Abstract
Objective To examine whether observed differences in dementia rates between black and white older people living in the community could be explained by measures of socioeconomic status (income, financial adequacy, education, and literacy) and health related factors.
Design Prospective cohort study.
Setting General community from two clinic sites in the United States (Pittsburgh, Pennsylvania and Memphis, Tennessee).
Participants 2457 older people (mean age 73.6 years; 1019 (41.5%) black; 1233 (50.2%) women), dementia-free at baseline, in the Health, Aging, and Body Composition study.
Main outcome measure Dementia was determined over 12 years (ending January 2011) by prescribed dementia drugs, hospital records, and decline in global cognitive scores. The influence of socioeconomic status and health related factors on dementia rates was examined in a series of Cox proportional hazard models in which these variables were added sequentially in covariate blocks.
Results Over follow-up, 449 (18.3%) participants developed dementia. Black participants were more likely than white participants to develop dementia (211 (20.7%) v 238 (16.6%), P<0.001; unadjusted hazard ratio 1.44, 95% confidence interval 1.20 to 1.74). The hazard ratio lessened somewhat after adjustment for demographics, apolipoprotein E e4, comorbidities, and lifestyle factors (1.37, 1.12 to 1.67) but was greatly reduced and no longer statistically significant when socioeconomic status was added (1.09, 0.87 to 1.37).
Conclusion These findings suggest that differences in the burden of risk factors, especially socioeconomic status, may contribute to the higher rates of dementia seen among black compared with white older people. Strategies aimed at reducing these disparities may favorably affect the incidence of dementia.
Introduction
Differences in rates of dementia among diverse populations have garnered recent attention. Older black people have a higher prevalence of Alzheimer’s disease and other dementias, and potentially a faster rate of cognitive decline, compared with older white people.1 2 3 4 5 Previous studies have shown that genetic mechanisms are unlikely to account entirely for the higher risk of dementia among older black people,6 7 8 suggesting that other factors may explain this variation. Compared with older white people, black people have a higher prevalence of socioeconomic status related risk factors for cognitive impairment, including less education and literacy, and poorer health.2 3 9 Studies of dementia rates among diverse adults have generally taken into account comorbidities and education,10 11 12 but they have overlooked other socioeconomic status factors such as financial stability and literacy.
Although socioeconomic status, quantified by education and financial indicators, has been associated with measures of cognitive function,13 little is known about its contribution to the risk for dementia. Low socioeconomic status, particularly less education, has been found to predict cognitive decline independently of biomedical factors.14 Quality of education, in addition to years of education, may also be an important determinant of cognitive outcomes,3 15 16 17 18 yet few studies have accounted for this. Low socioeconomic status has also been associated with worse physical and mental health outcomes,19 20 21 22 which may further increase the risk of cognitive impairment.23
We sought to determine whether black older adults had a higher incidence of dementia than white older adults and, if so, whether socioeconomic in addition to lifestyle and health related factors explained this variation.
Methods
Study population
Participants were part of the Health, Aging, and Body Composition study, a prospective cohort study, beginning in 1997, of 3075 community dwelling older adults aged 70-79 years and without dementia on recruitment to the study. To identify potential participants, a random sample of white Medicare eligible older people and all black older people within designated zip code areas surrounding the Memphis, Tennessee or Pittsburgh, Pennsylvania field centers were contacted. Eligibility requirements included no reported difficulties performing activities of daily living, walking a quarter of a mile, or climbing 10 steps; no life threatening cancers; and no intention of moving out of the study area. All participants signed an informed written consent. Our analytic cohort consisted of 2457 Health, Aging, and Body Composition study participants who had complete information for all variables of interest.
Measurements
Dementia assessment
Onset of dementia was determined by the date of the first available record of a diagnosis of dementia identified by any of the following criteria: hospital records indicating a dementia related hospital event (participants were queried about hospital admissions every six months, and hospital records were reviewed for either a primary or a secondary diagnosis of dementia related to the admission) and a modified mini-mental state examination score of 90 or below (completed at baseline and years 3, 5, 8, 10, and 11); record of prescribed dementia drugs (galantamine, rivastigmine, memantine, donepezil, or tacrine) determined with a drug inventory administered at the annual visit; or race stratified change of at least 1.5 standard deviations on the modified mini-mental state examination score from baseline to the last available visit. We were not able to assess diagnosis of dementia if hospital records and clinic data were missing for a participant. We defined time to dementia as the length of the interval between the study baseline and when the participant was classified with a diagnosis of dementia or censored from observation at the last available contact. Data were available through January 2011.
Demographic and socioeconomic characteristics
Participants’ race, age, and sex were based on self report at baseline. Socioeconomic status measures included family income (<$10 00; $10 000-49 000; ≥$50 000); perceived financial adequacy (categorized as financially inadequate if participants reported that their income did not meet financial needs, they did not have enough money for food, or they did not have money left at the end of the month); and education variables, which included level of education (categorized as less than or some high school; high school graduate or technical school; some college) and literacy level, determined by the rapid estimate of adult literacy in medicine (REALM) test,24 a word recognition test of 66 medical terms with varying degrees of difficulty, administered at year 3 and categorized by reading level (<9th grade; ≥9th grade).
Health related characteristics and comorbidities
Presence of diabetes mellitus was determined at baseline and during annual follow-up assessments by self report, use of hypoglycemic drugs, a fasting glucose of 126 mg/dL or above, or a two hour glucose tolerance test above 200 mg/dL, in accordance with the American Diabetes Association’s 2002 criteria. Presence of hypertension was determined by self report, antihypertensive drug use, and clinical measurements taken at the baseline examination. History of stroke and myocardial infarct was based on self report, clinic data, and drug use. Apolipoprotein E genotype was determined using standard methods,25 and participants were coded as e4 carriers or non-carriers. Depressive symptoms were assessed using the 20 item Center for Epidemiologic Studies-depression scale, and depression was defined as a score above 16.26
Lifestyle factors included baseline measures of number of alcoholic drinks per day (categorized as one or less versus more than one drink a day), current cigarette smoking, and self reported physical activity (total kcal/kg expended per week). Body mass index was calculated (as kg/m2) from direct height and weight measurements at baseline. The modified mini-mental state examination, a brief, general cognitive battery with components for orientation, concentration, language, praxis, and immediate and delayed memory,27 was administered at baseline and repeated at years 3, 5, 8, 10, and 11. Scores range from 0 to 100 points, with lower scores indicating poorer performance.
Statistical analysis
We firstly did bivariate analyses to test for associations between baseline characteristics and race by using χ2 tests for categorical variables and t tests for continuous variables. We then used Kaplan-Meier curves to examine the time to dementia by race. We used a set of Cox proportional hazard models and added explanatory variables in covariate blocks to examine the association between race and incidence of dementia. Models were constructed in the following order: demographics (age, sex) and apolipoprotein E e4 status; demographics, apolipoprotein E e4 status, and comorbidities (body mass index, history of stroke or myocardial infarction, hypertension, diabetes, depression); demographics, apolipoprotein E e4 status, comorbidities, and lifestyle (current smoker, alcohol use, physical activity); and demographics, apolipoprotein E e4 status, lifestyle, and socioeconomic status (education, literacy, income, perceived financial adequacy). Each component of the covariate block entered the model simultaneously. To investigate the possibility that a greater incidence of dementia may be explained by lower baseline cognition,17 we created an additional model that controlled for baseline modified mini-mental state examination score. We also calculated the excess hazard for each covariate block,28 and then calculated the hazard ratio for incidence of dementia associated with each individual socioeconomic status factor for the complete sample and stratified by race. We used SAS statistical software, version 9.2 for all analyses.
Results
Among the 2457 participants, 41.5% (n=1019) were black and 50.2% (n=1233) were female. At baseline, the mean age of the cohort was 73.6 years, 24.6% did not complete high school, and 12.8% had an annual family income less than $10 000. Black participants were younger and more likely to be female and apolipoprotein E e4 allele carriers than were white participants (table 1). Black participants were more likely to have a higher body mass index and history of hypertension and diabetes but not stroke, myocardial infarction, or depression. Black participants were also less physically active, more likely to smoke, and less likely to drink alcohol. Compared with white participants, black participants had a greater burden of low socioeconomic status measures, including lower education, literacy, family income, and financial adequacy (all P<0.001).
Table 1.
Baseline characteristics by race (n=2457). Values are numbers (percentages) unless stated otherwise
| Characteristic | White (n=1438) | Black (n=1019) | P value |
|---|---|---|---|
| Mean (SD) age, years | 73.7 (2.9) | 73.4 (2.9) | 0.004 |
| Female sex | 669 (46.5) | 564 (55.3) | <0.001 |
| Apolipoprotein E e4 | 339 (23.6) | 365 (35.8) | <0.001 |
| Stroke | 110 (7.7) | 90 (8.8) | 0.29 |
| Myocardial infarction | 172 (12.0) | 115 (11.3) | 0.61 |
| Hypertension | 781 (54.3) | 724 (71.1) | <0.001 |
| Depression | 59 (4.1) | 36 (3.5) | 0.47 |
| Diabetes | 281 (19.5) | 302 (29.6) | <0.001 |
| Mean (SD) body mass index (kg/m2) | 26.5 (4.1) | 28.6 (5.4) | <0.001 |
| >1 alcoholic drink/day | 135 (9.4) | 54 (5.3) | <0.001 |
| Current smoker | 90 (6.3) | 169 (16.6) | <0.001 |
| Mean* (SD) physical activity | 4.2 (0.8) | 4.1 (0.9) | <0.001 |
| Education: | |||
| <High school | 170 (11.8) | 434 (42.6) | <0.001 |
| High school graduate/technical school | 497 (34.6) | 314 (30.8) | |
| >High school | 771 (53.6) | 271 (26.6) | |
| Literacy† <9th grade | 323 (22.5) | 588 (57.7) | <0.001 |
| Family income ($): | |||
| <$10 000 | 56 (3.9) | 258 (25.3) | <0.001 |
| >$10 000-49 000 | 1039 (72.3) | 708 (69.5) | |
| ≥$50 000 | 343 (23.9) | 53 (5.2) | |
| Financial inadequacy | 96 (6.7) | 206 (20.2) | <0.001 |
| Mean (SD) modified mini-mental state examination score | 92.9 (5.8) | 86.3 (9.7) | <0.001 |
*Total kcal/kg/week, geometric mean (log transformed for normality).
†Measured at year 3.
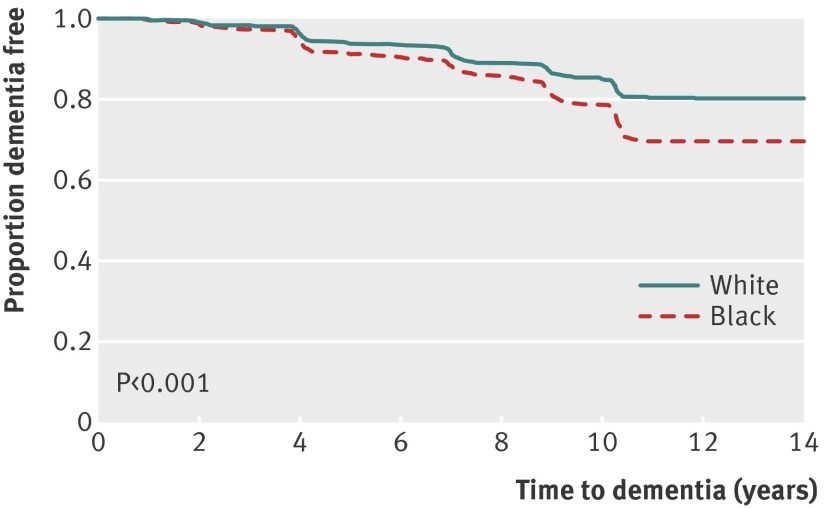
During 12 years of follow-up, 449 (18.3%) participants developed dementia. The incidence of dementia was higher among black than white participants (211 (20.7%) versus 238 (16.6%); P<0.001) (figure). Among those with dementia, 172 (38%) were on drug treatment, 280 (62%) had a hospital diagnosis, and 227 (51%) met the criteria for cognitive change. Results from unadjusted Cox proportional hazard models showed that black participants had a greater hazard ratio for dementia (1.44, 95% confidence interval 1.20 to 1.74) than white participants.
Unadjusted Kaplan-Meier curve for time to dementia associated with race
After adjustment for demographics and apolipoprotein E e4 status, the hazard ratio was reduced slightly but black participants still had a greater risk of developing dementia (table 2). Demographics and apolipoprotein E e4 status accounted for 18% of the excess hazard among black participants. The addition of comorbidities and lifestyle factors did not substantially alter the hazard ratio for race; 16% of the excess hazard was explained by including demographics, apolipoprotein E e4 status, comorbidities, and lifestyle factors in the model. When socioeconomic status was added to the model, the hazard ratio was greatly reduced and became non-significant (1.09, 0.87 to 1.37). The addition of socioeconomic status to the model accounted for 80% of the excess hazard among older black adults, suggesting that it accounted for a substantial proportion of the increased risk of dementia. The addition of baseline modified mini-mental state examination score to the model attenuated the effect size slightly (0.97, 0.77 to 1.22). We found no interactions between the effect of race and socioeconomic status measures on risk of dementia.
Table 2.
Cox proportional hazard ratios for time to dementia by race* (n=2457)
| Model | Cox proportional hazard ratio (95% CI) |
|---|---|
| Unadjusted | 1.44 (1.20 to 1.74) |
| Model 1: Demographics and apolipoprotein E e4 status | 1.36 (1.12 to 1.64) |
| Model 2: Demographics, apolipoprotein E e4, and comorbidities | 1.38 (1.14 to 1.67) |
| Model 3: Demographics, apolipoprotein E e4, comorbidities, and lifestyle | 1.37 (1.12 to 1.67) |
| Model 4: Demographics, apolipoprotein E e4, comorbidities, lifestyle, and socioeconomic measures | 1.09 (0.87 to 1.37) |
*White as reference.
In the models of the effect of each socioeconomic status factor on the incidence of dementia, among all the participants, having less than a high school education, less than a 9th grade literacy level, and a family income of less than $10 000 and reporting financial inadequacy were each associated with an increased hazard for dementia (table 3). Among white participants, having less than a 9th grade literacy level was associated with an increased hazard for dementia. Among black participants, having less than a high school education, less than a 9th grade literacy level, and a family income of less than $10 000 were each associated with an increased hazard of dementia. Participants who reported having all four low socioeconomic status indicators also had a greater hazard of dementia (1.32, 1.22 to 1.42) than did those who reported no low socioeconomic status indicators. This relation was consistent among black and white participants.
Table 3.
Associations between socioeconomic factors and dementia risk among black and white older adults participating in Health Aging, and Body Composition study
| Factors | Hazard ratio (95% CI) | ||
|---|---|---|---|
| All combined | White | Black | |
| Education: | |||
| <High school | 1.47 (1.17 to 1.86) | 0.99 (0.66 to 1.47) | 1.75 (1.26 to 2.43) |
| High school or technical school | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| ≥Some college | 0.80 (0.64 to 1.00) | 0.72 (0.55 to 0.96) | 0.99 (0.67 to 1.47) |
| Literacy: | |||
| <9th grade | 1.70 (1.41 to 2.05) | 1.42 (1.05 to 1.93) | 1.67 (1.25 to 2.21) |
| ≥9th grade | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Family income: | |||
| <$10 000 | 1.66 (1.29 to 2.15) | 1.49 (0.80 to 2.77) | 1.45 (1.07 to 1.96) |
| $10 000-49 000 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| ≥$50 000 | 0.92 (0.71 to 1.20) | 1.01 (0.75 to 1.36) | 1.00 (0.54 to 1.87) |
| Financial inadequacy | 1.46 (1.13 to 1.90) | 1.40 (0.88 to 2.21) | 1.29 (0.93 to 1.79) |
| Sum of worst case levels of above variables (range 0-4) | 1.32 (1.22 to 1.42) | 1.25 (1.06 to 1.47) | 1.31 (1.18 to 1.46) |
Discussion
Consistent with previous studies and national data,1 2 3 4 5 29 our results from this study of community dwelling older people with more than 12 years of follow-up showed higher incidence rates of dementia among black than white participants. The risk of dementia was reduced but remained 40% higher among black participants after adjustment for demographics, apolipoprotein E e4 status, lifestyle factors, and comorbidities. When we accounted for socioeconomic status, differences in the risk of dementia were greatly reduced and no longer significant. These results suggest that black-white disparities in risk of dementia may be largely attributed to socioeconomic differences.
Comparison with other studies
Previous studies have shown that socioeconomic status may influence cognitive measures and help to explain differences in cognitive performance between black and white adults.3 13 14 17 30 31 Previous cross sectional work conducted in the Health, Aging, and Body Composition study showed that socioeconomic status accounted for a substantial amount of the variation in baseline cognitive test scores between older black and white participants.17 Our results expand on these findings by providing evidence that low socioeconomic status may also increase the risk of dementia. Of the few known studies that have investigated differences in the risk of dementia among black and white older people, none has comprehensively evaluated the contribution of both financial status and quality of education.3 10 11 12 29 For example, in the Cardiovascular Health Study, after adjustment for age and education, rates of dementia were reduced but remained higher for black than white participants.10 11 However, education was measured only by years attained, and neither literacy level, which may reflect quality of education,15 16 nor income was examined. In our study, socioeconomic status, which included income, financial adequacy, educational attainment, and literacy level, accounted for a significant portion of the difference in black-white dementia risk among older adults.
Potential mechanisms
Low socioeconomic status has been associated with worse physical and mental health,21 22 32 33 both of which may increase the risk of cognitive impairment.23 People of low socioeconomic status may be less likely to access resources and appropriate medical care,32 which may exacerbate underlying conditions. Although black participants in our study had a greater prevalence of vascular risk factors, including a history of hypertension, diabetes, and current smoking, these factors did not seem to contribute substantially to the difference in the risk of dementia. Previous studies investigating racial differences in functional decline and disabilities among older adults have shown similar results that socioeconomic status may contribute more to disparities in functional status than in chronic conditions.34 35 The reasons for this finding are not well understood.
Socioeconomic status may influence cognition in late life through several other potential mechanisms. Low socioeconomic status may increase physiological stress and contribute to greater allostatic load, a cumulative measure of multisystem biological dysregulation that includes inflammatory, metabolic, and cardiovascular processes.36 37 38 Chronic stress has been shown to impair neurogenesis in the hippocampus in animal models and has been associated with reduced hippocampal volumes in human neuroimaging studies.36 37 Education may directly affect cognition through building a cognitive reserve to help protect against brain deterioration.39 40 41 Results from a previous Health ABC study found that among older adults with low plasma β amyloid concentration, those with greater cognitive reserve showed less cognitive decline than did those with less cognitive reserve.42 Education may also affect cognition indirectly through fostering healthier lifestyle choices and improving health literacy that may lead to better health management.32 43 Literacy, as a potential measure of quality of education, may affect cognition through many of the same mechanisms.
Strengths and limitations
Our study had several strengths, including a long follow-up period in a relatively large sample of black and white community dwelling older people. All participants were without dementia at baseline. We also had comprehensive measures of socioeconomic status and were able to evaluate literacy level. To account for differences in baseline cognition and the potential influence of race and socioeconomic status variables on cognitive change score, we used a 1.5 standard deviation cut-off to classify a participant’s personal change in modified mini-mental state examination performance relative to that of their race matched peers, in line with others’ definitions of cognitive impairment.44 45
Our study also had several limitations. Our study population was relatively well functioning at baseline, so the results may not be generalizable to older adults with disabilities. Although we believe that our method for identifying cases of dementia was sensitive to capturing people with dementia, our diagnosis was not based on a formal clinical assessment. Some participants with mild cognitive impairment or who were delirious may have been classified as having dementia. We also did not have measures of early life adversity.46 47
Conclusion
In summary, our results provide evidence that differences in participants’ characteristics, in particular socioeconomic status, may contribute to disparities in the incidence of dementia among black and white older people. These findings also suggest that socioeconomic status measures such as income and quality of education should be taken into account in studies of dementia and cognitive health. Reducing these disparities is a critical goal for both overall health and cognitive aging, and future studies should investigate whether improving lifelong education and other socioeconomic risk factors will reduce the risk of dementia.
What is already known on this topic
Black older people have a higher incidence of Alzheimer’s disease and other dementias than do white older people, but the reason for this difference is unclear
Previous studies have shown an association between cognitive outcomes and socioeconomic status, suggesting that socioeconomic status may influence cognition
However, whether socioeconomic status influences dementia risk, and whether differences in socioeconomic status can help to explain racial disparities in incidence of dementia, has not been determined
What this study adds
Differences in participants’ characteristics, in particular socioeconomic status, may contribute to disparities in the incidence of dementia among black and white older adults
These findings suggest that socioeconomic measures such as income and quality of education should be taken into account in studies of dementia and cognitive health
Reducing these disparities is a critical goal for both overall health and cognitive aging, and future studies should investigate whether improving lifelong education and other socioeconomic risk factors will reduce the risk of dementia
We acknowledge Jerin Ullah for her contributions to the manuscript.
Contributors: KY was responsible for the study concept and design, analysis and interpretation of data, drafting and revising the manuscript, study supervision, and obtaining funding. CF contributed to the analysis and interpretation of data and drafting/revising the manuscript. TBH and HA helped to acquired the data and contributed to drafting/revising the manuscript. AN, SS, and AK contributed to drafting/revising the manuscript. ES helped to acquire the data and contributed to the data analysis and interpretation and to drafting/revising the manuscript. KY is the guarantor.
Funding: National Institute on Aging (NIA) contract Nos N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106; NIA grant No R01-AG028050; NINR grant No R01-NR012459. This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging, and a grant from the American Health Assistance Foundation, grant number A201-0029. The Intramural Research Program of the NIA participated in the design and conduct of the study.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: this research was supported as detailed above; KY has served on data safety monitoring boards for Takeda, the NIH, Pfizer, and Medivation and served as a consultant for Novartis; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: This study was approved by the institutional review boards of the two field centers (University of Pittsburgh and University of Tennessee, Memphis) and that of the coordinating center, the University of California, San Francisco. All participants signed an informed written consent, approved by the institutional review boards at the clinical sites.
Data sharing: No additional data available.
Transparency declaration: The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Cite this as: BMJ 2013;347:f7051
References
- 1.Lee HB, Richardson AK, Black BS, Shore AD, Kasper JD, Rabins PV. Race and cognitive decline among community-dwelling elders with mild cognitive impairment: findings from the Memory and Medical Care Study. Aging Ment Health 2012;16:372-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alzheimer’s Association. 2012 Alzheimer’s disease facts and figures. Alzheimers Dement 2012;8:131-68. [DOI] [PubMed] [Google Scholar]
- 3.Sachs-Ericsson N, Blazer DG. Racial differences in cognitive decline in a sample of community-dwelling older adults: the mediating role of education and literacy. Am J Geriatr Psychiatry 2005;13:968-75. [DOI] [PubMed] [Google Scholar]
- 4.Adelman S, Blanchard M, Rait G, Leavey G, Livingston G. Prevalence of dementia in African-Caribbean compared with UK-born white older people: two-stage cross-sectional study. Br J Psychiatry 2011;199:119-25. [DOI] [PubMed] [Google Scholar]
- 5.Adelman S, Blanchard M, Livingston G. A systematic review of the prevalence and covariates of dementia or relative cognitive impairment in the older African-Caribbean population in Britain. Int J Geriatr Psychiatry 2009;24:657-65. [DOI] [PubMed] [Google Scholar]
- 6.Logue MW, Schu M, Vardarajan BN, Buros J, Green RC, Go RC, et al. A comprehensive genetic association study of Alzheimer disease in African Americans. Arch Neurol 2011;68:1569-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sawyer K, Sachs-Ericsson N, Preacher KJ, Blazer DG. Racial differences in the influence of the APOE epsilon 4 allele on cognitive decline in a sample of community-dwelling older adults. Gerontology 2009;55:32-40. [DOI] [PubMed] [Google Scholar]
- 8.Green RC, Cupples LA, Go R, Benke KS, Edeki T, Griffith PA, et al. Risk of dementia among white and African American relatives of patients with Alzheimer disease. JAMA 2002;287:329-36. [DOI] [PubMed] [Google Scholar]
- 9.Chin AL, Negash S, Hamilton R. Diversity and disparity in dementia: the impact of ethnoracial differences in Alzheimer disease. Alzheimer Dis Assoc Disord 2011;25:187-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fitzpatrick AL, Kuller LH, Ives DG, Lopez OL, Jagust W, Breitner JCS, et al. Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc 2004;52:195-204. [DOI] [PubMed] [Google Scholar]
- 11.Shadlen M, Siscovick D, Fitzpatrick A, Dulberg C, Kuller L, Jackson S. Education, cognitive test scores, and black-white differences in dementia risk. J Am Geriatr Soc 2006;54:898-905. [DOI] [PubMed] [Google Scholar]
- 12.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, et al. Prevalence of dementia in the United States: the Aging, Demographics, and Memory Study. Neuroepidemiology 2007;29:125-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee S, Kawachi I, Berkman LF, Grodstein F. Education, other socioeconomic indicators, and cognitive function. Am J Epidemiol 2003;157:712-20. [DOI] [PubMed] [Google Scholar]
- 14.Koster A, Penninx BW, Bosma H, Kempen GI, Newman AB, Rubin SM, et al. Socioeconomic differences in cognitive decline and the role of biomedical factors. Ann Epidemiol 2005;15:564-71. [DOI] [PubMed] [Google Scholar]
- 15.Manly JJ, Jacobs DM, Touradji P, Small SA, Stern Y. Reading level attenuates differences in neuropyschological test performance between African American and white elders. J Int Neuropsychol Soc 2002;8:341-8. [DOI] [PubMed] [Google Scholar]
- 16.Chin AL, Negash S, Xie S, Arnold SE, Hamilton R. Quality, and not just quantity, of education accounts for differences in psychometric performance between African Americans and white non-Hispanics with Alzheimer’s disease. J Int Neuropsychol Soc 2012;18:277-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta KM, Simonsick EM, Rooks R, Newman AB, Pope SK, Rubin SM, et al. Black and white differences in cognitive function test scores: what explains the difference? J Am Geriatr Soc 2004;52:2120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manly JJ, Touradji P, Tang MX, Stern Y. Literacy and memory decline among ethnically diverse elders. J Clin Exp Neuropsychol 2003;25:680-90. [DOI] [PubMed] [Google Scholar]
- 19.Sudore RL, Yaffe K, Satterfield S, Harris TB, Mehta KM, Simonsick EM, et al. Limited literacy and mortality in the elderly: the health, aging, and body composition study. J Gen Intern Med 2006;21:806-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haas SA, Krueger PM, Rohlfsen L. Race/ethnic and nativity disparities in later life physical performance: the role of health and socioeconomic status over the life course. J Gerontol B Psychol Sci Soc Sci 2012;67:238-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thorpe RJ, Koster A, Kritchevsky SB, Newman AB, Harris T, Ayonayon HN, et al. Race, socioeconomic resources, and late-life mobility and decline: findings from the Health, Aging, and Body Composition Study. J Gerontol A Biol Sci Med Sci 2011;66:1114-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thorpe RJ Jr, Koster A, Bosma H, Harris TB, Simonsick EM, van Eijk JT, et al. Racial differences in mortality in older adults: factors beyond socioeconomic status. Ann Behav Med 2012;43:29-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol 2011;10:819-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis TC, Long SW, Jackson RH, Mayeaux EJ, George RB, Murphy PW, et al. Rapid estimate of adult literacy in medicine: a shortened screening instrument. Fam Med 1993;25:391-5. [PubMed] [Google Scholar]
- 25.Livak K. SNP genotyping by the 5’-nuclease reaction. Methods Mol Biol 2003;212:129-47. [DOI] [PubMed] [Google Scholar]
- 26.Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385-401. [Google Scholar]
- 27.Teng E, Chui H. The modified mini-mental state (3MS) examination. J Clin Psychiatry 1987;48:314-8. [PubMed] [Google Scholar]
- 28.Szklo M, Nieto J. Epidemiology: beyond the basics. Jones and Bartlett Learning, 2008.
- 29.Tang MX, Cross P, Andrews H, Jacobs DM, Small S, Bell K, et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology 2001;56:49-56. [DOI] [PubMed] [Google Scholar]
- 30.Manly JJ, Jacobs DM, Sano M, Bell K, Merchant CA, Small SA, et al. Cognitive test performance among nondemented elderly African Americans and whites. Neurology 1999;50:1238-45. [DOI] [PubMed] [Google Scholar]
- 31.Whitfield KE, Fillenbaum GG, Pieper C, Albert MS, Berkman LF, Blazer DG, et al. The effect of race and health-related factors on naming and memory: the MacArthur Studies of Successful Aging. J Aging Health 2000;12:69-89. [DOI] [PubMed] [Google Scholar]
- 32.Schiller JS, Lucas JW, Ward BW, Peregoy JA. Summary health statistics for U.S. adults: National Health Interview Survey, 2010. Vital Health Stat 10 2012;252:1-207. [PubMed] [Google Scholar]
- 33.Hemingway H, Nicholson A, Stafford M, Roberts R, Marmot M. The impact of socioeconomic status on health functioning as assessed by the SF-36 questionnaire: the Whitehall II Study. Am J Public Health 1997;87:1484-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kington RS, Smith JP. Socioeconomic status and racial and ethnic differences in functional status associated with chronic diseases. Am J Public Health 1997;87:805-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moody-Ayers SY, Mehta KM, Lindquist K, Sands L, Covinsky KE. Black-white disparities in functional decline in older persons: the role of cognitive function. J Gerontol A Biol Sci Med Sci 2005;60:933-9. [DOI] [PubMed] [Google Scholar]
- 36.McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annu Rev Med 2011;62:431-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci 2010;1186:190-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beckie TM. A systematic review of allostatic load, health, and health disaprities. Biol Res Nurs 2012;14:311-46. [DOI] [PubMed] [Google Scholar]
- 39.Meng X, D’Arcy C. Education and dementia in the context of the cognitive reserve hypothesis: a systematic review with meta-analyses and qualitative analyses. PLoS One 2012;7:e38268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Honer WG, Barr AM, Sawada K, Thornton AE, Morris MC, Leurgans SE, et al. Cognitive reserve, presynaptic proteins and dementia in the elderly. Transl Psychiatry 2012:e114. [DOI] [PMC free article] [PubMed]
- 41.Jefferson AL, Gibbons LE, Rentz DM, Carvalho JO, Manly J, Bennett DA, et al. A life course model of cognitive activities, socioeconomic status, education, reading ability, and cognition. J Am Geriatr Soc 2011;59:1403-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yaffe K, Weston A, Graff-Radford NR, Satterfield S, Simonsick EM, Younkin SG, et al. Association of plasma beta-amyloid level and cognitive reserve with subsequent cognitive decline. JAMA 2011;305:261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sudore R, Mehta K, Simonsick E, Harris T, Newman A, Satterfield S, et al. Limited literacy in older people and disparities in health and healthcare access. J Am Geriatr Soc 2006;54:770-6. [DOI] [PubMed] [Google Scholar]
- 44.Artero S, Petersen R, Touchon J, Ritchie K. Revised criteria for mild cognitive impairment: validation within a longitudinal population study. Dement Geriatr Cogn Dis 2006;22:465-70. [DOI] [PubMed] [Google Scholar]
- 45.Loewenstein DA, Acevedo A, Agron J, Duara R. Stability of neurocognitive impairment in different subtypes of mild cognitive impairment. Dement Geriatr Cogn Dis 2007;23:82-6. [DOI] [PubMed] [Google Scholar]
- 46.Fors S, Lennartsson C, Lundberg O. Childhood living conditions, socioeconomic position in adulthood, and cognition in later life: exploring the associations. J Gerontol B Psychol Sci Soc Sci 2009;64:750-7. [DOI] [PubMed] [Google Scholar]
- 47.Barnes LL, Wilson RS, Everson-Rose SA, Hayward MD, Evans DA, Mendes de Leon CF. et al. Effects of early-life adversity on cognitive decline in older African Americans and whites. Neurology 2012;79:2321-7. [DOI] [PMC free article] [PubMed] [Google Scholar]