Summary
Objectives
The incretin hormone glucagon-like peptide-1 (GLP-1) retards gastric emptying and decreases caloric intake. It is unclear whether increased GLP-1 concentrations achieved by inhibition of the inactivating enzyme dipeptidyl peptidase-4 (DPP-4) alter gastric volumes and satiation in people with type 2 diabetes.
Methods
In a double-blind, placebo-controlled crossover design, 14 subjects with type 2 diabetes received vildagliptin (50 mg bid) or placebo for 10 days in random order separated by a 2-week washout. On day 7, fasting and postmeal gastric volumes were measured by a 99mTc single-photon emission computed tomography (SPECT) method. On day 8, a liquid Ensure® meal was consumed at 30 ml/min, and maximum tolerated volume (MTV) and symptoms 30 min later were measured using a visual analogue scale (VAS) to assess effects on satiation. On day 10, subjects ingested water until maximum satiation was achieved. The volume ingested was recorded and symptoms similarly measured using a VAS.
Results
Vildagliptin raised plasma GLP-1 concentrations. However, fasting (248 ± 21 vs. 247 ± 19 ml, P = 0·98) and fed (746 ± 28 vs. 772 ± 26 ml, P = 0·54) gastric volumes did not differ when subjects received vildagliptin or placebo. Treatment with vildagliptin did not alter the MTV of Ensure® (1657 ± 308 vs. 1389 ± 197 ml, P = 0·15) or water compared to placebo (1371 ± 141 vs. 1172 ± 156 ml, P = 0·23). Vildagliptin was associated with decreased peptide YY (PYY) concentrations 60 min after initiation of the meal (166 ± 27 vs. 229 ± 34 pmol/l, P = 0·01).
Conclusions
Vildagliptin does not alter satiation or gastric volume in people with type 2 diabetes despite elevated GLP-1 concentrations. Compensatory changes in enteroendocrine secretion could account for the lack of gastrointestinal symptoms.
Introduction
The relative stability of body weight in human subjects requires close matching of overall caloric ingestion and expenditure over long periods of time.1 Adaptive alteration of energy intake in response to changing energy expenditure is regulated to a large part by the brain, which modulates appetite in response to multiple indicators of energy status.2 Signals originating in the gastrointestinal tract ultimately lead to postprandial fullness (satiation) and possibly to appetite regulation. Several gut hormones interact with the brain in a complex manner to regulate gastrointestinal functions and the perception of gastrointestinal signals and satiation. These processes ultimately lead to meal termination. Understanding the mechanisms involved in satiation is important to the development of therapies for obesity and diabetes.
Glucagon-like peptide-1 (GLP-1) is produced by the enteroendocrine L cells of the intestinal mucosa and is released into the portal circulation in response to meal ingestion. It arises from the post-translational processing of proglucagon by prohormone convertase 1 (PC1) in the enteroendocrine L cells of the intestinal mucosa.3 GLP-1 enhances insulin secretion and inhibits glucagon release in a glucose-dependent manner.4 In addition, it delays gastric emptying5 and increases gastric volumes.6,7 When infused in pharmacological concentrations, it enhances satiation and facilitates weight loss in people with type 2 diabetes.8 However, its utility as a therapeutic agent in diabetes has been limited by its extremely short half-life, and by the development of nausea, which results in discontinuation of therapy.9
The insulinotrophic activity of the major form of secreted GLP-1, GLP-1-(7-36)-amide, requires the two N-terminal amino acids. The widely distributed enzyme dipeptidyl peptidase-4 (DPP-4) rapidly converts the intact peptide to the metabolite GLP-1-(9-36)-amide. Therefore, GLP-1-based therapy for type 2 diabetes has required the development of GLP-1 receptor agonists that resist the action of DPP-4.10 These compounds have similar effects to GLP-1, inducing satiety and weight loss in clinical practice.11
An alternative therapeutic strategy to enhance glycaemic control has been to inhibit DPP-4, thereby raising endogenous concentrations of active GLP-1.12 DPP-4 inhibitors lower fasting and postprandial glucose concentrations, with few reported gastrointestinal symptoms.13 We have recently reported that, at a dose sufficient to lower postprandial glucose concentrations, the DPP-4 inhibitor vildagliptin achieved the expected effect on postprandial glucose after a mixed meal, and increased plasma GLP-1 levels but did not slow the gastric emptying of solids.14 It is unknown whether these effects represent the lower concentrations of GLP-1 observed in the peripheral circulation or the effect of DPP-4 inhibitors on other components of the enteroendocrine system.
Inhibition of DPP-4 raises concentrations of other gut hormones, such as peptide YY (PYY), which is also produced by L cells, and glucose-dependent insulinotrophic polypeptide (GIP), produced by the enteroendocrine K cells.15 These or other unknown hormones may also affect satiation and appetite,16–18 independent of the effects of DPP-4 inhibition on GLP-1. The aim of this study was to examine the effects of vildagliptin on gastric volume while fasting and in response to meal ingestion (accommodation), as well as enteroendocrine secretion and satiation in people with type 2 diabetes.
Methods
Subjects
After approval from the Mayo Institutional Review Board, 14 subjects with type 2 diabetes gave written informed consent to participate in the study. All subjects were in good health and were at a stable weight and did not engage in regular vigorous exercise. Subjects were not taking medication known to alter gastric emptying such as narcotics or calcium channel blockers. None of the subjects had microvascular complications of diabetes. At the time of screening, a bowel disease questionnaire revealed no gastrointestinal symptoms.19 All subjects were instructed to follow a weight maintenance diet containing 55% carbohydrate, 30% fat and 15% protein for the period of study. All oral agents used for the treatment of diabetes were discontinued 3 weeks before the study. The mean age was 53·1 ± 2·0 years, body mass index was 33·9 ± 1·5 kg/m2 and mean glycosylated haemoglobin (HbA1c) was 6·1 ± 0·2%.
Experimental design
Protocol
Subjects were admitted to the Clinical Research Unit (CRU) on the evening of the sixth day of the treatment period. After an overnight fast, gastric volume during fasting and the change in volume after a standard meal (volume accommodation) were measured on the seventh day of the treatment period. The maximum tolerated volume (MTV) of caloric or noncaloric liquids was measured, respectively, on the eighth and tenth day of the treatment period to examine the effect of DPP-4 inhibition on satiation and postprandial gastrointestinal symptoms. Glucose turnover and gastric emptying were measured simultaneously on the ninth day of the treatment period; those results have been reported previously.14
Assignment
We used a randomized, double-blind, placebo-controlled crossover design. Subjects received either vildagliptin 50 mg or placebo taken before breakfast and the evening meal over a 10-day treatment period, with the two treatment intervals being separated by at least a 2-week washout period. The order of treatment was random. The trial was registered at www.clinicaltrials.gov (identifier NCT00351507).
The investigators were unblinded after all data were collected and analysed. No adverse events, such as nausea, that might have led to unmasking were experienced by the subjects.
Subjects were directly observed when taking their first dose of study drug/placebo. The tablets of study medication were counted prior to dispensing, at the time of admission on the sixth day and at the end of the treatment period. While admitted to the CRU, medication was dispensed by the nursing staff.
Gastric volume measurement (day 7)
On the morning of day 7 following an 8-h fast, fasting and postprandial gastric volumes were measured noninvasively using single-photon emission computed tomography (SPECT). The gastric mucosa is able to take up intravenously administered 99mTc-pertechnetate from the circulating blood pool.6 Subjects received the morning dose of study drug (vildagliptin 50 mg or placebo) at −30 min. Ten minutes later (−20 min), 10 mCi 99mTc-pertechnetate was injected intravenously. SPECT imaging was performed 10 min later (starting at −10 min) and after a 300 ml nutrient drink (Ensure®, 1 kcal/ml) had been ingested through a straw (0 min). Gastric volumes were assessed during two postprandial periods: 0–10 min and 10–20 min after the meal.
Tomographic images were acquired using a large field-of-view, dual-headed gamma camera system. Transaxial images of the stomach were obtained with the SPECT Analyse PC 2·5 (Biomedical Imaging Resource, Mayo Foundation, Rochester, MN) software system. Three-dimensional images of the stomach were reconstructed, enabling measurement of gastric volumes during the fasting and postprandial periods. Volume changes and ratios between the fasting and postprandial periods were subsequently calculated.
Satiation in response to caloric liquid ingestion (day 8)
On the morning of day 8 following an 8-h fast, an 18-gauge cannula was inserted retrogradely into a vein on the dorsum of the nondominant hand. This was subsequently placed in a heated Plexiglas box maintained at 55 °C to allow sampling of arterialized venous blood. Subjects received the morning dose of study drug (vildagliptin 50 mg or placebo) at −30 min. After a baseline blood draw (0 min), subjects ingested a liquid nutrient (Ensure®, Abbott Laboratories; 1 kcal/ml, 11% fat, 73% carbohydrate, 16% protein) from a 120 ml cup that was refilled every 4 min (30 ml/min) until maximum satiation was achieved. The start time was recorded. Subjects recorded the actual time when each level of fullness was reached using a graphic rated scale graded 0–5 (0 = no symptoms, 5 = maximum unbearable fullness). The MTV was then recorded, and patient symptom scores for nausea, fullness, bloating and abdominal pain were recorded using a visual analogue scale (VAS) 30 min after MTV was achieved. The 100-mm VAS for each symptom consisted of horizontal lines anchored with the words ‘none’ and ‘worst ever’ at the left and right ends of the lines.
Analytical techniques
Plasma samples were placed on ice, centrifuged at 4 °C, separated, and stored at −20 °C until assayed. Glucose concentrations were measured using a reflectance meter (Accucheck, Roche Diagnostics, Indianapolis, IN). Samples tubes used for measurement of GLP-1 and GIP had 100 μM of DPP-4 inhibitor (Linco Research, St Louis, MO) added. Intact GLP-1 concentrations were measured using an N-terminal immunoassay (Linco Research). Active GIP concentrations were measured using an N-terminal radioimmunoassay (RIA) as described previously.20 In addition, active ghrelin, leptin and total PYY concentrations were measured by RIA using reagents supplied by Linco Research.
Satiation in response to water ingestion (day 10)
On the morning of day 10, following an 8-h fast, subjects received the morning dose of study drug (vildagliptin 50 mg or placebo) at −30 min. At 0 min subjects started to drink water poured into a glass from a 1000 ml volumetric flask. The flask was not visible to the subject. The glass was refilled whenever empty. Subjects were instructed to drink as much water as possible, at their own rate. Water ingestion continued until 5 min had elapsed or until maximum satiation was achieved. The MTV was then recorded. Thirty minutes after MTV was achieved, symptom scores for nausea, fullness, bloating and abdominal pain were recorded using a VAS as described above.
To ensure that the data were representative for each individual, the water satiation test was repeated 2 h later; during the time between the two water satiation tests, the subjects continued to fast.
Statistical analysis
All data are presented as means ± SEM. Paired comparisons between the treatment and placebo group were made using Student’s two-way t-test for paired samples. A P-value of less than 0·05 was considered to be statistically significant.
Power statement
The study was designed to test the hypothesis that vildagliptin altered gastric emptying and accommodation in people with type 2 diabetes. Taking account of the variance in measurement of gastric emptying in people with type 2 diabetes,21 14 subjects would provide 100% power to detect a 20% change in gastric emptying T1/2 at a P < 0·05. This change in gastric emptying is deemed to be clinically significant as it approximates the degree of delay observed in diabetic patients with upper gastrointestinal symptoms.22 Data from a previous experiment using SPECT to measure gastric volume accommodation6 showed that, with similar variance in measurement, 14 subjects would provide > 99% power to detect a 20% change in gastric volume accommodation at a P < 0·05.
Results
Gastric volumes (Fig. 1)
Fig. 1.
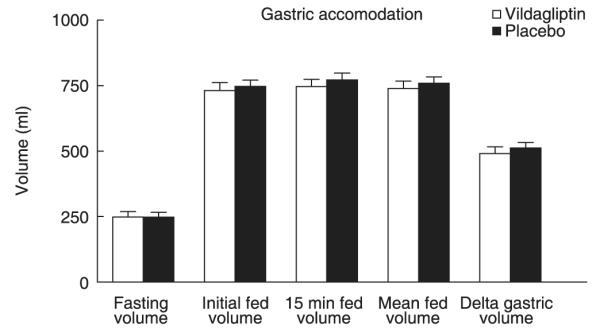
Fasting and fed gastric volumes in the presence of vildagliptin (open bars) and placebo (solid bars).
Fasting gastric volumes (248 ± 21 vs. 247 ± 19 ml, P = 0·98) did not differ when subjects received vildagliptin or placebo. Ingestion of the liquid meal led to an increase in gastric volume that did not differ in the presence or absence of vildagliptin (739 ± 28 vs. 759 ± 24 ml, P = 0·57). The change in gastric volume (mean fed volume – fasting volume) likewise did not differ (491 ± 25 vs. 512 ± 21 ml, P = 0·56).
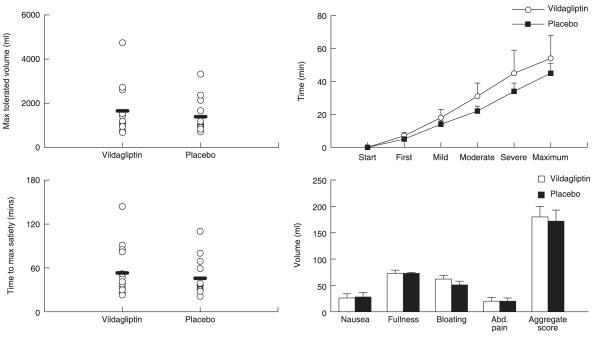
MTV and symptom scores in the nutrient drink test (Fig. 2)
Fig. 2.
Maximum tolerated volume of a caloric liquid (left upper panel), time to achieve maximum satiety (left lower panel), time taken to achieve different degrees of satiety (right upper panel) and individual and aggregate symptom scores (right lower panel) in the presence of vildagliptin (open circles) and placebo (solid squares).
Treatment with vildagliptin did not alter the MTV of ingested Ensure® compared to placebo (1657 ± 308 vs. 1389 ± 197 ml, P = 0·15). The time taken to experience the first sensation of fullness (7 ± 2 vs. 5 ± 1 min, P = 0·24) or a mild sensation of fullness during ingestion of Ensure® (18 ± 4 vs. 14 ± 2 min, P = 0·28) did not differ significantly in the presence or absence of vildagliptin. Similarly, there was no significant delay in the perception of moderate (31 ± 7 vs. 22 ± 3 min, P = 0·09) and severe (45 ± 10 vs. 34 ± 6 min, P = 0·06) fullness with vildagliptin compared to placebo.
Aggregate (180 ± 20 vs. 172 ± 21, P = 0·72) and individual symptom scores for nausea (26 ± 8 vs. 28 ± 9, P = 0·79), fullness (73 ± 6 vs. 73 ± 2, P = 0·99), bloating (62 ± 7 vs. 51 ± 7, P = 0·11) and abdominal pain (20 ± 7 vs. 20 ± 6, P = 0·99) did not differ in the presence or absence of vildagliptin.
Plasma glucose concentrations in the nutrient drink test
Treatment with vildagliptin resulted in lower fasting glucose (8·9 ± 0·6 vs. 9·3 ± 0·4 mmol/l, P = 0·05), lower postmeal peak glucose (14·2 ± 0·97 vs. 15·5 ± 0·8 mmol/l, P < 0·01) and lower glycaemic area above basal (2328 ± 78 vs. 2550 ± 88 mmol/l per 3 h, P = 0·01) than occurred after administration of placebo.
Plasma PYY, ghrelin, GLP-1 and GIP concentrations (Fig. 3)
Fig. 3.
PYY (left upper panel), ghrelin (left lower panel), active GLP-1 (right upper panel) and GIP (right lower panel) concentrations in the presence of vildagliptin (open circles) and placebo (solid squares).
Fasting plasma PYY concentrations did not differ significantly when subjects received vildagliptin or placebo (85 ± 9 vs. 97 ± 11 pmol/l,P = 0·17). After meal ingestion, the net area under the curve did not differ in the presence of vildagliptin as opposed to placebo (29·3 ± 5·3 vs. 38·5 ± 5·2 nmol/l per 3 h, P = 0·08). However, vildagliptin was associated with decreased PYY concentrations 60 min after initiation of the meal (166 ± 27 vs. 229 ± 34 pmol/l, P = 0·01).
Plasma ghrelin concentrations did not differ significantly when subjects received vildagliptin or placebo before (760 ± 72 vs. 742 ± 92 μU/ml, P = 0·92) meal ingestion. During the caloric intake, the associated suppression of ghrelin did not differ in the presence or absence of vildagliptin (102·4 ± 6·7 vs. 8·0 ± 5·2 mU/ml per 3 h, P = 0·75)
In the presence of vildagliptin, fasting GLP-1 did not differ compared to placebo (5·1 ± 1·47 vs. 5·2 ± 1·69 pmol/l, P = 0·13). As expected, in the presence of vildagliptin, GLP-1 concentrations also rose to a higher concentration after caloric ingestion and remained elevated compared to placebo over the period of study (16·5 ± 1·1 vs. 7·9 ± 0·4 nmol/l per 3 h, P < 0·001).
Fasting concentrations of GIP did not differ in the presence or absence of vildagliptin (16·1 ± 1·76 vs. 14·0 ± 1·01 pmol/l, P = 0·34). As expected, in the presence of vildagliptin, GIP concentrations rose to a higher concentration after caloric ingestion (110·4 ± 10·9 vs. 49·1 ± 5·3 pmol/l, P < 0·001) and remained elevated over the period of study compared to placebo, as expressed by the area under the curve over time (16·5 ± 1·1 vs. 7·9 ± 0·4 nmol/l per 3 h, P < 0·001).
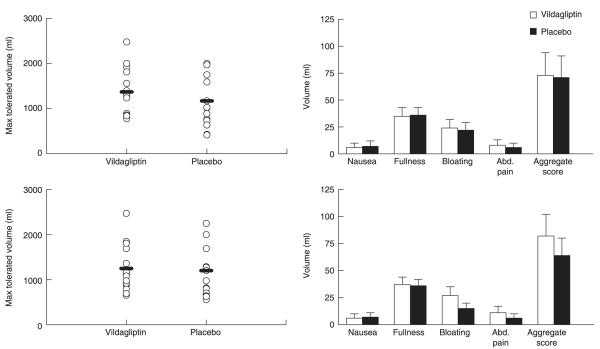
MTV and symptom scores with water ingestion (Fig. 4)
Fig. 4.
Maximum tolerated volume of water ingested on initial (left upper panel) and repeat testing (left lower panel) together with individual and aggregate symptom scores on initial (right upper panel) and repeat testing (right lower panel) in the presence of vildagliptin (open bars) and placebo (solid bars).
Treatment with vildagliptin did not alter the MTV of ingested water compared to placebo both in the initial water satiation test (1371 ± 141 vs. 1172 ± 156 ml, P = 0·23) and in the study repeated 2 h later (1247 ± 141 vs. 1201 ± 154 ml, P = 0·77).
Aggregate symptom scores did not differ 30 min after completion of the first study (73 ± 21 vs. 71 ± 21, P = 0·91) and the second study (82 ± 20 vs. 64 ± 16, P = 0·24) in the presence or absence of vildagliptin.
Discussion
In this study, we have demonstrated that DDP-4 inhibition does not influence satiation or gastric volume. This is concordant with a previous observation that vildagliptin does not alter gastric emptying of solids.14 In contrast, when GLP-1 was infused at a rate of 1·2 pmol/kg/min for 60 min in healthy volunteers fed an Ensure® meal (median of 1119 kcal in the presence of GLP-1 and 1350 kcal in the presence of placebo), there was a slight and transient delay in gastric emptying of the liquid meal.6 Exogenous GLP-1 also increased fasting and postprandial gastric volumes.5,7 Continuous subcutaneous infusion of GLP-1 over 1 month has been associated with weight loss, although the mechanism is uncertain because gastrointestinal function and satiation were not studied directly.8 GLP-1 receptor agonists also decrease caloric intake and are associated with weight loss and an increased incidence of gastrointestinal symptoms in clinical practice.11,23 This is in marked contrast to the experience reported for DPP-4 inhibitors, where no increase in the frequency of gastrointestinal side-effects, or significant weight loss, has been noted.24
It has been suggested that lack of gastrointestinal effects of DPP-4 inhibitors occurs because the resulting rise in peripheral active GLP-1 concentrations is not elevated or sustained, in marked contrast to concentrations observed during peripheral GLP-1 infusion. Indeed, there has been some controversy as to the mode of action of DPP-4 inhibitors.25,26 However, in double incretin receptor knockout (DIRKO) mice (GLP1R−/−, GIPR−/−), DDP-4 inhibition does not lower blood glucose. This implies that GLP-1 and/or GIP are necessary for DPP-4 inhibitors to mediate their glucose-lowering effects.27
We observed that in response to a liquid nutrient meal of ~1500 kcal, DPP-4 inhibition led to a very marked elevation of circulating concentrations of active GLP-1. The reason for this marked increase in GLP-1 levels, which exceeded concentrations previously reported from our laboratory,28 is unclear. It is conceivable that the nature of the liquid nutrient meal and its caloric content may have stimulated a larger segment of bowel containing the enteroendocrine L cells.29,30 It is important to note, however, that despite this elevation of GLP-1 with vildagliptin treatment, participants did not experience greater degrees of bloating, nausea and abdominal pain compared to placebo. Indeed, peak concentrations of active GLP-1 observed in response to a mixed meal in the presence of vildagliptin14 are similar to concentrations of infused GLP-1, known to alter gastric emptying.31 These observations make it less likely that the absence of gastrointestinal symptoms during DPP-4 inhibition can be completely explained by the peripheral circulating concentrations of active GLP-1.
As expected, vildagliptin significantly raised circulating concentrations of GIP. GIP fails to stimulate insulin secretion in people with diabetes, in contrast to its actions in healthy humans,32 and it does not alter the gastric emptying of solids.33 To date, there are no reports on the effects of GIP on gastric accommodation or satiation in humans. In this study, the expected elevation of GIP concentrations in the presence of DPP-4 inhibition did not significantly alter satiation or gastric accommodation.
A potential explanation for the absence of effects of vildagliptin on satiation or gastrointestinal motility is that it may alter concentrations of other gut hormones, with effects on appetite or motility (such as PYY or ghrelin) that neutralize the effect of GLP-1 elevation.
PYY, like GLP-1, is produced by the intestinal L cells.3,34 The secreted form of PYY, PYY1-36, undergoes proteolysis by DPP-4 to PYY3-36, which is bioactive and may reduce appetite.16 Exogenous infusion of PYY (leading to circulating levels around 55 pmol/l) delays gastric emptying.35 In our study there was a slight decrease in net postprandial PYY concentrations in the presence of vildagliptin. The C-terminal immunoassay used in this study could not differentiate between PYY1-36 and PYY3-36 (i.e. it measures total PYY) but suggests that PYY secretion by the L cells is decreased in the presence of vildagliptin. This is consistent with the observation that negative-feedback inhibition of the L cell by increased GLP-1 concentrations may occur in the presence of DPP-4 inhibition,36 and suggests that the same applies to L-cell secretion of PYY. Future experiments will be required to determine whether decreased PYY concentrations (or specifically decreased concentrations of PYY3-36) can explain the paucity of gastrointestinal effects of vildagliptin.
Ghrelin, currently the only known orexigenic gut hormone, is not a substrate for DPP-4 and the meal-induced decrease in ghrelin did not differ in the presence or absence of vildagliptin, suggesting that compensatory rises in ghrelin concentrations cannot explain the lack of increased gastrointestinal symptoms or change in satiation associated with vildagliptin in this study.
There are some limitations in our study. Our study focused on postprandial satiation and we did not examine the effects of DPP-4 inhibition on appetite or hunger during the interval between meals. Although significant weight loss has not been a feature of DPP-4 administration in long-term clinical studies,13 determination of the effects of DPP-4 inhibitors on hunger, caloric intake at subsequent meals and meal frequency would require a different study design.
In addition, although we had previously shown that the actions of GLP-1 on the stomach require intact vagal function,7 we did not directly exclude the presence of cardiovagal neuropathy in the current studies. However, there was no evidence of microvascular complications in the subjects studied and all reported the absence of gastrointestinal symptoms.
Finally, we did not formally assess the potential effects of changes in blood glucose as a confounder in the interpretation of our data. It is possible that lower glucose concentrations during vildagliptin administration may override any potential effect of DPP-4 inhibition on gastric accommodation and satiation. The effect of glycaemia, if any, on gastric accommodation is unknown.37 Hyperglycaemia delays gastric emptying38 whereas hypoglycaemia accelerates gastric emptying.39 It is possible that the lower glucose levels during vildagliptin administration may override any inhibitory effect on gastric accommodation. However, Schvarcz et al. demonstrated that raising blood glucose concentrations by ~4 mmol/l delayed gastric emptying T1/2 of a liquid meal by 10 min.40 We do not perceive that the observed differences in glucose concentrations between study days were sufficient, in the absence of hypoglycaemia, to stimulate the vagus nerve and accelerate gastric emptying39 or to explain the observed lack of effects of DPP-4 inhibition on satiation.
In conclusion, as part of a detailed examination of the gastrointestinal effects of DPP-4 inhibition, we report that vildagliptin does not alter fasting gastric volume or volume accommodation in response to a meal. We also measured satiation and gastrointestinal symptoms in response to caloric and noncaloric liquids. Vildagliptin did not alter the MTV or the symptom score compared to placebo. It remains to be determined why, in marked contrast to GLP-1 or GLP-1 receptor agonists, DPP-4 inhibitors do not alter gastric emptying, accommodation or satiation. These experiments lend additional support to the notion that DPP-4 inhibitors improve glycaemic control by stimulating insulin secretion and by inhibiting glucagon release rather than by altering gastrointestinal function or satiation.
Acknowledgements
We thank Novartis Pharmaceuticals for providing financial support for these studies. We acknowledge the support of the Mayo Clinic General Clinical Research Center by NIH grant RR024150. Dr Vella is supported by DK078646, Dr Camilleri by DK02638 and NIH P01-DK068055 and Dr Rizza by DK29953.
A.V. has received research grants from Merck, Eli Lilly and Novartis. R.A.R. is a member of the advisory boards of Merck, Novo Nordisk, Takeda, Mankind and Eli Lilly and a consultant for Abbott and Eli Lilly. C.F.D. has served as a member of the Novartis, BMS, Takeda and GSK advisory boards and has received lecture fees from Merck, Astra Zeneca and Prosidion. J.E.F., M.L.S. and D.B.S. are employees of and hold stock in Novartis.
References
- 1.Levine JA, Schleusner SJ, Jensen MD. Energy expenditure of nonexercise activity. American Journal of Clinical Nutrition. 2000;72:1451–1454. doi: 10.1093/ajcn/72.6.1451. [DOI] [PubMed] [Google Scholar]
- 2.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. Journal of Clinical Investigation. 2007;117:13–23. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holst JJ, Bersani M, Johnsen AH, Kofod H, Hartmann B, Orskov C. Proglucagon processing in porcine and human pancreas. Journal of Biological Chemistry. 1994;269:18827–18833. [PubMed] [Google Scholar]
- 4.Holst JJ, Toft-Nielsen MB, Orskov C, Nauck M, Willms B. On the effects of glucagon-like peptide-1 on blood glucose regulation in normal and diabetic subjects. Annals of the New York Academy of Sciences. 1996;805:729–736. doi: 10.1111/j.1749-6632.1996.tb17549.x. [DOI] [PubMed] [Google Scholar]
- 5.Schirra J, Wank U, Arnold R, Goke B, Katschinski M. Effects of glucagon-like peptide-1 (7-36) amide on motility and sensation of the proximal stomach in humans. Gut. 2002;50:341–348. doi: 10.1136/gut.50.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delgado-Aros S, Kim DY, Burton DD, Thomforde GM, Stephens D, Brinkmann BH, Vella A, Camilleri M. Effect of GLP-1 on gastric volume, emptying, maximum volume ingested, and postprandial symptoms in humans. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2002;282:G424–G431. doi: 10.1152/ajpgi.2002.282.3.G424. [DOI] [PubMed] [Google Scholar]
- 7.Delgado-Aros S, Vella A, Camilleri M, Low PA, Burton DD, Thomforde GM, Stephens D. Effects of glucagon-like peptide-1 and feeding on gastric volumes in diabetes mellitus with cardiovagal dysfunction. Neurogastroenterological Motility. 2003;15:435–443. doi: 10.1046/j.1365-2982.2003.00422.x. [DOI] [PubMed] [Google Scholar]
- 8.Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet. 2002;359:824–830. doi: 10.1016/S0140-6736(02)07952-7. [DOI] [PubMed] [Google Scholar]
- 9.Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Annals of Internal Medicine. 2005;143:559–569. doi: 10.7326/0003-4819-143-8-200510180-00006. [DOI] [PubMed] [Google Scholar]
- 10.Nauck MA, Meier JJ. Glucagon-like peptide 1 and its derivatives in the treatment of diabetes. Regulatory Peptides. 2005;128:135–148. doi: 10.1016/j.regpep.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 11.Kendall DM, Riddle MC, Rosenstock J, Zhuang D, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28:1083–1091. doi: 10.2337/diacare.28.5.1083. [DOI] [PubMed] [Google Scholar]
- 12.Drucker DJ. Therapeutic potential of dipeptidyl peptidase IV inhibitors for the treatment of type 2 diabetes. Expert Opinion on Investigating Drugs. 2003;12:87–100. doi: 10.1517/13543784.12.1.87. [DOI] [PubMed] [Google Scholar]
- 13.Ahren B, Simonsson E, Larsson H, Landin-Olsson M, Torgeirsson H, Jansson PA, Sandqvist M, Bavenholm P, Efendic S, Eriksson JW, Dickinson S, Holmes D. Inhibition of dipeptidyl peptidase IV improves metabolic control over a 4-week study period in type 2 diabetes. Diabetes Care. 2002;25:869–875. doi: 10.2337/diacare.25.5.869. [DOI] [PubMed] [Google Scholar]
- 14.Vella A, Bock G, Giesler PD, Burton DB, Serra DB, Saylan ML, Dunning BE, Foley JE, Rizza RA, Camilleri M. Effects of dipeptidyl peptidase-4 inhibition on gastrointestinal function, meal appearance, and glucose metabolism in type 2 diabetes. Diabetes. 2007;56:1475–1480. doi: 10.2337/db07-0136. [DOI] [PubMed] [Google Scholar]
- 15.De Meester I, Durinx C, Bal G, Proost P, Struyf S, Goossens F, Augustyns K, Scharpe S. Natural substrates of dipeptidyl peptidase IV. Advances in Experimental Medicine and Biology. 2000;477:67–87. doi: 10.1007/0-306-46826-3_7. [DOI] [PubMed] [Google Scholar]
- 16.Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, Ghatei MA, Bloom SR. Inhibition of food intake in obese subjects by peptide YY3-36. New England Journal of Medicine. 2003;349:941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 17.Batterham RL, Le Roux CW, Cohen MA, Park AJ, Ellis SM, Patterson M, Frost GS, Ghatei MA, Bloom SR. Pancreatic polypeptide reduces appetite and food intake in humans. Journal of Clinical Endocrinology and Metabolism. 2003;88:3989–3992. doi: 10.1210/jc.2003-030630. [DOI] [PubMed] [Google Scholar]
- 18.Flint A, Raben A, Ersboll AK, Holst JJ, Astrup A. The effect of physiological levels of glucagon-like peptide-1 on appetite, gastric emptying, energy and substrate metabolism in obesity. International Journal of Obesity and Related Metabolic Disorders. 2001;25:781–792. doi: 10.1038/sj.ijo.0801627. [DOI] [PubMed] [Google Scholar]
- 19.Talley NJ, Phillips SF, Melton J, 3rd, Wiltgen C, Zinsmeister AR. A patient questionnaire to identify bowel disease. Annals of Internal Medicine. 1989;111:671–674. doi: 10.7326/0003-4819-111-8-671. [DOI] [PubMed] [Google Scholar]
- 20.Deacon CF, Nauck MA, Meier J, Hucking K, Holst JJ. Degradation of endogenous and exogenous gastric inhibitory polypeptide in healthy and in type 2 diabetic subjects as revealed using a new assay for the intact peptide. Journal of Clinical Endocrinology and Metabolism. 2000;85:3575–3581. doi: 10.1210/jcem.85.10.6855. [DOI] [PubMed] [Google Scholar]
- 21.Cremonini F, Mullan BP, Camilleri M, Burton DD, Rank MR. Performance characteristics of scintigraphic transit measurements for studies of experimental therapies. Alimentary Pharmacology and Therapeutics. 2002;16:1781–1790. doi: 10.1046/j.1365-2036.2002.01344.x. [DOI] [PubMed] [Google Scholar]
- 22.Bredenoord AJ, Chial HJ, Camilleri M, Mullan BP, Murray JA. Gastric accommodation and emptying in evaluation of patients with upper gastrointestinal symptoms. Clinical Gastroenterology and Hepatology. 2003;1:264–272. [PubMed] [Google Scholar]
- 23.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28:1092–1100. doi: 10.2337/diacare.28.5.1092. [DOI] [PubMed] [Google Scholar]
- 24.Ahren B. Dipeptidyl peptidase-4 inhibitors: clinical data and clinical implications. Diabetes Care. 2007;30:1344–1350. doi: 10.2337/dc07-0233. [DOI] [PubMed] [Google Scholar]
- 25.Holst JJ, Deacon CF. Glucagon-like peptide-1 mediates the therapeutic actions of DPP-IV inhibitors. Diabetologia. 2005;48:612–615. doi: 10.1007/s00125-005-1705-7. [DOI] [PubMed] [Google Scholar]
- 26.Nauck MA, El-Ouaghlidi A. The therapeutic actions of DPP-IV inhibition are not mediated by glucagon-like peptide-1. Diabetologia. 2005;48:608–611. doi: 10.1007/s00125-005-1704-8. [DOI] [PubMed] [Google Scholar]
- 27.Hansotia T, Baggio LL, Delmeire D, Hinke SA, Yamada Y, Tsukiyama K, Seino Y, Holst JJ, Schuit F, Drucker DJ. Double incretin receptor knockout (DIRKO) mice reveal an essential role for the enteroinsular axis in transducing the glucoregulatory actions of DPP-IV inhibitors. Diabetes. 2004;53:1326–1335. doi: 10.2337/diabetes.53.5.1326. [DOI] [PubMed] [Google Scholar]
- 28.Vazquez Roque MI, Camilleri M, Stephens DA, Jensen MD, Burton DD, Baxter KL, Zinsmeister AR. Gastric sensorimotor functions and hormone profile in normal weight, overweight, and obese people. Gastroenterology. 2006;131:1717–1724. doi: 10.1053/j.gastro.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 29.Reimann F, Gribble FM. Glucose-sensing in glucagon-like peptide-1-secreting cells. Diabetes. 2002;51:2757–2763. doi: 10.2337/diabetes.51.9.2757. [DOI] [PubMed] [Google Scholar]
- 30.Gribble FM, Williams L, Simpson AK, Reimann F. A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes. 2003;52:1147–1154. doi: 10.2337/diabetes.52.5.1147. [DOI] [PubMed] [Google Scholar]
- 31.Meier JJ, Gallwitz B, Salmen S, Goetze O, Holst JJ, Schmidt WE, Nauck MA. Normalization of glucose concentrations and deceleration of gastric emptying after solid meals during intravenous glucagon-like peptide 1 in patients with type 2 diabetes. Journal of Clinical Endocrinology and Metabolism. 2003;88:2719–2725. doi: 10.1210/jc.2003-030049. [DOI] [PubMed] [Google Scholar]
- 32.Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. Journal of Clinical Investigation. 1993;91:301–307. doi: 10.1172/JCI116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meier JJ, Goetze O, Anstipp J, Hagemann D, Holst JJ, Schmidt WE, Gallwitz B, Nauck MA. Gastric inhibitory polypeptide does not inhibit gastric emptying in humans. American Journal of Physiology. Endocrinology and Metabolism. 2004;286:E621–E625. doi: 10.1152/ajpendo.00499.2003. [DOI] [PubMed] [Google Scholar]
- 34.Cohen MA, Ellis SM, Le Roux CW, Batterham RL, Park A, Patterson M, Frost GS, Ghatei MA, Bloom SR. Oxyntomodulin suppresses appetite and reduces food intake in humans. Journal of Clinical Endocrinology and Metabolism. 2003;88:4696–4701. doi: 10.1210/jc.2003-030421. [DOI] [PubMed] [Google Scholar]
- 35.Chelikani PK, Haver AC, Reidelberger RD. Comparison of the inhibitory effects of PYY (3-36) and PYY (1-36) on gastric emptying in rats. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2004;287:R1064–R1070. doi: 10.1152/ajpregu.00376.2004. [DOI] [PubMed] [Google Scholar]
- 36.Deacon CF, Wamberg S, Bie P, Hughes TE, Holst JJ. Preservation of active incretin hormones by inhibition of dipeptidyl peptidase IV suppresses meal-induced incretin secretion in dogs. Journal of Endocrinology. 2002;172:355–362. doi: 10.1677/joe.0.1720355. [DOI] [PubMed] [Google Scholar]
- 37.Chaikomin R, Rayner CK, Jones KL, Horowitz M. Upper gastrointestinal function and glycemic control in diabetes mellitus. World Journal of Gastroenterology. 2006;12:5611–5621. doi: 10.3748/wjg.v12.i35.5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hebbard GS, Samsom M, Sun WM, Dent J, Horowitz M. Hyperglycemia affects proximal gastric motor and sensory function during small intestinal triglyceride infusion. American Journal of Physiology. 1996;271:G814–G819. doi: 10.1152/ajpgi.1996.271.5.G814. [DOI] [PubMed] [Google Scholar]
- 39.Schvarcz E, Palmer M, Aman J, Berne C. Hypoglycemia increases the gastric emptying rate in healthy subjects. Diabetes Care. 1995;18:674–676. doi: 10.2337/diacare.18.5.674. [DOI] [PubMed] [Google Scholar]
- 40.Schvarcz E, Palmer M, Aman J, Horowitz M, Stridsberg M, Berne C. Physiological hyperglycemia slows gastric emptying in normal subjects and patients with insulin-dependent diabetes mellitus. Gastroenterology. 1997;113:60–66. doi: 10.1016/s0016-5085(97)70080-5. [DOI] [PubMed] [Google Scholar]