Abstract
BACKGROUND
To determine the potential efficacy of targeting both the tumor and bone microenvironment in patients with castration-resistant prostate cancer (CRPC), we conducted a phase 1/2 trial combining docetaxel with dasatinib, an oral SRC inhibitor.
METHODS
In phase 1, 16 men received dasatinib 50–120 mg once daily (QD) and docetaxel 60–75 mg/m2 every 21 days (Q21D). In phase 2, 30 additional men received dasatinib 100 mg QD/docetaxel 75 mg/m2 Q21D. Efficacy endpoints included changes in prostate-specific antigen (PSA), measurable disease, bone scans, and markers of bone metabolism. Safety and pharmacokinetics were also studied.
RESULTS
Combination dasatinib and docetaxel therapy was generally well tolerated. Thirteen of 46 patients (28%) had a grade 3/4 toxicity. Drug–drug interactions and a maximum tolerated dose were not identified. Durable 50% PSA declines occurred in 26/46 patients (57%). Of 30 patients with measurable disease, 18 (60%) had a partial response. Fourteen patients (30%) had disappearance of a lesion on bone scan. In bone-marker assessments, 33/38 (87%) and 26/34 (76%) had decreases in urinary N-telopeptide or bone-specific alkaline phosphatase levels, respectively. Twenty-eight patients (61%) received single-agent dasatinib following docetaxel discontinuation and had stabilization of disease for an additional 1–12 months.
CONCLUSIONS
The high objective response rate and favorable toxicity profile are promising and justify randomized studies of docetaxel and dasatinib in CRPC. Parallel declines in levels of PSA and bone markers are consistent with co-targeting of epithelial and bone compartments of the cancer. Treatment with single-agent dasatinib following docetaxel cessation warrants further study.
Keywords: Dasatinib, Docetaxel, Prostate Cancer, Metastases, Bone
INTRODUCTION
Mortality in patients with advanced prostate cancer is associated with osseous spread of bone-forming metastases.1-4 Clinical observations and mechanistic-based studies implicate signaling by paracrine factors from the tumor microenvironment in prostate cancer progression.5-8 These findings suggest that successful therapeutic strategies will require targeting of signaling pathways central to both the tumor and bone microenvironment.
SRC-family kinases (SFKs) are nonreceptor protein tyrosine kinases that are rational therapeutic targets for castration-resistant prostate cancer (CRPC). The SFKs SRC, LYN, and FYN have established roles in prostate cancer growth, invasion, and metastasis.9-14 Elevated SFK activity in tumors from patients with prostate cancer is associated with a shorter responses to androgen-ablation therapy, metastasis to the bone, and shorter survival.15 SRC also has key roles in regulating osteoclast function and in the pathogenesis of bone metastases.16-20
Dasatinib is a tyrosine kinase inhibitor that potently inhibits SFKs and also has activity against ABL1, platelet-derived growth factor receptor (PDGFR), KIT,10,21 EPHA2,22 and focal adhesion kinase.23 In vitro, dasatinib decreased proliferation and migration of prostate cancer cells,11,15 including the hormone-refractory cell line LNCaP-SDM.15 In a mouse model, dasatinib treatment significantly reduced prostate tumor size and number of lymph node metastases compared with control mice.11 In mice with prostate tumor cells injected intratibially, dasatinib significantly lowered serum prostate-specific antigen (PSA) concentrations and increased bone mineral density, and treatment with dasatinib plus docetaxel had greater activity than either agent alone.12 These preclinical studies led to our hypothesis that combining dasatinib with docetaxel would improve treatment of patients with metastatic CRPC by targeting both the tumor and bone microenvironment.
To test this hypothesis, a phase 1/2 study was conducted to define the toxicity profile, pharmacokinetics (PK), and maximum tolerated dose (MTD) of oral dasatinib combined with docetaxel in patients with metastatic CRPC. Secondary objectives were to assess tumor responses, PSA responses, progression-free survival (PFS), bone-scan changes, and modulation of bone turnover markers.
PATIENTS AND METHODS
Patients and Eligibility Criteria
The study was conducted in accordance with institutional and federal regulations including informed consent. Men aged ≥18 years were eligible if they had metastatic prostate cancer that had progressed despite castrate levels of serum testosterone (≤50 ng/dL). Progression was defined as: increased size or appearance of one or more new radiographic lesion; two or more new lesions on bone scan or one new lesion on bone scan with rising PSA; or two consecutive PSA rises (≥5 ng/mL) separated by 2 weeks. Exclusion criteria were: brain metastases; clinically significant cardiovascular disease; existing pleural/pericardial effusion; second malignancy (excluding nonmelanoma skin cancer); and prior treatment with more than one chemotherapy (including docetaxel) or course of palliative radiotherapy or any radioisotope. There was no limit on prior hormonal therapy. Bisphosphonate therapy could be continued but could not be initiated immediately before or on study. The registered study number was CA180-086 (clinicaltrials.gov NCT00439270).
Study Design
This was an open-label phase 1/2 study. In phase 1, cohorts of patients were treated with escalating doses of docetaxel and dasatinib. Docetaxel was administered intravenously every 21 days (Q21D) from Day 1 of Cycle 1 with twice-daily (BID) oral prednisone 5 mg. Oral dasatinib was initiated on Day 3 of Cycle 1 and administered once daily (QD) continuously. Coadministered doses of dasatinib (mg QD)/docetaxel (mg/m2 Q21D) were: 50/60, 50/75, 70/75, 100/75, and 120/75. To enable accurate evaluation of safety and PK, patients without disease progression or significant toxicity received a minimum of 6 cycles of therapy. Therapy was discontinued for disease progression (defined below) after a minimum of 2 cycles of combination therapy, or earlier for serious adverse events (AEs), rapid progression, or withdrawal of consent. Patients with stable or responding disease after 6 cycles were permitted to receive further dasatinib with or without docetaxel at the investigator’s discretion.
Pharmacokinetic Evaluations
Blood samples for PK analysis were collected on Day 1 (Cycle 1) for docetaxel alone, Day 14 (Cycle 1) for dasatinib alone, and Day 21 (Cycle 2, Day 1) for the combination. PK parameters were derived from plasma concentration versus time and included maximum plasma concentration (Cmax), area under curve (AUC) for a dosing interval or from time zero to infinity, time to Cmax (Tmax), plasma half-life (T1/2), and clearance (CL). PK interactions were assessed using point estimates and 90% confidence interval (CI) of Cmax and AUC with dasatinib and docetaxel alone or in combination.
Safety Evaluations
AEs were assessed continuously and graded according to National Cancer Institute Common Terminology Criteria (v3.0). Dose-limiting toxicity was defined between Day 3 (Cycle 1) and Day 42 (Cycle 2, Day 21) as: grade 4 neutropenia causing treatment interruption for >14 days; febrile neutropenia; grade 4 thrombocytopenia; grade 3 thrombocytopenia with a bleeding episode requiring platelet transfusion; nausea and/or vomiting despite medical intervention/prophylaxis causing treatment interruption for >14 days; grade 3–4 asthenia/fatigue; any other grade ≥3 nonhematologic toxicity except alopecia or transient arthralgia/myalgia (unless unresponsive to intervention); or interruption of study drug for >14 days due to toxicity.
Efficacy Evaluations
Serum PSA concentrations were determined every 3 weeks. PSA response was defined as ≥50% decrease in PSA concentration from baseline sustained for ≥6 weeks. PSA progression was defined as three consecutive PSA increases from baseline/nadir observed at ≥1-week intervals, including PSA increase to ≥5 ng/mL and by ≥50%, as per Prostate Cancer Working Group (PCWG) 1 recommendations.24 Duration of PSA response was measured from the first of two consecutive measurements confirming response until the first of three consecutive measurements confirming PSA progression, disease progression (defined below), or death. Time to PSA progression was not a specified endpoint and was not determined.
For patients with measurable disease, Response Evaluation Criteria in Solid Tumors (RECIST) were used to define patients with a complete response or partial response (PR) or who had not met criteria for response or tumor progression after ≥18 weeks of treatment. Tumor progression was defined as either ≥20% increase in sum of longest diameters of target lesions from nadir, progression of nonmeasurable lesions, or detection of new lesions. As per PCWG2 recommendations, response determination excluded pelvic lymph nodes measuring <2 cm.25 Tumor assessments were performed every 6 weeks.
Pretreatment bone scans were performed within 28 days prior to treatment and every 6 weeks after starting docetaxel. Bone scans were classified as improved (disappearance of at least one lesion and no new lesion or pain), stable (no new lesions/pain), or progressed (at least two areas of new focal uptake or new adverse clinical symptoms). Confirmatory bone scans were not required. A subset of patients treated at MD Anderson Cancer Center, Texas, were categorized as having high (20 or more lesions), intermediate (10–20 lesions), or low (1–9 lesions) bone-lesion volume at baseline.
Concentrations of serum bone alkaline phosphatase (BAP; marker of osteoblast activity/differentiation) and urinary N-telopeptide (uNTX; marker of osteoclast activity) were measured prior to treatment and on study.
Progression was defined as either bone-scan progression or at least two of: tumor progression (RECIST), PSA progression, or investigator-defined clinical progression. The protocol-specified definition of progression therefore differs from recommendations published after the study was initiated.25 As appropriate for an early-phase trial, the protocol did not require that patients should be followed for progression after discontinuing study treatment, due to PFS not being the primary endpoint of the study and the likelihood that patients would receive additional therapies after discontinuing that may have impacted PFS. Because of a relatively high proportion of patients in whom the date of progression could not be obtained and difficulties of data interpretation, PFS could not be accurately ascertained and is not reported.
For patients who remained on single-agent dasatinib for >21 days after discontinuing docetaxel treatment, duration of additional single-agent therapy was measured from the date of last docetaxel dose to the date of progression or death, discontinuation, or last efficacy assessment.
RESULTS
Patients and Treatment
Forty-six patients were treated (Table 1). Median age was 65 years. Thirty-nine patients (85%) had bone metastases and 30 patients (65%) had RECIST-evaluable disease. Fifteen patients (33%) had received prior chemotherapy, including docetaxel in 8 (17%).
Table 1.
Baseline Demographics and Disease Characteristics
| Phase 1 | Phase 2 | Total | |
|---|---|---|---|
|
| |||
| Treated patients (n) | 16 | 30 | 46 |
|
| |||
| Median age, years (range) | 69 | 62.5 | 65 (48-83) |
|
| |||
| Age ≥65 years, n (%) | 11 (69) | 12 (40) | 23 (50) |
|
| |||
| Median time since diagnosis, months (range) | 46 (11-184) | 44 (6-210) | 44 (6-210) |
|
| |||
| ECOG status, n (%) | |||
| 0 | 9 (56) | 13 (43) | 22 (48) |
| 1 | 7 (44) | 15 (50) | 22 (48) |
| 2 | 0 (0) | 2 (7) | 2 (4) |
|
| |||
| Prior therapy, n (%) | |||
| Surgery or radiotherapy | 14 (88) | 24 (80) | 38 (83) |
| Chemotherapy | 11 (69) | 4 (13) | 15 (33) |
| Docetaxel | 7 (44) | 1 (3) | 8 (17) |
|
| |||
| Current bisphosphonate use, n (%)* | 3 (19) | 9 (30) | 12 (26) |
|
| |||
| Bone metastases, n (%) | 14 (88) | 25 (83) | 39 (85) |
|
| |||
| uNTX concentration, n (%) | |||
| ≤ULN | 10 (63) | 20 (67) | 30 (65) |
| >ULN | 5 (31) | 8 (27) | 13 (28) |
| Not reported | 1 (6) | 2 (7) | 3 (7) |
|
| |||
| Bone-specific alkaline phosphatase concentration, n (%) | |||
| Higher than normal range | 5 (31) | 12 (40) | 17 (37) |
| Normal | 2 (13) | 10 (33) | 12 (26) |
| Lower than normal range | 1 (6) | 1 (3) | 2 (4) |
| No normal range defined | 6 (38) | 4 (13) | 10 (22) |
| Not reported | 2 (13) | 3 (10) | 5 (11) |
|
| |||
| RECIST-evaluable disease, n (%) | 11 (70) | 19 (63) | 30 (65) |
|
| |||
| Target lesions, n (%) | |||
| Lymph node | 9 (56) | 17 (57) | 26 (57) |
| Pelvis | 1 (6) | 1 (3) | 2 (4) |
| Visceral, liver | 2 (13) | 1 (3) | 3 (7) |
| Visceral, lung | 1 (6) | 3 (10) | 4 (9) |
Started prior to protocol entry and ongoing during dasatinib and docetaxel treatment.
ECOG indicates Eastern Cooperative Oncology Group; RECIST, Response Evaluation Criteria in Solid Tumors; ULN, upper limit of normal; uNTX, urinary N-telopeptide.
In phase 1, 16 patients were treated, comprising three per dose cohort except cohort 4, in which one patient was withdrawn in Cycle 1 after docetaxel hypersensitivity requiring an additional patient to be treated. Thirty additional patients were treated in phase 2. At data capture (March 2010), median treatment duration was 6.2 months (range 0.1–17.2 months) for dasatinib and 6 cycles for docetaxel. Excluding the single patient who had docetaxel hypersensitivity, the range of docetaxel cycles administered was 2–24. Thirty-four patients (74%) received ≥6 cycles of docetaxel, including 11 patients (24%) who received ≥10 cycles. Twenty-eight patients (61%) received single-agent dasatinib after discontinuing docetaxel for a median of 2.9 months (range 0.9–11.7+ months) up to data capture. Nine patients (20%) remain on study: seven on single-agent dasatinib (current treatment duration 13.9–17.0 months) and two on combination therapy (14.7/17.25 months of dasatinib and 22/24 cycles of docetaxel). Of 37 patients (80%) who are off study, reasons for discontinuation were: protocol-defined disease progression in 20 (43%), investigator decision due to patient not likely to benefit from further treatment in 3 (7%), study drug toxicity in 7 (15%), patient request in 2 (4%), and other reason (unrelated AE, noncompliance, no longer meets study criteria, or not specified) in 5 (11%).
Safety
No dose-limiting toxicities occurred and MTD was not reached. Combination treatment was generally well tolerated at the dose levels tested and treatment-related AEs were mostly mild to moderate in severity (Table 2). Thirteen patients (28%) experienced at least one grade ≥3 AE, of which only fatigue (n = 3) and pleural effusion (n = 2) occurred in more than one patient. Dose reductions of dasatinib or docetaxel were required in four and five patients, respectively. Docetaxel was delayed in 13 patients and dasatinib was interrupted in 30 patients.
Table 2.
Treatment-related Adverse Events (Worst Grade) Occurring in ≥10% of Patients (N = 46)
| Patients, n (%) | |||||
|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | All grades | |
| Fatigue | 16 (35) | 13 (28) | 3 (7) | - | 32 (70) |
| Alopecia | 23 (50) | 3 (7) | - | - | 26 (57) |
| Diarrhea | 20 (43) | 2 (4) | - | - | 22 (48) |
| Nausea | 17 (37) | 4 (9) | - | - | 21 (46) |
| Dysgeusia | 19 (41) | 1 (2) | - | - | 20 (44) |
| Peripheral edema | 11 (24) | 5 (11) | - | - | 16 (35) |
| Decreased appetite | 12 (26) | 3 (7) | - | - | 15 (33) |
| Anemia | 4 (9) | 6 (13) | 1 (2) | - | 11 (24) |
| Dyspnea | 8 (17) | 2 (4) | - | - | 10 (22) |
| Vomiting | 6 (13) | 3 (7) | - | - | 9 (20) |
| Dry skin | 9 (20) | - | - | - | 9 (20) |
| Nail disorder | 8 (17) | 1 (2) | - | - | 9 (20) |
| Headache | 6 (13) | 1 (2) | - | - | 7 (15) |
| Hypersensitivity | 5 (11) | 2 (4) | - | - | 7 (15) |
| Pleural effusion | 2 (4) | 3 (7) | 2 (4) | - | 7 (15) |
| Constipation | 4 (9) | 2 (4) | - | - | 6 (13) |
| Hypokalemia | 6 (13) | - | - | - | 6 (13) |
| Insomnia | 4 (9) | 1 (2) | - | - | 5 (11) |
| Peripheral sensory neuropathy | 2 (4) | 2 (4) | 1 (2) | - | 5 (11) |
| Peripheral neuropathy | 4 (9) | 1 (2) | - | - | 5 (11) |
Pharmacokinetic Analysis
PK parameters for dasatinib and docetaxel given alone or in combination were similar. Figure 1 and Table 3 show data for dasatinib 100 mg QD and docetaxel 75 mg/m2. Point estimates (90% CI) of Cmax and AUC values for each agent given alone/in combination are 0.96 (0.74, 1.24) and 1.08 (0.91, 1.29) for dasatinib and 1.02 (0.92, 1.14) and 0.97 (0.88, 1.06) for docetaxel, respectively, indicating no PK interaction.
Figure 1.
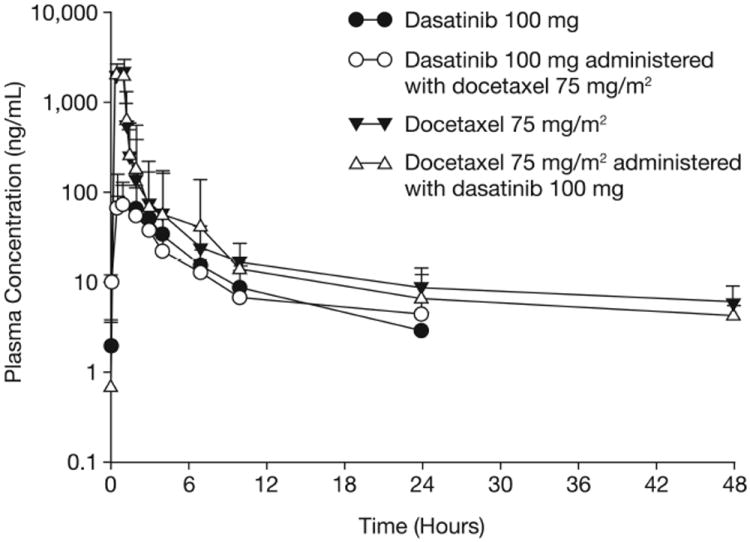
Mean plasma concentrations (+ standard deviations) of dasatinib and docetaxel administered alone or in combination.
Table 3.
Pharmacokinetic Parameters of Dasatinib and Docetaxel Given Alone or in Combination
| Analyte | Treatment | N | Cmax (ng/mL), geomean (% CV) | Tmax (h), median (min, max) | AUC* (ng.h/mL), geomean (% CV) | T1/2 (h), mean (SD) | CL (L/h), mean (SD) |
|---|---|---|---|---|---|---|---|
| Dasatinib | Dasatinib 100 mg | 28 | 84 (75) | 2.0 (0.5, 7.0) | 318 (57) | 5.0 (2.3) | NA |
| Dasatinib | Dasatinib 100 mg with docetaxel 75 mg/m2 | 31 | 76 (69) | 1.6 (0.5, 24) | 328 (64) | 4.9 (2.8) | NA |
| Docetaxel | Docetaxel 75 mg/m2 | 33 | 2125 (33) | NA | 2664 (44) | 24.3 (11.2) | 69.3 (50.5) |
| Docetaxel | Docetaxel 75 mg/m2 with dasatinib 100 mg | 31 | 2159 (30) | NA | 2563 (48) | 22.5 (9.9) | 66.6 (22.1) |
The AUC column shows AUC for a dosing interval for dasatinib and AUC from zero to infinity for docetaxel.
AUC indicates area under the curve; CL, clearance; Cmax, maximum plasma concentration; CV, coefficient of variation; NA, not applicable; SD, standard deviation; T1/2, plasma half-life; Tmax, time to Cmax.
Dasatinib 100 mg QD and docetaxel 75 mg/m2 Q21D were chosen as recommended phase 2 dose based on safety observations and PK analysis, plus prior studies of dasatinib in chronic myeloid leukemia.26
Efficacy
Of 46 treated patients, 37 (80%) had any decrease in PSA from baseline, including 26 (57%) who had a confirmed PSA response (sustained ≥50% decline for ≥6 weeks; Figure 2A). Median duration of PSA response for the 13/26 responding patients who had PSA progression, disease progression, or death on study was 4.9 months (range 1.4–9.5 months); this median value was not calculated using Kaplan-Meier methodology and does not include patients who remained in PSA response or who discontinued from the study without PSA progression, disease progression, or death.
Figure 2.
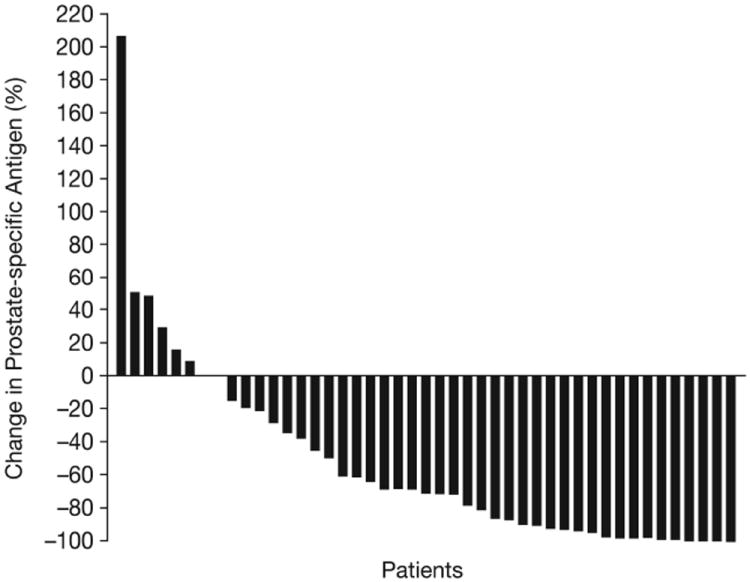
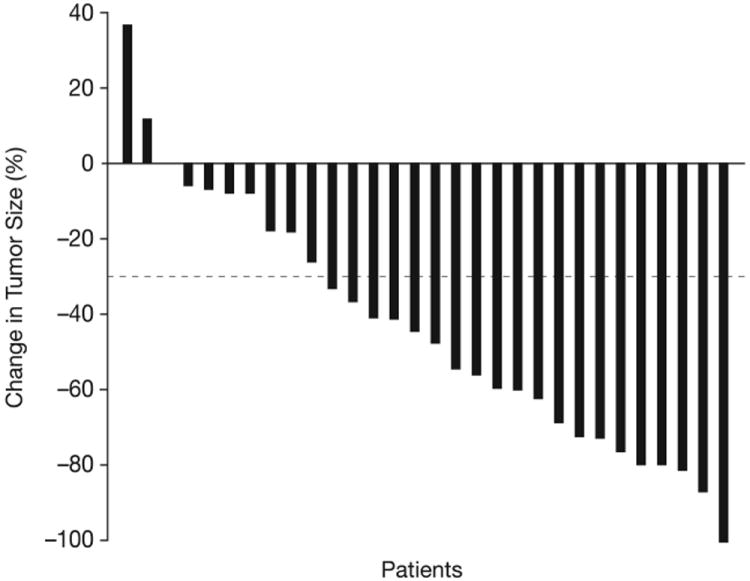
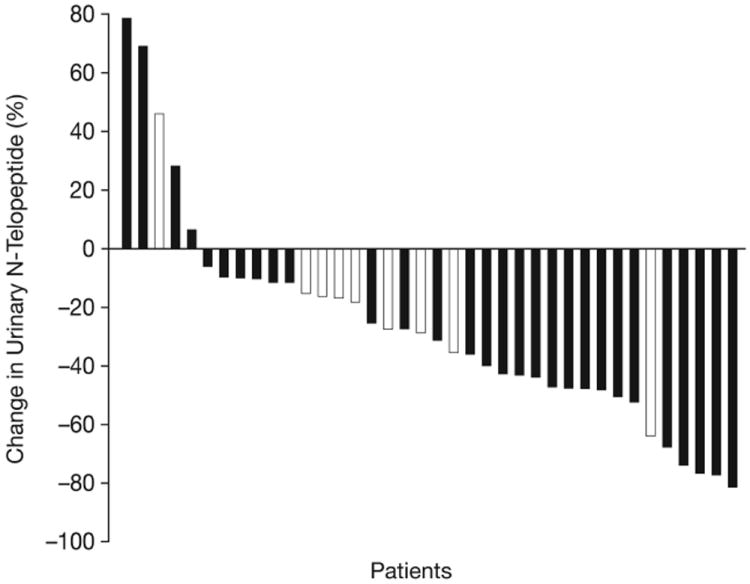
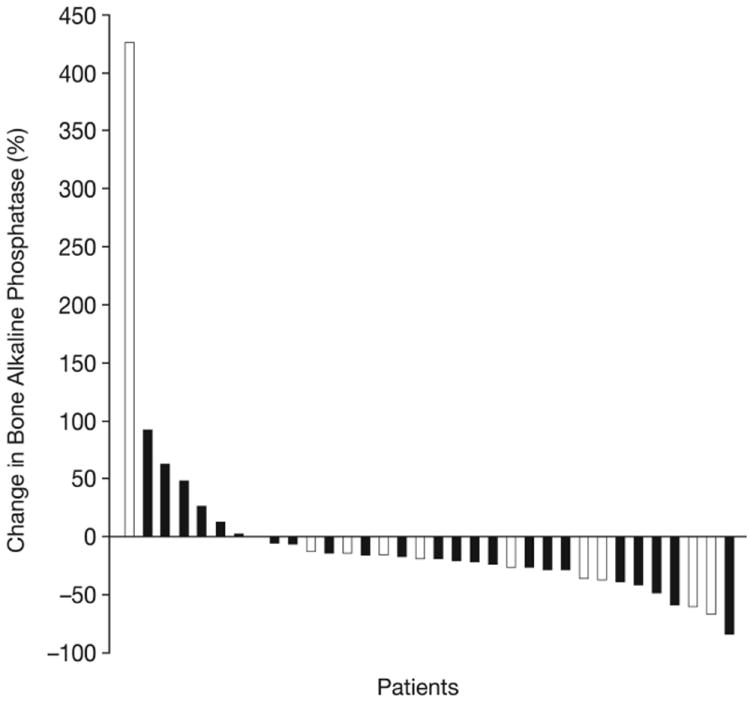
Waterfall plots showing maximal percentage changes from baseline in individual patients. (A) PSA. (B) Tumor size. (C) uNTX. (D) BAP. In Figures (C) and (D), patients who were receiving ongoing bisphosphonate therapy are shown by white bars.
Among RECIST-evaluable patients, 18/30 (60%) had a PR and 5/30 (17%) remained on therapy without response or progression for ≥18 weeks, resulting in an overall disease control rate of 77%. PRs occurred in 4/8 (50%) patients with visceral metastases and 14/22 (64%) patients with target lesions in lymph nodes only. Figure 2B shows maximum changes in tumor size.
Among all 46 patients, bone scan response was improved (disappearance of at least one lesion) in 14 patients (30%) and stable (no new bone lesions at ≥18 weeks) in 19 patients (41%). Nine patients (20%) had no new bone lesions at 6 and/or 12 weeks but were not scanned further because of discontinuation from the study. Three patients (7%) showed bone scan progression. One patient was not evaluable. As described in the Methods section, results on bone scan were not required to be confirmed.
Of patients evaluable for bone markers, 33/38 (87%) had a reduction in uNTX (Figure 2C). This included 18 patients (47%) who achieved a ≥35% uNTX reduction, of which two were receiving bisphosphonates. Furthermore, 26/33 patients (76%) had a reduction in BAP (Figure 2D), including nine receiving bisphosphonates. Median decreases from baseline (range) in patients with reductions were 36% (6–81%) for uNTX and 26% (6–85%) for BAP.
Post-hoc analyses were performed to determine if bone effects correlated with antitumor activity. Of evaluable patients with bone-scan improvement, 12/13 (92%) had a uNTX decrease, 10/12 (83%) had a BAP decrease, and 13/14 (93%) had a PSA decrease. Of evaluable patients with stable bone scan, uNTX, BAP, or PSA decrease occurred in 15/16 (94%), 12/13 (92%), and 17/19 (90%), respectively. In patients with low, medium and high numbers of bone scan lesions at baseline, all three biomarkers showed variable decreases (Table 4). In a further analysis, 25 patients were classified as responders based on achievement of PSA response (patients with nonmeasurable disease) or RECIST PR without PSA progression (patients with measurable disease). Of evaluable responders, 19/21 (90%) and 16/18 (89%) had a decrease in uNTX and BAP, respectively, and 23/25 (92%) had improved bone scans or no new lesion at ≥18 weeks.
Table 4.
Subset Analysis of Best Percentage Changes in uNTX, BAP, and PSA According to Bone Scan Volume at Baseline
| Bone Scan Volume | |||
|---|---|---|---|
| Low (1–9 lesions) n = 13 | Intermediate (10–20 lesions) n = 6 | High (>20 lesions) n = 8 | |
| Median uNTX at baseline, ng/mL (min, max) | 41 (9, 98) | 58 (26, 203) | 104 (46, 238) |
| Best percentage change in uNTX from baseline, median (min, max) | -39 (-77, 10) | -47 (-81, -27) | -28 (-64, 69) |
| Median BAP at baseline, ng/mL (min, max) | 17 (7, 33) | 17 (10, 584) | 95 (22, 318) |
| Best percentage change in BAP from baseline, median (min, max) | -25 (-59, 12) | -19 (-61, 24) | -23 (-85, 62) |
| Median PSA at baseline, ng/mL (min, max) | 17 (1, 50) | 135 (29, 379) | 96 (19, 700) |
| Best percentage change in PSA from baseline, median (min, max) | -78 (-98, 50) | -85 (-100, -34) | -20 (-95, 49) |
BAP indicates bone alkaline phosphatase; PSA, prostate-specific antigen; uNTX, urinary N-telopeptide.
Protocol-defined disease progression was recorded in 23 patients, including eight defined by bone scan. Of 28 patients who continued on single-agent dasatinib after stopping docetaxel, 16 patients subsequently progressed (after 0.9–8.1 additional months of single-agent dasatinib), including five patients who had stabilization of disease for at least 3 months. Six patients discontinued dasatinib without progression due to: study drug toxicity (n = 2), AE unrelated to study drug, patient request, investigator decision due to patient not likely to benefit from further treatment, and social reasons (n=1 each). Six additional patients remained on single-agent dasatinib without progression at data capture and had stabilization of disease for 3.4+ to 11.7+ months after the last docetaxel dose.
DISCUSSION
The results of this phase 2 study establish the feasibility and safety of combining dasatinib with docetaxel and prednisone at therapeutically relevant doses in patients with metastatic CRPC. The high rates of soft tissue responses, bone effects (as captured by bone scanning), and modulation of bone turnover markers are promising and support our hypothesis that co-targeting the tumor and associated microenvironment may increase the efficacy of docetaxel.27,28
Dasatinib plus docetaxel had a favorable toxicity profile, allowing escalation to dasatinib 120 mg QD with docetaxel 75 mg mg/m2 Q21D and prednisone. In conjunction with noninteracting PK profiles, these results strongly suggest that the combination can be given safely without compromising dose or schedule. The rate of fluid retention (pleural effusion, edema) was comparable to experience with docetaxel alone. Only 15% of patients experienced pleural effusion (grade 3 in 4%) and 1 patient (2%) had grade 1 pericardial effusion, whereas in a phase 2 study of 47 patients with CRPC who received BID dasatinib monotherapy, pleural and pericardial effusion occurred in 51% and 23%, respectively.29 Oral prednisone, combined with the improved safety of QD versus BID dasatinib,30 may have minimized fluid retention in our study.
Notwithstanding prior chemotherapy exposure in 33% of patients, the objective tumor response rate (60%), which is higher than those seen with docetaxel Q21D alone in the TAX327 and SWOG9916 trials (12–17%), is very encouraging. Furthermore, the PSA response rate of 57% compares favorably with TAX327/SWOG9916 (45–50%).27,28 The finding that 33/46 patients (72%) had either improved bone scan (disappearance of at least one lesion) or stable bone scan during the study was also of interest. These observations might be the result of the antitumor and bone-targeted effects of docetaxel and dasatinib treatment, which has been suggested by preclinical studies.11,12,15 Whether dasatinib increases antitumor effects of docetaxel in patients and prolongs survival requires evaluation in a randomized setting.
In this study, 23/46 patients had experienced disease progression by last follow-up, defined using radiographic and symptomatic criteria. Nine patients remained on-study at last follow-up. Of patients who discontinued treatment, one-third discontinued for reasons other than disease progression (eg, drug toxicity) and were not followed further for progression or survival. Because of proportion of patients for whom date of progression could not be determined, PFS could not be accurately calculated and this represents a limitation of the study.
Of particular interest was the duration of stable disease in patients who received “maintenance” single-agent dasatinib after stopping chemotherapy. Experimental observations in murine prostate cancer models suggest that dasatinib inhibits tumor proliferation, invasion, and metastasis, indicating that the antitumor effects of dasatinib are likely to be cytostatic rather than cytotoxic. Supporting these studies, 28 patients (61% of study participants), who responded by PSA or RECIST, continued on single-agent dasatinib after completing docetaxel therapy. Among these patients, durations of stabilization of disease of up to 12 months after docetaxel was stopped were recorded by data cut-off. The median of 6 docetaxel cycles administered was lower than published experience with docetaxel Q21D alone (9.5 cycles in TAX327),28 and additional cycles of docetaxel might have also resulted in ongoing stabilization of disease. The comparatively shorter duration of docetaxel use in this study likely reflects early cessation of chemotherapy after the protocol-specified minimum of 6 cycles based on investigator/patient choice. The occurrence of ongoing stabilization of disease after discontinuation of docetaxel has been reported infrequently within published literature. In the ASCENT trial of docetaxel with or without calcitriol, patients who had a confirmed 50% PSA response and PSA level ≤4.0 ng/mL were allowed to receive intermittent chemotherapy, ie, docetaxel was suspended until the PSA level rose (by 50% and to ≥2 ng/mL) or other evidence of progression was seen. Among 250 randomized patients, 45 (18%) had their chemotherapy suspended, and median duration until resumption was ~4.2 months (18 weeks), with a reported range of ~1–16.2 months (4–70 weeks).31 Although these data indicate that patients can experience periods of stabilized disease without continuous docetaxel, in the ASCENT trial only a minority of patients who had achieved relatively stringent response criteria had their therapy suspended. Whether single-agent dasatinib has the potential to prolong responses without ongoing docetaxel therapy warrants further investigation.
Treatment with dasatinib targeted both osteoclastic and osteoblastic components of bone disease, as shown by most patients having decreases in concentrations of uNTX and BAP. Interestingly, bisphosphonate-treated patients had additional reductions in bone-marker levels, suggesting that SRC inhibition may have bone-targeted effects beyond those achieved with bisphosphonates. Previous studies have assessed bone-marker effects of novel agents combined with docetaxel in patients with metastatic prostate cancer. In a phase 1/2 trial of docetaxel and atrasentan, an endothelin-A receptor antagonist, concentrations of BAP but not uNTX decreased in patients not receiving bisphosphonates.32 In a randomized phase 2 trial of docetaxel plus imatinib, a PDGFR inhibitor, combination treatment was associated with significantly greater declines in uNTX compared with docetaxel alone whereas docetaxel-related declines in BAP were impaired.33 Together with bone-marker outcomes with zoledronic acid,34 these results suggest that modulating bone turnover alone is insufficient to meaningfully alter the course of prostate cancer bone metastases in most patients.
Post-hoc analyses were performed to investigate the correlation between bone-scan responses and standard PSA/tumor responses or effects on bone markers. Of patients who responded by PSA and/or RECIST criteria, 92% also had an improved or stable bone scan versus 71% in the overall group. Of those with improved or stable bone scans, the proportions of evaluable patients with decreases in uNTX, BAP, or PSA were 93%, 88%, and 91%, respectively, versus 87%, 76%, and 80% in the overall group. No correlation was seen in comparisons between volume of bone scan lesions at baseline and effects of treatment on PSA or bone markers.
In summary, this study demonstrates that dasatinib and docetaxel combination therapy is well tolerated and has encouraging efficacy. Parallel bone scan improvements, uNTX/BAP decreases, and tumor effects (RECIST and PSA responses) are consistent with the hypothesis that the efficacy of dasatinib and docetaxel combination therapy is attributable to co-targeting of the tumor and the soft tissue and bone microenvironments. These data have provided the rationale for an ongoing randomized phase 3 study.
Acknowledgments
The study was funded by Bristol-Myers Squibb and conducted in part through the Department of Defense Prostate Cancer Clinical Trials Consortium. Medical writing assistance was provided by Fiona Bolland and Jeremy Gardner of StemScientific (funded by Bristol-Myers Squibb).
Footnotes
Conflicts of Interest:
GCT is an employee of and owns stocks in Bristol-Myers Squibb. PP and SA are employees of Bristol-Myers Squibb. AJA has received research funding from and acted in a consultant/advisory role for Bristol-Myers Squibb, has received research funding and honoraria from sanofi-aventis, and received research funding from ImClone and Medivation. CJL has acted in a consultant/advisory role for and received research funding and honoraria from Bristol-Myers Squibb. JCA, PM, ELB, EP, ML, and GEG have no conflicts of interest to disclose.
Clinical trial identifiers: CA180-086; NCT00439270
References
- 1.Soloway MS, Ishikawa S, van der Zwaag R, Todd B. Prognostic factors in patients with advanced prostate cancer. Urology. 1989;33:53–56. doi: 10.1016/0090-4295(89)90107-6. [DOI] [PubMed] [Google Scholar]
- 2.Ernst DS, Hanson J, Venner PM. Analysis of prognostic factors in men with metastatic prostate cancer. Uro-Oncology Group of Northern Alberta. J Urol. 1991;146:372–376. doi: 10.1016/s0022-5347(17)37797-2. [DOI] [PubMed] [Google Scholar]
- 3.Sabbatini P, Larson SM, Kremer A, et al. Prognostic significance of extent of disease in bone in patients with androgen-independent prostate cancer. J Clin Oncol. 1999;17:948–957. doi: 10.1200/JCO.1999.17.3.948. [DOI] [PubMed] [Google Scholar]
- 4.Noguchi M, Kikuchi H, Ishibashi M, Noda S. Percentage of the positive area of bone metastasis is an independent predictor of disease death in advanced prostate cancer. Br J Cancer. 2003;88:195–201. doi: 10.1038/sj.bjc.6600715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tu SM, Millikan RE, Mengistu B, et al. Bone-targeted therapy for advanced androgen-independent carcinoma of the prostate: a randomised phase II trial. Lancet. 2001;357:336–341. doi: 10.1016/S0140-6736(00)03639-4. [DOI] [PubMed] [Google Scholar]
- 6.Chen N, Ye XC, Chu K, et al. A secreted isoform of ErbB3 promotes osteonectin expression in bone and enhances the invasiveness of prostate cancer cells. Cancer Res. 2007;67:6544–6548. doi: 10.1158/0008-5472.CAN-07-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson JB, Hedican SP, George DJ, et al. Identification of endothelin-1 in the pathophysiology of metastatic adenocarcinoma of the prostate. Nat Med. 1995;1:944–949. doi: 10.1038/nm0995-944. [DOI] [PubMed] [Google Scholar]
- 8.Kimura T, Kuwata T, Ashimine S, et al. Targeting of bone-derived insulin-like growth factor-II by a human neutralizing antibody suppresses the growth of prostate cancer cells in a human bone environment. Clin Cancer Res. 2010;16:121–129. doi: 10.1158/1078-0432.CCR-09-0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Recchia I, Rucci N, Festuccia C, et al. Pyrrolopyrimidine c-Src inhibitors reduce growth, adhesion, motility and invasion of prostate cancer cells in vitro. Eur J Cancer. 2003;39:1927–1935. doi: 10.1016/s0959-8049(03)00394-0. [DOI] [PubMed] [Google Scholar]
- 10.Nam S, Kim D, Cheng JQ, et al. Action of the Src family kinase inhibitor, dasatinib (BMS-354825), on human prostate cancer cells. Cancer Res. 2005;65:9185–9189. doi: 10.1158/0008-5472.CAN-05-1731. [DOI] [PubMed] [Google Scholar]
- 11.Park SI, Zhang J, Phillips KA, et al. Targeting SRC family kinases inhibits growth and lymph node metastases of prostate cancer in an orthotopic nude mouse model. Cancer Res. 2008;68:3323–3333. doi: 10.1158/0008-5472.CAN-07-2997. [DOI] [PubMed] [Google Scholar]
- 12.Koreckij T, Nguyen H, Brown LG, Yu EY, Vessella RL, Corey E. Dasatinib inhibits the growth of prostate cancer in bone and provides additional protection from osteolysis. Br J Cancer. 2009;101:263–268. doi: 10.1038/sj.bjc.6605178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldenberg-Furmanov M, Stein I, Pikarsky E, et al. Lyn is a target gene for prostate cancer: sequence-based inhibition induces regression of human tumor xenografts. Cancer Res. 2004;64:1058–1066. doi: 10.1158/0008-5472.can-03-2420. [DOI] [PubMed] [Google Scholar]
- 14.Posadas EM, Al-Ahmadie H, Robinson VL, et al. FYN is overexpressed in human prostate cancer. BJU Int. 2009;103:171–177. doi: 10.1111/j.1464-410X.2008.08009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tatarov O, Mitchell TJ, Seywright M, Leung HY, Brunton VG, Edwards J. SRC family kinase activity is up-regulated in hormone-refractory prostate cancer. Clin Cancer Res. 2009;15:3540–3549. doi: 10.1158/1078-0432.CCR-08-1857. [DOI] [PubMed] [Google Scholar]
- 16.Miyazaki T, Sanjay A, Neff L, Tanaka S, Horne WC, Baron R. Src kinase activity is essential for osteoclast function. J Biol Chem. 2004;279:17660–17666. doi: 10.1074/jbc.M311032200. [DOI] [PubMed] [Google Scholar]
- 17.Boyce BF, Yoneda T, Lowe C, Soriano P, Mundy GR. Requirement of pp60c-src expression for osteoclasts to form ruffled borders and resorb bone in mice. J Clin Invest. 1992;90:1622–1627. doi: 10.1172/JCI116032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Myoui A, Nishimura R, Williams PJ, et al. C-SRC tyrosine kinase activity is associated with tumor colonization in bone and lung in an animal model of human breast cancer metastasis. Cancer Res. 2003;63:5028–5033. [PubMed] [Google Scholar]
- 19.Rucci N, Recchia I, Angelucci A, et al. Inhibition of protein kinase c-Src reduces the incidence of breast cancer metastases and increases survival in mice: implications for therapy. J Pharmacol Exp Ther. 2006;318:161–172. doi: 10.1124/jpet.106.102004. [DOI] [PubMed] [Google Scholar]
- 20.Zhang XH, Wang Q, Gerald W, et al. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell. 2009;16:67–78. doi: 10.1016/j.ccr.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lombardo LJ, Lee FY, Chen P, et al. Discovery of N-(2-chloro-6-methyl-phenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47:6658–6661. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- 22.Huang F, Reeves K, Han X, et al. Identification of candidate molecular markers predicting sensitivity in solid tumors to dasatinib: rationale for patient selection. Cancer Res. 2007;67:2226–2238. doi: 10.1158/0008-5472.CAN-06-3633. [DOI] [PubMed] [Google Scholar]
- 23.Bantscheff M, Eberhard D, Abraham Y, et al. Quantitative chemical proteomics reveals mechanisms of action of clinical ABL kinase inhibitors. Nat Biotechnol. 2007;25:1035–1044. doi: 10.1038/nbt1328. [DOI] [PubMed] [Google Scholar]
- 24.Bubley GJ, Carducci M, Dahut W, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17:3461–3467. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]
- 25.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah NP, Kantarjian HM, Kim DW, et al. Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and -intolerant chronic-phase chronic myeloid leukemia. J Clin Oncol. 2008;26:3204–3212. doi: 10.1200/JCO.2007.14.9260. [DOI] [PubMed] [Google Scholar]
- 27.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 28.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 29.Yu EY, Wilding G, Posadas E, et al. Phase II study of dasatinib in patients with metastatic castration-resistant prostate cancer. Clin Cancer Res. 2009;15:7421–7428. doi: 10.1158/1078-0432.CCR-09-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu EY, Massard C, Gross M, et al. A phase II study of once-daily dasatinib for patients with castration-resistant prostate cancer (CA180085) J Clin Oncol. 2009;27(suppl 15):270s. Abstract 5147. [Google Scholar]
- 31.Beer TM, Ryan CW, Venner PM, et al. Intermittent chemotherapy in patients with metastatic androgen-independent prostate cancer: results from ASCENT, a double-blinded, randomized comparison of high-dose calcitriol plus docetaxel with placebo plus docetaxel. Cancer. 2008;112:326–330. doi: 10.1002/cncr.23163. [DOI] [PubMed] [Google Scholar]
- 32.Armstrong AJ, Creel P, Turnbull J, et al. A phase I-II study of docetaxel and atrasentan in men with castration-resistant metastatic prostate cancer. Clin Cancer Res. 2008;14:6270–6276. doi: 10.1158/1078-0432.CCR-08-1085. [DOI] [PubMed] [Google Scholar]
- 33.Mathew P, Thall PF, Bucana CD, et al. Platelet-derived growth factor receptor inhibition and chemotherapy for castration-resistant prostate cancer with bone metastases. Clin Cancer Res. 2007;13:5816–5824. doi: 10.1158/1078-0432.CCR-07-1269. [DOI] [PubMed] [Google Scholar]
- 34.Saad F, Gleason DM, Murray R, et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96:879–882. doi: 10.1093/jnci/djh141. [DOI] [PubMed] [Google Scholar]


