was first reported in 1989 from an Australian repatriated from Papua New Guinea (Rieckman et al., 1989). Resistance was subsequently reported from Sumatra and Papua, Indonesia in 1991 (Baird et al., 1991; Schwartz et al., 1991; Murphy et al., 1993), Myanmar in 1993 and 1995 (Myat-Phone et al., 1993; Than et al., 1995), India in 1995 (Garg et al., 1995; Singh, 2000), Malaysian Borneo in 1996 (Clas et al., 1996), Guyana, South America in 1996 (Phillips et al., 1996), parts of the Amazon Brazil (Alecrim et al., 1999; de Santana et al., 2007; Simoes et al., 2007), Colombia in 2001 (Soto et al., 2001), Vietnam in 2002 (Tasanor et al., 2002), Peru in 2003 (Ruebush et al., 2003), Turkey in 2004 (Kurcer et al., 2004), Ethiopia in 2008 (Teka et al., 2008), and Republic of Korea in 2009 (Lee et al., 2009). A 2003 report from northeastern Indonesian Papua showed 84% risk of therapeutic failure with CQ against P. vivax (Sumawinata et al., 2003). More recent reports from eastern Indonesia show failure rates routinely exceeding 50% (Tjitra et al., 2008). Chloroquine-resistant P. vivax (CRPV) represents a widespread and apparently worsening problem.
Despite the importance of CRPV to public health, no standardized means of ascertaining resistance has been developed. An in vivo test procedure was described over a decade ago (WHO, 2000) and has been applied, at least partially, in some studies of this problem. Unlike P. falciparum, some sporozoites of P. vivax stay dormant in the liver as forms called hypnozoites, while others initiate the primary parasitemia and the consequent acute attack of vivax malaria. Hypnozoites later activate, develop and cause a secondary parasitemia and acute disease called a relapse. In endemic settings it is not known if any given patient presenting with acute vivax malaria is experiencing a primary or secondary parasitemia. This represents the fundamental problem for estimating therapeutic efficacy (Imwong et al., 2007): uncertainty regarding the origin of the new parasitemia as a consequence of therapeutic failure as opposed to relapse unrelated to treatment of the primary attack.
The in vivo test format (WHO, 2000) showed promise in solving this ambiguity in the specific instance of CQ by avoiding the necessity of appropriate classification of recurrent parasitemia as relapse, reinfection or recrudescence. It was reasoned that parasitemia despite CQ levels exceeding the minimally effective concentration (MEC) for CQ-sensitive P. vivax must be resistant to CQ regardless of its origin (Baird et al., 1996, 1997). However, such potential classification bias remains, especially for studies correlating CRPV phenotype and P. vivax genotype(s). The aim of the current study was to establish a means of phenotyping CQ resistance among parasites using an in vivo test format.
2. Materials and methods
2.1. Study site
The study was conducted at Sentani (latitude 2°34′0″S, longitude 140°29′0″E) northeastern Papua, Indonesia, from June to August 2007. The area is typically meso- to hyper-endemic with perennial falciparum and vivax malarias. The Anopheles punctulatus group of mosquitoes are the overwhelmingly dominant vector species (An. punctulatus, Anopheles farauti, and Anopheles koliensis) in this region.
2.2. Subject enrolment
Cross-sectional microscopic surveys of blood films from participating subjects in endemic communities identified persons with P. vivax asexual parasitemia. Potential study participants were excluded from study if found to be: (1) also positive for falciparum or any other species of malaria; (2) positive for signs of severe or complicated malaria; (3) pregnant; (4) positive for history of allergy to the study drugs; (5) admitting to completion of antimalarial therapy within past 72 h; or (6) having a medical history of untreated hypertension or chronic heart, kidney or liver disease. All adult subjects and the parents of child participants individually signed informed consent to participate. The study protocol was reviewed and approved by the Eijkman Institute Research Ethics Commitee for the use of human subjects in medical research.
2.3. Treatment
All study subjects were given directly observed treatment consisting of a total dose of 25 mg/kg body weight of CQ over 48 h (10 mg/kg on D0 and D1, and 5 mg/kg on D2). Subjects vomiting within 30 min were given another dose. Paracetamol was provided to subjects with an axillary temperature of ⩾ 37.5 °C. Primaquine therapy against relapse was not provided until discontinuation from the study, i.e., day of recurrence or day 28.
2.4. Follow up
Study subjects were followed for up to 28 days. Subjects were evaluated on Days 1, 2, 3, 7, 14, 21 and 28, or at any time they complained of illness. Evaluation consisted of an axillary temperature along with a blood sample collected by finger prick for both microscopic and molecular examination (using filter paper; Whatman, Schleicher & Schuell, Whatman International Ltd., Maidstone, UK). Subjects not clearing parasitemia or developing recurrent parasitemia were offered unsupervised oral artemisinin combined therapy (ACT; artesunate with amodiaquine) for 3 days plus primaquine for 14 days (0.25 mg/kg/day) and were not further evaluated. Subjects were discharged from the study upon treatment failure, loss to follow-up, or reaching Day 28 without recurrent parasitemia.
2.5. Treatment outcome classification
Parasites infecting study subjects of the 28-day test were phenotyped for clinical responsiveness to CQ as follows.
-
(i)
Sensitive: Asexual parasitemia not detectable by qualified microscopy within 72 h of initiating therapy and failing to recur up to day 28 after therapy.
-
(ii)
Resistant: Asexual parasitemia remains detectable by qualified microscopy at 72 h after initiating therapy, or becoming sub-patent but reappearing within 28 days of treatment in the presence of blood levels of CQ+DCQ > 100 ng/ml, the estimated minimally effective concentration (MEC) of CQ-sensitive P. vivax (Patchen et al., 1983; Baird et al., 1996, 1997).
Resistant infections were further classified as direct treatment failures if the microscopically patent asexual parasitemia on Day 2 was similar to or higher than that of Day 0 (⩾25%). Resistant infections were classified as early treatment failures if they showed any level of microscopically patent asexual parasitemia between Days 3–7. Subjects with asexual parasitemia that became undetectable but reappeared at any time between Days 8 and 28 were provisionally classified as having recurrent treatment failures.
-
(iii)
Indeterminate: Infections were classified as indeterminate CQ resistance phenotypes when the study subject was withdrawn from analysis for any reason, including loss to follow-up, protocol violations, inter-current parasitemia by other plasmodia, or recurrent P. vivax parasitemia with < 100 ng/ml CQ+DCQ.
2.6. Whole blood CQ+DCQ levels
Blood levels of CQ and its major metabolite DCQ were measured using high-performance liquid chromatography (HPLC) according to the method previously described (Patchen et al., 1983). A micropipette set to deliver 100 μl whole blood was used to spot this blood onto filter paper on day of enrollment and at the time of recurrence of parasitemia. Blood spots were air dried, placed in ziplock bags and stored at ambient temperature until later testing. Prior to extraction, an isopropyl analog of CQ was added to the blood blot in a volume 50 μl and allowed to dry, to allow for an extraction control. Following mincing of the filter paper, extraction was performed using 0.5 ml of 20% Na3PO4·12H2O and 0.5 ml methyl-tertbutylether. The solution was vortexed for 30 s and centrifuged to separate the organic phase. The organic phase was then transferred to another tube and evaporated to dryness with air. The sample was reconstituted with HPLC mobile phase and a portion was injected into the HPLC system (Waters™, USA).
2.7. Laboratory analysis
A finger prick was performed at enrolment to make thick and thin blood smears, blots on filter paper for parasite genotyping, and hemoglobin measurement (Hemocue™ Hb201+, Angelholm, Sweden). Smears and filter paper blood samples were also collected from finger pricks on days 1, 2, 3, 7, 14, 21 and 28. Smears were read by expert microscopists and confirmed by polymerase chain reaction (PCR) (25). Parasite density was determined by counting the number of parasites per leukocytes and assuming an average of 8000 leukocytes/μl of blood to convert to volume units (Gilles, 1993). Discordant findings between microscopy and PCR were resolved by independent PCR confirmation.
3. Results
3.1. Recruitment
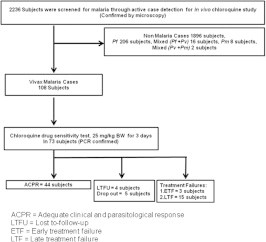
Of 2236 subjects screened, 340 (15%) were found positive for malaria. Falciparum malaria dominated at 60% (206/340), followed by vivax malaria at 32% (108/340). An additional 8 subjects (2%) were positive for Plasmodium malariae, 16 (5%) had P. falciparum and P. vivax co-infection, and 2 had P. vivax and P. malariae co-infection. A total of 73 (68%) of the 108 P. vivax cases met inclusion criteria. The 35 remaining subjects were excluded due to age, inadequate asexual parasitemia, or refusal of informed consent (Fig. 1). During follow up, 4 subjects were lost from the study and 5 dropped out between Days 7–14 without citing reasons.
Fig. 1.
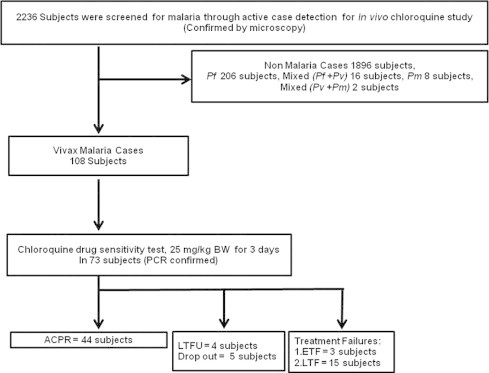
Flow chart of subject enrollment. ACPR = Adequate clinical and parasitological response; LTFU = Lost to-follow-up; ETF = Early treatment failure; LTF = Late treatment failure. ACPR = Adequate clinical and parasitological response; LTFU = Lost to-follow-up; ETF = Early treatment failure; LTF = Late treatment failure.
3.2. Subjects
Table 1 lists the demographic characteristics of the study subjects at enrolment. Of 73 enrolled subjects, 36 were males and 37 were females, with ages ranging from 2 to 60 years (mean 38 years). At enrolment only 3 subjects had evidence of fever (⩾37.5 °C). Hemoglobin levels ranged from 7.3 to 15.3 g/dl and 24 subjects (33%) had Hb levels < 11 g/dl. The density of asexual forms ranged from 40 to 2080/μl, whereas sexual stages (gametocytes) occurred in 8 subjects (40–320/μl).
Table 1.
Characteristics of the subjects at enrollment in the 28 days in vivo CQ test in Jayapura District.
| Number of subjects | 73 |
| Median (range) age (year) | 13.2 (2–60) |
| Median (range) hemoglobin level (D0) (g/dl) | 11.8 (7–15) |
| Geometric mean asexual parasitemia per μl blood | 560 (40–2080) |
| Mean CQ+DCQ level (ng/ml of blood) | 40 (0–252) |
3.3. Parasite clearance
46 subjects cleared parasitemia before Day 3 and without again becoming microscopically patent for vivax malaria up to Day 28 after enrollment. 18 other subjects had persistent or recurrent parasitemia during the test: 1 (6%) had persistent parasitemia at Day 3; another 2 (11%) cleared parasitemia before Day 3 but recurred by Day 7; by day 14 another 7 subjects (39%) had recurrent parasitemia, as did 8 others (44%) by Day 28. The balance of 9 subjects of the 73 enrolled were either lost (4) or dropped out (5). The 28-day cumulative incidence of therapeutic failure was 17% (Table 2).
Table 2.
Cumulative incidence of CQ therapeutic failure in vivax malaria at Sentani, northeastern Papua, Indonesia.
| Day | Number at risk (N) | Incident cases (I) | Withdrawn (W) | Interval risk (IR) | Cumulative risk (CR) |
|---|---|---|---|---|---|
| 0 | 73 | 0 | 0 | 0 | 0 |
| 3 | 73 | 1 | 3 | 0.0143 | 0.0143 |
| 5 | 69 | 1 | 1 | 0.0147 | 0.0288 |
| 7 | 67 | 1 | 0 | 0.0149 | 0.0433 |
| 11 | 66 | 0 | 2 | 0 | 0.0433 |
| 13 | 64 | 0 | 1 | 0 | 0.0433 |
| 14 | 63 | 4 | 4 | 0.0678 | 0.1081 |
| 15 | 55 | 2 | 0 | 0.0364 | 0.1406 |
| 17 | 53 | 1 | 0 | 0.0189 | 0.1568 |
| 18 | 52 | 0 | 1 | 0 | 0.1568 |
| 20 | 51 | 0 | 1 | 0 | 0.1568 |
| 21 | 50 | 1 | 3 | 0.0213 | 0.1747 |
| 28 | 46 | 0 | 0 | 0 | 0.1747 |
Day = day of the test.
N = number of subjects remaining at risk.
I = incident of resistant cases.
W = withdrawals due to intercurrent parasitemia by the other species, drop out and CQ level less than 100 ng/ml at positive malaria.
IR = interval risk i{N − (w/2)}−1.
CIRn = cumulative incidence of resistant case.
=1 − ((1 − IRn) × (1 − CIFn−1)) where n is day of test and n − 1 is the prior interval, e.g., for calculating CIR day 14, use IR day 14 and CIR day 7.
3.4. Adverse reactions
The most frequent clinical complaints reported by subjects at the time of enrolment were headache (24%), fever (20%), rigors (11%), dizziness (11%), weakness (2%), nausea (2%) and vomiting (1%). No severe side effects were noted in any of the subjects enrolled and all recovered uneventfully within 3 days of treatment.
3.5. CQ+DCQ blood levels
A total of 24 subjects (33%) had detectable CQ in blood before treatment, with concentrations ranging from 19 to 90 ng/ml and 3 (4%) of these had ⩾ 100 ng/ml. The remaining 46 samples had undetectable CQ at the day before treatment. Table 3 summarizes the analysis of CQ+DCQ levels among the 18 subjects with recurrent parasitemia. Samples from 5 subjects were unavailable. Among the remaining 13, CQ+DCQ blood levels were ⩾ 100 ng/ml in 11 and < 100 ng/ml in 2.
Table 3.
HPLC analysis of the CQ plus DCQ blood level on recurrent days among the subjects with treatment failure.
| Subject’s code | Drug level (ng/ml of blood) |
Phenotype⁎ | |
|---|---|---|---|
| D0 | DR | ||
| CRPV-005 | 38 | 454 | Resistant |
| CRPV-007 | ND | 231 | Resistant |
| CRPV-008 | ND | 181 | Resistant |
| CRPV-011 | 41 | 439 | Resistant |
| CRPV-012 | ND | 423 | Resistant |
| CRPV-013 | 97 | 204 | Resistant |
| CRPV-014 | 56 | 516 | Resistant |
| CRPV-030 | 16 | 238 | Resistant |
| CRPV-033 | ND | ND | NA |
| CRPV-036 | 22 | 409 | Resistant |
| CRPV-041 | ND | 147 | Resistant |
| CRPV-042 | 164 | ND | NA |
| CRPV-045 | ND | 43 | NA |
| CRPV-046 | 252 | 100 | Resistant |
| CRPV-061 | 31 | ND | NA |
| CRPV-062 | 106 | ND | NA |
| CRPV-063 | 96 | 24 | NA |
| CRPV-064 | ND | ND | NA |
D0 = day of enrollment; DR = day of recurrence; ND = not determined; NA = not applicable.
MEC: minimum effective concentration = 100 ng/ml.
4. Discussion
This study applied a relatively simple algorithm to classify P. vivax infection as clinically resistant or sensitive to CQ. Minimizing the probability of misclassification requires completion of the 28-day in vivo test, along with ensuring directly observed therapy and addressing other potential confounders of therapeutic outcome like poor drug absorbtion (Sumawinata et al., 2003). The amount of drug on day of recurrent parasitemia represents a critical tool in phenotyping CQ resistance; the measurement avoids confounding due to uncertainty regarding the possible causes of recurrence: recrudescence, reinfection, or relapse (Baird et al., 1997).
Parasites in subjects clearing asexual parasitemia within 3 days who had no recurrent asexual parasitemia up to day 28 were phenotyped, a priori, as sensitive to CQ. Likewise, parasites in subjects failing to clear microscopically patent asexual parasitemia within 3 days or reappearing within 7 days were phenotyped, a priori, resistant to CQ. However, infections that reappeared between days 8 and 28 were only provisionally classified as resistant. These infections required measurement of CQ+DCQ in whole blood on the day of recurrence: parasitemias recurring with ⩾ 100 ng/ml CQ+DCQ were confirmed as resistant, and those recurring with < 100 ng/ml were classified as indeterminate.
Asexual parasitemia recurring in the 28 days following treatment with CQ may originate from recrudescence, reinfection or relapse. The algorithm for phenotyping clinical resistance to CQ we applied in this study does not attempt to distinguish these sources of parasitemia. Instead, we classified recurrences appearing with ⩾ 100 ng/ml CQ+DCQ as unambiguously resistant on the weight of evidence provided by development to microscopic patency despite drug levels known to be lethal to CQ sensitive P. vivax.
Among the 73 subjects evaluated at the study site in Sentani, Papua, Indonesia, 13 parasitemias were classified as indeterminate, 49 as sensitive, and 11 as resistant. Because the indeterminate infections contribute person-time at risk of treatment failure prior to withdrawal, a life table analysis provides the ideal means of estimating overall risk of therapeutic failure in the study population. In this study, that method provided the estimate of 17% as the 28-day cumulative incidence of resistance at the study site in 2007. This is much lower risk than an estimate of 84% derived from non-immune migrants to the nearby Arso region (Sumawinata et al., 2003). Naturally acquired immunity conferred by repeated infection among life-long indigenous residents of Sentani may largely explain the sharp difference in these estimates.
This study generated 49 populations of parasites confirmed to be clinically sensitive to CQ, and 11 others confirmed as clinically resistant. The parasite populations classified as sensitive were represented by those present at enrollment. The same may be said of the parasite populations classified as direct or early treatment failures. However, among recurrent treatment failures, the parasite populations present at enrollment cannot be phenotyped – it is only the parasite populations appearing on the day of recurrence that represent that phenotype.
In conclusion, this study successfully phenotyped 11 CQ-resistant P. vivax isolates in Papua Indonesia and this might be a vital step in studies leading to identification of the underlying genetic polymorphisms. Further study to determine the extent of CQ resistance in this and other populations at risk, especially given the high frequency of P. vivax infection and its newly appreciated potential to cause severe outcomes, is required.
Acknowledgments
The authors wish to thank Prof. S. Marzuki of the Eijkman Institute for Molecular Biology for his encouragement and support, Sofyan Masbar, Alert Asia Foundation, Jakarta, Gurid P. Eko Mulyo, Biomedic Program, Graduate Program in Faculty of Medicine, University of Indonesia, for their assistance during sample collection, Budhi Leksana and Amir Faisal, U.S. Naval Medical Research Unit No. 2, Jakarta, Indonesia, for their assistance during HPLC analysis. We thank Paula Maguina of UCSD for her expertise and assistance in coordinating ethics, logistics, and administrative research matters with the oversight and compliance bodies in Indonesia and La Jolla, California, USA.
This study is part of Puji B.S. Asih Ph.D Program in University of Indonesia through PRIOR Program and Funded through A Grant-In-Aid from The Netherlands Foundation for the Advancement of Tropical Research, The Netherlands Foundation for Health Research and Development. Samples collection in Sentani District, Papua Province was supported by U.S. Public Health Service, National Institutes of Health: 1U19AI089681, R01AI067727 and K24 AI068903. J. Kevin Baird is supported by a grant from the Wellcome Trust #B9RJIXO.
References
- Alecrim M.C., Alecrim W., Macedo V. Plasmodium vivax resistance to chloroquine (R2) and mefloquine (R3) in Brazilian Amazon region. Rev. Soc. Bras. Med. Trop. 1999;32:67–68. doi: 10.1590/s0037-86821999000100013. [DOI] [PubMed] [Google Scholar]
- Baird J.K., Basri H., Purnomo B.M.J., Subianto B., Patchen L.C., Hoffman S.L. Resistance to chloroquine by Plasmodium vivax in Irian Jaya. Indonesia Am. J. Trop. Med. Hyg. 1991;44:547–552. doi: 10.4269/ajtmh.1991.44.547. [DOI] [PubMed] [Google Scholar]
- Baird J.K., Nalim M.F.S., Basri H., Masbar S., Leksana B., Tjitra E., Dewi R.M., Khairani M., Wignal F.S. Survey of resistance to chloroquine by Plasmodium vivax in Indonesia. Trans. R. Soc. Trop. Med. Hyg. 1996;90:409–411. doi: 10.1016/s0035-9203(96)90526-x. [DOI] [PubMed] [Google Scholar]
- Baird J.K., Leksana B., Masbar S., Sutanihardja M.A., Fryauff D.J., Subianto B. Diagnosis of resistance to chloroquine by Plasmodium vivax timing of recurrence and whole blood chloroquine levels. Am. J. Trop. Med. Hyg. 1997;56:621–626. doi: 10.4269/ajtmh.1997.56.621. [DOI] [PubMed] [Google Scholar]
- Baird, J.K., 2004. Chloroquine resistance in Plasmodium vivax. Antimicrob. Agents. Chemother. 11, 4075–4083. [DOI] [PMC free article] [PubMed]
- Baird, J.K., 2009. Resistance to therapies for infection by Plasmodium vivax. Clinc. Microbiol. Rev. 508–534. [DOI] [PMC free article] [PubMed]
- Clas, A., Johan, W., Carlson, H., 1996. Chloroquine-resistant Plasmodium vivax malaria in Borneo. J. Travel. Med. 3, 24. [DOI] [PubMed]
- de Santana F.S., Arcanjo A.R., Chehuan Y.M., Costa M.R., Martinez-Espinosa F.E., Vieira J.L., Barbosa M.G., Alecrim W.D., Alecrim M.G. Chloroquine-resistant Plasmodium vivax, Brazilian Amazon. Emerg. Infect. Dis. 2007;13:1125–1126. doi: 10.3201/eid1307.061386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg M., Gopinathan N., Bodhe P., Kshirsagar N.A. Vivax malaria resistant to chloroquine: case reports from Bombay. Trans. R. Soc. Trop. Med. Hyg. 1995;89:656–657. doi: 10.1016/0035-9203(95)90432-8. [DOI] [PubMed] [Google Scholar]
- Gilles, H.M., 1993. Diagnostic method in malaria. In: Gilles, H.M., Warrel, D.A. (Eds.), Bruce-Chwatt”s Essential Malariology. Oxford University Press, New York, pp. 79–95.
- Guerra, C.A., Howes, R.E., Patil, A.P., Gething, P.W., Van Boeckel, T.P., Temperley, W.H., Kabaria, C.W., Tatem, A.J., Manh, B.H., Elyazar, I.R.F., Baird, J.K., Snow, R.W., Hay, S.I., 2010. The international limits and population at risk of Plasmodium vivax transmission in 2009. PLoS Negl. Trop. Dis. 4, e774. [DOI] [PMC free article] [PubMed]
- Imwong M., Snounou G., Pukrittayakamee S., Tanomsing N., Kim J.R., Nandy A., Guthmann J.P., Nosten F., Carlton J., Looareesuwan S., Nair S., Sudimack D., Day N.P.J., Anderson T.J.C., White N.J. Relapses of Plasmodium vivax infection usually result from activation of heterologous hypnozoites. J. Infect. Dis. 2007;195:927–933. doi: 10.1086/512241. [DOI] [PubMed] [Google Scholar]
- Kurcer M.A., Simsek Z., Zeyrek F.Y., Atay S., Celik H., Kat I., Topluoglu A. Efficacy of chloroquine in the treatment of Plasmodium vivax malaria in Turkey. Ann. Trop. Med. Parasitol. 2004;98:447–451. doi: 10.1179/000349804225021343. [DOI] [PubMed] [Google Scholar]
- Lee K.S., Kim T.H., Kim E.S., Lim H.S., Yeom J.S., Jun G., Park J.W. Chloroquine-resistant Plasmodium vivax in the republic of Korea. Am. J. Trop. Med. Hyg. 2009;80:215–217. [PubMed] [Google Scholar]
- Than Marlar, Kyaw Myat-Phone, Soe Aye-Yu, Ge Khaing-Khaing, Sabai Ma, Oo Myin. Development of resistance to chloroquine by Plasmodium vivax in Myanmar. Trans. R. Soc. Trop. Med. Hyg. 1995;89:307–308. doi: 10.1016/0035-9203(95)90556-1. [DOI] [PubMed] [Google Scholar]
- Murphy G.S., Basri H., Purnomo, Anderson E.M., Bangs M.J., Mounts D.L., Gorden J., Lal A.A., Purwokusumo A.R., Hardjosuwarno S. Vivax malaria resistant to treatment and prophylaxis with chloroquine. Lancet. 1993;341:96–100. doi: 10.1016/0140-6736(93)92568-e. [DOI] [PubMed] [Google Scholar]
- Myat-Phone Kyaw, Oo Myint, Zin Thaw, Lwin Myint, Aye Kyin-Hla, Yin Nwe-Nwe. Emergence of chloroquine-resistant Plasmodium vivax in Myanmar (Burma) Trans. R. Soc. Trop. Med. Hyg. 1993;87:687. doi: 10.1016/0035-9203(93)90294-z. [DOI] [PubMed] [Google Scholar]
- Patchen L.C., Mount D.L., Schwartz I.K., Churchill F.C. Analysis of filter-paper-absorbed, finger-stick blood samples for chloroquine and its major metabolite using high-performance liquid chromatography with fluorescence detection. J. Chrom. 1983;273:81–89. doi: 10.1016/s0378-4347(00)84758-1. [DOI] [PubMed] [Google Scholar]
- Phillips E.J., Keystone J.S., Kain K.C. Failure of combined chloroquine and high-dose primaquine therapy for Plasmodium vivax malaria acquired in Guyana, South America. Clin. Infect. Dis. 1996;23:1171–1173. doi: 10.1093/clinids/23.5.1171. [DOI] [PubMed] [Google Scholar]
- Price R.N., Nosten F. Drug resistant falciparum malaria: Clinical consequences and strategies for prevention. Drug. Res. Update. 2001;4:187–196. doi: 10.1054/drup.2001.0195. [DOI] [PubMed] [Google Scholar]
- Rieckman H., Davis D.R., Hutton D.C. Plasmodium vivx resistance to chloroquine? Lancet ii. 1989;1183:1184. doi: 10.1016/s0140-6736(89)91792-3. [DOI] [PubMed] [Google Scholar]
- Ruebush T.K., Zegarra J., Cairo J., Andersen E.M., Green M., Pillai D.R., Marquino W., Huilca M., Arevalo E., Garcia C., Solary L., Kain K.C. Chloroquine resistant Plasmodium vivax malaria in Peru. Am. J. Trop. Med. Hyg. 2003;69:548–552. [PubMed] [Google Scholar]
- Schwartz I.K., Lackritz E.M., Patchen L.C. Chloroqune-resistanct Plasmodium vivax from Indonesia. N. Engl. J. Med. 1991;324:927. doi: 10.1056/NEJM199103283241317. [DOI] [PubMed] [Google Scholar]
- Simoes F., de Filho S., Arcanjo A.R., Chehuan Y.M., Costa M.R., Martinez-Espinosa F.E., Vieira J.L., Barbosa M.V., Alecrim W.D., Alecrim M.G. Chloroquine-resistant Plasmodium vivax, Brazilian Amazon. Emerg. Infec. Dis. 2007;13:7. doi: 10.3201/eid1307.061386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R.K. Emergence of chloroquine-resistant vivax malaria in south Bihar (India) Trans. R. Soc. Trop. Med. Hyg. 2000;94:327. [PubMed] [Google Scholar]
- Soto J., Toledo J., Gutierrez P., Luzz M., Llinas N., Cedeno N., Dunne M., Berman J. Plasmodium vivax clinically resistant to chloroquine in Colombia. Am. J. Trop. Med. Hyg. 2001;65:90–93. doi: 10.4269/ajtmh.2001.65.90. [DOI] [PubMed] [Google Scholar]
- Sumawinata I.W., Bernadeta B.L., Awalludin S., Purnomo B., Subianto S., Fryauff D.J., Baird J.K. Very high risk of therapeutic failure with chloroquine for uncomplicated Plasmodium falciparum and Plasmodium vivax malaria in Indonesian Papua. Am. J. Trop. Med. Hyg. 2003;68:416–420. [PubMed] [Google Scholar]
- Tasanor O., Noedl H., Na-Bangchang K., Congpuong K., Sirichaisinthop J., Wernsdorfer W.H. An in vitro system for assessing the sensitivity of Plasmodium vivax to chloroquine. Acta Trop. 2002;83:49–61. doi: 10.1016/s0001-706x(02)00056-6. [DOI] [PubMed] [Google Scholar]
- Teka H., Petros B., Yamuah L., Tesfaye G., Elhassan I., Muchohi S., Kokwaro G., Aseffa A., Engers H. Chloroquine-resistant Plasmodium vivax malaria in Debre Zeit, Ethiopia. Malar. J. 2008;7:220. doi: 10.1186/1475-2875-7-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjitra E., Anstey N.M., Sugiarto P., Warikar N., Kenangalem E., Karyana M., Lampah D.A., Price R.N. Multidrug-resistant Plasmodium vivax associated with severe and fatal malaria: a prospective study in Papua, Indonesia. Plos. Medicine. 2008;5:128. doi: 10.1371/journal.pmed.0050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellems T.E., Plowe C.V. Chloroquine-resistant malaria. J. Infect. Dis. 2001;184:770–776. doi: 10.1086/322858. [DOI] [PubMed] [Google Scholar]
- World Health Organisation, 2000. Assesment of Therapeutic Efficacy of Chloroquine for Uncomplicated Vivax Malaria in Asia.


