Abstract
Flavonoids, found in a wide diversity of plant foods from fruits and vegetables, herbs and spices, essential oils, and beverages, have the most potential of dietary components for promotion of bone health beyond calcium and vitamin D. Recent epidemiological studies show flavonoid consumption to have a stronger association with bone than general fruit and vegetable consumption. Bioactive flavonoids are being assessed for properties beyond their chemical anti-oxidant capacity, including anti-inflammatory actions. Some have been reported to enhance bone formation and to inhibit bone resorption through their action on cell signaling pathways that influence osteoblast and osteoclast differentiation. Future research is needed to determine which of the flavonoids and their metabolites are most effective and at what dose, as well as the mechanism of modulating cellular events, in order to set priorities for clinical trials.
Keywords: anti-inflammation, antioxidant, bone, flavonoids, soy isoflavones
Osteoporosis is a debilitating and painful condition of low bone mineral density (BMD) and high fracture risk. There are an estimated 2 million new fractures each year in the USA and 9 million worldwide (1), with an estimated annual cost to our society of ~$100 billion (1). Strategies to prevent osteoporosis include reducing bone loss induced by acute estrogen deficiency post-menopause and more gradual declines in sex steroid levels with age in both men and women. As for other chronic diseases, there is increasing evidence that inflammation is part of the etiology of osteoporosis. Flavonoids as a class of phytochemicals have promise in protecting against bone loss, likely related in part to their anti-inflammatory properties. In a large observational study in Scotland, total flavonoid intake was positively associated with BMD and increase in BMD of the spine and hip. Flavonoids in the catechin family had the strongest association with bone (2). The relationship between flavonoid intake and bone health was stronger in general than what has been reported previously for fruits and vegetables (3,4). The most studied of the flavonoids in relation to bone health are the isoflavones, especially those derived from soy beans. Isoflavones, along with flavonoids from other plant foods showing promise for bone health, will be described in this review.
A variety of methods are available to assess bone health. BMD, as determined by dual energy x-ray absorptiometry (DXA), is the most common measure of fracture risk. It can be used to monitor response to intervention in either humans or animal models. The ovariectomized (OVX) rodent is an FDA-approved model for osteoporosis in postmenopausal women. Interventions, such as dietary flavonoids, can be tested for their ability to suppress OVX-induced bone loss if the intervention is started subsequent to OVX or after bone has been stabilized subsequent to OVX; the ability to recover lost bone can then be determined. Because bone strength is determined by microarchitecture and bone geometry, as well as BMD, more recent studies use three-dimensional imaging techniques, such as peripheral quantitative computed tomography (pQCT) in humans or micro-computed tomography (μCT) in animal models. Excised bones of animals can be tested using breaking tests such as 3-point bending for energy to failure. Bone turnover rate is also predictive of fracture. Thus, measures of bone turnover, including biochemical markers of bone turnover or calcium isotope kinetics, are also useful to evaluate interventions for their effect on bone.
Tests such as oxidative radical absorbency capacity (ORAC) have been utilized historically to evaluate the potential of a food or compound to quench free radicals and protect against inflammation-related chronic diseases such as osteoporosis. However, we are beginning to understand, as summarized by Finley et al. (5), that the protective effect of diets high in antioxidant-rich foods goes beyond simply quenching oxygen radicals. Natural compounds act through gene expression and cell signaling pathways to activate enzymes that eliminate oxygen radicals that initiate inflammatory events. Antioxidants can also sequester potential oxidants, such as iron, that regulate oxygen radicals. Also antioxidants can influence redox capacity to regulate oxidation pathways. A cellular antioxidant activity assay using a cell culture model was recently developed to screen foods for biologically relevant antioxidant activity to supersede the chemically based assays to account for these additional functions (6). Cell culture studies can also be used to identify affected pathways. This review briefly summarizes the state of knowledge for the role of flavonoids on bone health and their role in modulating cell signaling pathways.
Soy Isoflavones
The main class of flavonoids that has been studied for their role in bone health is soy isoflavones. Isoflavones are structurally similar to estrogen and bind to estrogen receptors (7). Observational studies suggest that soy consumption contributes to low rates of hip fractures in Asians (8,9). In a large study of approximately 75,000 women participating in the Shanghai Women's Study, hip fracture prevalence was inversely related to soy consumption (10). The relationship is stronger in women who are in early (i.e., <10 y) rather than late menopause, possibly related to greater responsiveness of bone turnover to soy isoflavones during the early phase of estrogen deficiency.
In contrast to observational studies in Asia, the most comprehensive intervention studies using isoflavones have largely been negative. A meta-analysis of randomized control trials (RCTs) showed soy isoflavones (~82 mg aglycones/day) increased spine BMD by 2.4% (p=0.0001) in the short term (6–12 mo)(11), but randomized controlled trials (RCTs) of 2 or 3 y in duration showed no benefit of soy isoflavones on BMD of fracture-prone bone sites in postmenopausal women (12–14). The longest (3 y) RCT (13) showed no overall treatment effect for lumbar spine, proximal femur, or whole body BMD in either the intent-to-treat or compliant models. However, after adjustment for age, whole body fat mass, and bone resorption, the 120 mg isoflavone (aglycone form) dose compared with placebo was modestly protective (p=0.024) for femoral neck BMD. Further evaluation of volumetric BMD and bone strength at the 1/3 midshaft femur and distal tibia using pQCT in the Alekel trial (15) indicated a protective effect of 120 mg isoflavone/day on cortical volumetric BMD of the femur as time since last menstrual period increased, but no benefit to trabecular bone. There are many differences that may explain the discrepancy between the negative findings of the RCTs and the positive association in the observational studies (16). The RCTs were conducted in western women with heterogeneous genetic backgrounds, as well as low habitual soy food intake. Additionally, because osteoporosis is a long latency disease, the primary outcome measure of the RCTs was BMD rather than fracture. Properties other than BMD may protect against fracture. For example, soy isoflavones may reduce net bone turnover in postmenopausal women (17). All of the RCTs published to date used mixed isoflavones isolated from the soybean, an entirely different form than the whole soy foods consumed in Asia, which contain plant proteins and more than 100 associated phytochemicals in addition to isoflavones (18). Yet, a RCT in Italian women used a purified form of the dominant soy isoflavone, aglycone genistein, demonstrating this isoflavone to be as effective as estrogen therapy in reducing postmenopausal bone loss (19).
Animal studies examining the effect of soy protein or isolated isoflavones on bone health show the strongest benefit during growth rather than in the older OVX rodent model that mimics postmenopausal women (20). Animal models have the advantage of feeding a controlled diet for sufficiently long periods to effect changes in bone properties. Moreover, femurs and vertebra can be excised at the end of long-term feeding trials and direct fracture testing at common sites of fracture (femur neck, vertebra), as well as sites that contain almost exclusively cortical bone (femur midpoint) can be performed. While BMD is the gold standard for assessing fracture risk, other factors such as bone structure, including trabecular thickness and separation, influence bone strength. Thus, combining bone strength measurements with BMD and outcomes of bone structure, obtained by μCT, provide a more comprehensive understanding of how a dietary intervention alters bone at the tissue level. Furthermore, a larger number of comparisons, such as dose response studies or those comparing alternative forms, can be made than is practical in clinical trials. For example, 9 treatments were compared for multiple bone outcomes in an OVX rat model that included a dose response of mixed isoflavones with and without a soy protein background (21). Estrogens, but not soy isoflavones, benefited bone properties and calcium metabolism.
An interesting life-stage that has received recent attention is neonatal exposure to soy isoflavones. Using a mouse model, exposure to purified soy isoflavones (genistein and daidzein) during the first five days of life resulted in higher BMD, improved bone structure, and greater bone strength at adulthood in females but not males (22). Moreover, these benefits to bone development in females protected against ovariectomy-induced bone loss (23). The level of genistein and daidzein used in these mouse studies resulted in similar total serum isoflavone levels in infants consuming soy infant formula (24). Potential differences in metabolism of isoflavones between rodents and human infants and the fact that isolated isoflavones may have different biological effects than when present in the soy matrix suggest that prospective studies of human infants fed soy infant formula are needed to confirm if consumption of soy protein based infant formula benefits bone health at adulthood.
Plant Flavonoids Other Than Soy Isoflavones
Higher intakes of fruits and vegetables have been associated with improved BMD or bone mineral content (3,4), although a systematic review of studies in women aged 45 y and older was inconclusive (25). Benefits to bone have been attributed to several potential constituents, including acid-base balance, potassium or other micronutrients (such as boron or vitamin K), and particular bioactive ingredients. In a comparative evaluation of 53 food items, Mühlbauer et al. (26) used urinary excretion of [3H]-tetracycline following pre-labeling of rat bones to measure bone resorption in response to serial exposures to each food for 10 days. This approach allows several foods to be tested in each rat for direct comparison against a positive reference control of onion. Half of the food items tested showed bone turnover inhibitory capacity. Using this same approach, bone resorption suppression activity was not altered after correcting for the alkaline load of a mixed plant diet with potassium citrate (27), which raises doubt about postulated acid-base mechanisms of action of plant foods and the benefit of extra potassium. In support, a meta-analysis of human studies showed no relation between dietary acid load and osteoporotic bone disease (28), nor did potassium intake influence calcium balance (29). Further, the content of boron or vitamin K in fruits and vegetables is not likely to be the complete explanation for their benefit to bone since boron is typically adequate in human diets and vitamin K has not been shown to attenuate postmenopausal bone loss (30,31).
Instead, Mühlbauer et al. (26) concluded that particular bioactive compounds in some food items have anti-resorptive properties. Total polyphenolic content was not related to efficacy, but specific flavonoids appeared to confer benefits to bone. Onion was used as the positive control because of its strong effect to inhibit bone resorption. Separately, onion powder was shown to dose-dependently prevent OVX-induced bone loss in rats and to increase bone volume and trabecular number (32). Rutin was identified initially as the bioactive compound, but later this group reported that the likely bioactive constituent in onion using bioactivity directed fractionation is Γ-L-glutamyl-trans-S-1-propenyl-L-cysteine sulfoxide (33). However, this requires confirmation in vivo. Other vegetables in the onion family, including garlic and leek, and members of the celeriac family, including cabbage, lettuce, and green beans, were also effective in suppressing bone resorption (26).
The most effective fruit tested in the pre-labeled bone rat model of Mühlbauer et al. (26) described above was plum. Dried plum has been shown to increase vertebral and femoral BMD and to restore trabecular microarchitecture in orchidectomized, male rats (34), but the benefits are even stronger in young male rats (35). Other than soy, plum is the only other flavonoid-rich food that has been tested in a RCT of reasonable duration to study effects on bone properties. In a one-year study of 160 postmenopausal women, loss of BMD of the major fracture sites was prevented by feeding plums (36). Plum contains high concentrations of rutin (3.3 ng/100 g) (35), but the specific bone bioactive compound(s) in plum is uncertain. Rutin is hydrolyzed to its aglycone, quercetin, prior to absorption and can be converted to glucuro- or sulfoconjugates during absorption. Quercetin has antioxidant activity and binds to estrogen receptor-beta (ERβ) (38).
Another fruit with high flavonoid content and high antioxidant capacity that has received recent attention by bone researchers is blueberry. In the OVX rat model, blueberry prevented whole body bone loss, but no change in fracture prone sites (39). No clinical trials with blueberries and bone outcomes have been reported. Hippuric acid, phenylacetic, and hydroxybenzoic acids are likely the bioactive compounds in blueberries, as will be discussed later in the section on mechanisms.
Other fruit products shown by Mühlbauer et al. (26) to be effective in inhibiting bone resorption were orange juice and red wine. Orange juice and pulp prevented bone loss in castrated male rats and increased cortical thickness in a dose-dependent manner (40). The active compound is thought to be hesperidin, the most abundant flavonone in citrus fruits, since similar bone protective effects in OVX rats were reported with this purified compound (41). Resveratrol has been proposed as the bioactive polyphenolic in red wine, but the in vitro evidence for an effect on bone formation (42) is at a non-physiological level. Other constituents, such as anthocyanins, should be evaluated for bone health properties.
Antiresorptive food items identified by Mühlbauer et al. (26) also included mushrooms, herbs, and essential oils. A candidate for a bioactive compound in herbs is luteolin, a flavonoid found in celery, green pepper, parsley, perilla leaf and seeds, and chamomile. It has anti-inflammatory properties, inhibits osteoclast differentiation, and protects against OVX-induced bone loss (43). Extracts of king oyster mushroom (Pleuratus ergngii) have been shown to be protective against OVX-induced bone loss (44,45). Bioactive herbs and their oils include parsley, sage, rosemary, thyme, and chili. The effective dose will determine whether inclusion of these herbs have practical bone health benefits.
The studies of soy isoflavones and other plant flavonoids described above collectively suggest positive effects on bone mineral density and content. However, effects on risk of fragility fracture in humans, or overall effect on bone structure and strength properties has been scarcely studied. The magnitude of the effect appears to depend on the flavonoid source; context of exposure (pure compound vs. diet); route of exposure; and age and sex of the target population. Moreover, as discussed below, no single mechanism has been put forward for flavonoid actions on bone.
Potential Mechanisms of Action of Flavonoids on Bone
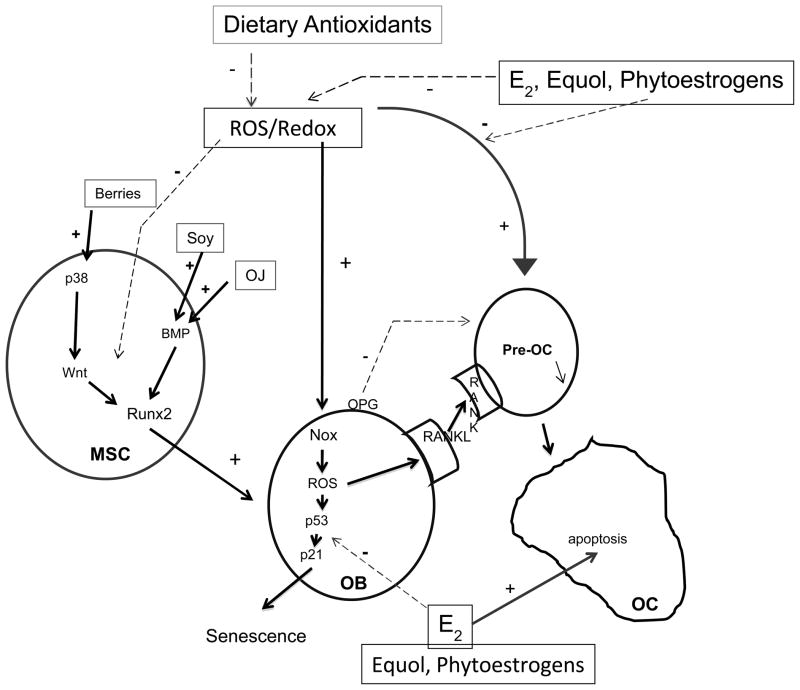
Recent research has identified molecular targets in cell signaling pathways that affect bone. Some flavonoids appear to have bone anabolic activity, which has exciting implications beyond merely inhibiting bone resorption through suppressing osteoclast activation. Although many gaps remain, we have attempted to develop a unifying model for how flavonoids from various plant sources might affect bone (Figure 1).
Figure 1.
Dietary intake and factors impact bone turnover and bone cell survival via common signal transduction pathways. BMP, bone morphogenic protein; E2, 17β-estradiol; MSC, mesenchymal stem cell; NEFA, nonesterified free fatty acids; Nox, NADPH oxidase; OB, osteoblast; OC, osteoclast; OJ, orange juice; OPG, osteoprotegrin; ROS, reactive oxygen species.
The most studied mechanism for benefits of flavonoids to bone has been in the estrogenic actions of phytoestrogens, including soy isoflavones, lignans, and coumesterol. Because of their weak binding to the estrogen receptor ER, and relatively higher affinity for ERβ than ERβ, these phytoestrogens have been largely studied in cell culture for their estrogenic properties (56). Effective concentrations required to produce classical ER activation, nuclear translocation and gene expression cannot be achieved at doses found after eating soy foods. Nevertheless, phytoestrogens ability to bind to ERs may have a positive effect on bone through their antioxidant and anti-inflammatory effects in individuals who have high reactive oxygen species production, such as occurs during aging, menopause, and arthritis, since estrogens also antagonize reactive oxygen species actions in bone cells via non-classical signaling pathways (Figure 1) (48,57). Newer reports show that soy isoflavones may also have roles independent of their estrogenic properties. For example, soy containing diets have been shown to activatesignaling through bone morphogenic proteins (BMPs) (58,59) (Figure 1). Interestingly, the major metabolite of hesperidin in orange juice, hesperetin-7-O-glucuronide, also activates BMP signaling, which increases expression of the major transcription factor Runx2 which drives osteoblast differentiation from multipotent mesenchymal stem cells (MSCs) in bone marrow (60).
The anabolic action on bone of flavonoids from berries is through upstream regulation of osteoblast differentiation through the molecular target, the mitogen activated protein MAP kinase p38. MAP kinases are a complex cascade of enzymes which regulate protein phosphorylation and which are themselves regulated via redox and phosphorylation state. Phosphorylation of p38 results in activation of the Wnt signaling pathway. This pathway involves a series of soluble growth factors (Wnts) which bind to a series of cell surface receptors (LRP5/6-Frizzled proteins) which signal to the cytosolic kinase GSK3β to become phosphorylated. Phosphorylation of GSK3β inactivates it and prevents phosphorylation of a cytosolic master regulator protein β-catenin. As a result of reduced phosphorylation β-catenin is stabilized, translocates to the nucleus and acts in conjunction with transcription factors LEF/TCF to increase transcription of genes involved in bone cell proliferation and differentiation such as Runx2 (61) (Figure 1). After observing increased serum concentrations of hippuric acid, phenylacetic acid, and hydrobenzoic acid after blueberry feeding, Chen et al. (61) tested an artificial mixture of these compounds and found that they stimulated Wnt signaling and differentiation in osteoblast precursors in vitro in a similar manner to serum from animals fed whole blueberry. How these phenolic acids activate p38 is not yet understood. Myosin production seems to be involved in the effect of blueberry on osteoblasts, since myosin-related genes are down-regulated following OVX. Feeding blueberry blunts this effect and protects against OVX-induced osteoblast death through a senescence pathway which involves reactive oxygen species (ROS) and the signaling proteins p53 and p21 (Figure 1) (62). In mouse-derived bone marrow stem cells, serum from blueberry fed rats increased osteoblast differentiation and lineage commitment. Moreover, silencing of myosin 2 expression using shRNA inhibited Runx2 mRNA expression in osteoblastic cells and stimulated cell senescence.
Osteoclasts are the primary cells responsible for bone resorption. When activated, these cells release proteolytic enzymes to digest connective tissue proteins and acids that solubilize bone mineral. They attach to bone surfaces and their action forms pits. Osteoblast cells form bone in the pits produced by the osteoclasts. This concerted process is normal and occurs throughout life to model and shape bone and to repair microarchitecture damage. However, estrogen deficiency accelerates bone resorptive activity, which outpaces bone formation. Several signaling proteins produced by osteoblasts including osteoprotegrin (OPG) and the TNFα family member, receptor activator of NFkB ligand (RANKL) are primary regulators of osteoclast activation. ROS and the redox status of bone cells are now thought to have important roles in regulation of bone turnover and survival of osteoblasts, osteoclasts, and osteocytes. ROS produced through the action of NADPH oxidase (Nox) enzymes appears to regulate osteoclastogenesis through control of expression of RANKL in osteoblastic cells and signaling through its receptor RANK on the surface of osteoclast precursors (Figure 1). Chronic bone loss is often accompanied by conditions of oxidative stress and/or inflammation, such as aging, estrogen deficiency, rheumatoid arthritis, inflammatory bowel disease, and alcohol abuse (46). Oxidative stress in bone cells results in production of reactive oxygen species from lipoxygenases and oxidases such as the Nox enzymes. ROS can affect bone cells in many ways, including stimulation of osteoblast apoptosis and senescence (47) and by up-regulation of RANKL, to activate osteoclast differentiation and bone resorption (48). Inflammatory conditions in which there is enhanced production of proinflammatory cytokines, such as TNFα and increased T cell expression of RANKL, are associated with lower BMD (49,50), likely due to up-regulation of osteoclast formation and activation of mature osteoclasts (51–53). Moreover, effects on osteoblast activity include lower production of bone matrix proteins and stimulation of osteoblast apoptosis (54,55).
Dried plum polyphenol extract has been shown to inhibit increases in RANKL expression in response to TNFα, oxidative stress induced by TNFα or by inflammatory response to lipopolysaccharide (that may be mediated in part through modulation of TNFα), and osteoclast differentiation (63,64). Similarly, resveratrol inhibited reactive oxygen species production and subsequently RANKL signaling and osteoclast activation (65) and up-regulated Runx2 gene expression (66). Green tea polyphenols may offer protection in states of chronic inflammation (67). In rats, administration of green tea polyphenols resulted in higher urinary epigallocatechin and epicatechin, and attenuated the inflammation-induced bone loss that results from administration of lipopolysaccharide in rats (67). Specifically, green tea polyphenols helped preserve femur bone mass and structure, and resulted in lower tartrate resistant acid phosphatase, a marker of bone resorption. These effects were associated with lower mRNA expression of TNFα and cyclooxygenase (COX)-2. It is important to note that the doses used in the plum, resveratrol, and green tea studies were pharmacological and these may not necessarily represent the bioactive metabolites produced upon consuming plum, red wine, or green tea.
Conclusions and Future Studies
Flavonoids in a variety of plant foods hold promise in promoting bone health, both in the primary prevention of bone loss in later life and as a complementary therapy during conditions of high oxidative stress or chronic inflammation. We are beginning to understand their roles in cell signaling, including Wnt-β-catenin and BMP pathways that stimulate bone formation, in addition to their anti-resorptive roles in inhibiting osteoclast activation. This represents an advance in our understanding of flavonoids beyond the classic estrogen-like actions of soy isoflavones and beyond evaluating flavonoids only for their chemical antioxidant properties. The interaction of dietary factors with these signaling pathways is a rich area for future research.
Although we have a growing body of descriptive evidence of various flavonoid-rich foods on bone health in animal models, we have scant clinical data beyond studies on soy isoflavones. To translate these animal data to dietary interventions in humans, we also need comparative data of the various sources of flavonoids. The work by Mühlbauer's group using urinary excretion of bone-seeking tracers from pre-labeled rat bone to screen various fruits, vegetables, and herbs, discussed extensively in this review, has contributed greatly to our understanding of their relative bioactivity. A similar approach has been developed for humans whereby bone is labeled with the rare isotope, 41Ca, and urinary excretion of the isotope measured by Accelerator Mass Spectroscopy is used to compare multiple interventions for their effect on bone balance (17,68). Such an approach will be useful to determine dose response effects and effectiveness of combination drug therapies, as well as to compare various plant flavonoids and their metabolites. Determining the most effective foods/constituents, identifying the bioactive ingredient(s), and their effective doses is prudent prior to investing in large, clinical trials to formulate public health recommendations.
Contributor Information
Connie M. Weaver, Email: weavercm@purdue.edu.
D. Lee Alekel, Email: lee.alekel@nih.gov.
Wendy E. Ward, Email: wward@brocku.ca.
Martin J. Ronis, Email: ronismartinj@uams.edu.
References
- 1.Compston J. Osteoporosis: Social and economic impact. Radiol Clin N Am. 2010;48:477–482. doi: 10.1016/j.rcl.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Hardcastle AC, Aucott L, Reid DM, Macdonald HM. Associations between dietary flavonoid intakes and bone health in a Scottish population. J Bone Miner Res. 2011;26:941–947. doi: 10.1002/jbmr.285. [DOI] [PubMed] [Google Scholar]
- 3.Prynne CJ, Mishra GD, O'Connell MA, Muniz G, Laskey MA, Yan L, Prentic A, Ginty F. Fruit and vegetable intakes and bone mineral status: a cross-sectional study in 5 age and sex cohorts. Am J Clin Nutr. 2006;83:1420–1428. doi: 10.1093/ajcn/83.6.1420. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y-M, Ho SC, Woo JLF. Greater fruit and vegetable intake is associated with increase bone mass among postmenopaual Chinese women. Br J Nutr. 2006;96:745–751. [PubMed] [Google Scholar]
- 5.Finley JW, Kong A-N, Hintze KJ, Jeffery EH, Ji LL, Lei XG. Antioxidants in foods: State of the science important to the food industry. J Agric Food Chem. 2011;59:6837–46. doi: 10.1021/jf2013875. [DOI] [PubMed] [Google Scholar]
- 6.Song W, Derito CM, Liu MK, He X, Doug M, Liu RH. Cellular antioxidant activity of common vegetables. J Agric Food Chem. 2010;58:6621–9. doi: 10.1021/jf9035832. [DOI] [PubMed] [Google Scholar]
- 7.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor-β. Endocrinology. 1998;139:4252–63. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 8.Ho SC, Bacon E, Harris T, Looker A, Muggi S. Hip fracture rates in Hong Kong and the United States, 1988 through 1989. Am J Publ Health. 1993;83:694–697. doi: 10.2105/ajph.83.5.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ross PD, Norimatsu H, Davis JW, Yano K, Wasnich RD, Fujiwara S, Hosoda Y, Melton J., III A comparison of hip fracture incidence among native Japanese, Japanese Americans, and American Caucasians. Am J Epidemiol. 1991;133:801–809. doi: 10.1093/oxfordjournals.aje.a115959. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Shu X, Li H, Yang G, Li Q, Gao Y, Zheng W. Prospective cohort study of soy food consumption and risk of bone fracture among postmenopausal women. Arch Intern Med. 2005;165:1890–95. doi: 10.1001/archinte.165.16.1890. [DOI] [PubMed] [Google Scholar]
- 11.Taku K, Melby MK, Kurzer MS, Mizuno S, Watanabe S, Ishimi Y. Effects of soy isoflavone supplements on bone turnover markers in menopausal women: Systematic review and meta-analysis of randomized controlled trials. Bone. 2010;47(2):413–423. doi: 10.1016/j.bone.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Wong WW, Lewis RD, Steinberg FM, Murray MJ, Cramer MA, Amrato P, Young RL, Barnes S, Ellis KJ, Shypailo RJ, Fraley JK, Konzelmann KL, Fischer JG, O’Brian Smith E. Soy isoflavone supplementation and bone mineral density in menopausal women: a 2-y multicenter clinical trial. Am J Clin Nutr. 2009;90:1433–9. doi: 10.3945/ajcn.2009.28001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alekel DL, Van Loan MD, Koehler KJ, Hanson LN, Stewart JW, Hanson KB, Kurzer MS, Peterson CT. The soy isoflavones for reducing bone loss (SIRBL) study: a 3-y randomized controlled trial in postmenopausal women. Am J Clin Nutr. 2010;91:218–230. doi: 10.3945/ajcn.2009.28306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levis S, Strickman-Stein N, Ganjei-Azar P, Xu P, Deorge DR, Krischer J. Soy isoflavones in the prevention of menopausal bone loss and menopausal symptoms. Arch Intern Med. 2011;171:1363–1369. doi: 10.1001/archinternmed.2011.330. [DOI] [PubMed] [Google Scholar]
- 15.Shedd-Wise KM, Alekel DL, Hofmann H, Hanson KB, Schiferl DJ, Hanson LN, Van Loan MD. The Soy Isoflavones for Reducing Bone Loss (SIRBL) Study: Three year effects on pQCT bone mineral density and strength measures in postmenopausal women. J Clin Densit: Assessment of Skeletal Health. 2011;14(1):47–57. doi: 10.1016/j.jocd.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reinwald S, Weaver CM. Soy components vs. whole soy: Are we betting our bones on a long shot? J Nutr. 2010;140:2312S–17S. doi: 10.3945/jn.110.124008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weaver CM, Martin BR, Jackson GS, McCabe GP, Nolan JR, McCabe LD, Barnes S, Reinwald S, Boris ME, Peacock M. Antiresorptive effects of phytoestrogen supplements compared to estradiol or risedronate in postmenopausal women using 41Ca methodology. J Clin Endocrin Metabol. 2009;94(10):3798–3805. doi: 10.1210/jc.2009-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang N, Yu S, Badger TM. Comprehensive phytochemical profile of soy protein isolate. J Agric Food Chem. 2004;52(12):4012–20. doi: 10.1021/jf049842y. [DOI] [PubMed] [Google Scholar]
- 19.Morabito N, Crisafulli A, Vergara C, Gaudio A, Lasco A, Frisina N, D’Anna R, Corrado F, Pizzoleo MA, Cincotta M, Altavilla D, Ientile R, Squadrito F. Effects of genistein and hormone-replacement therapy on bone loss in early postmenopausal women: A randomized double-blind placebo-controlled study. J Bone Miner Res. 2002;17(10):1904–1912. doi: 10.1359/jbmr.2002.17.10.1904. [DOI] [PubMed] [Google Scholar]
- 20.Reinwald S, Weaver CM. Soy isoflavones and bone health: a double-edged sword? J Natural Products. 2006;69:450–459. doi: 10.1021/np058104g. [DOI] [PubMed] [Google Scholar]
- 21.Cai DJ, Zhao Y, Glasier J, Cullen D, Barnes S, Turner CH, Wastney M, Weaver C. Comparative effect of soy protein, soy isoflavones and 17β-estradiol on bone metabolism in adult ovariectomized rats. J Bone Miner Res. 2005;20:828–39. doi: 10.1359/JBMR.041236. [DOI] [PubMed] [Google Scholar]
- 22.Kaludjerovic J, Ward WE. Neonatal exposure to daidzein, genistein, or the combination modulates bone development in female CD-1mice. J Nutr. 2009;139:467–473. doi: 10.3945/jn.108.100115. [DOI] [PubMed] [Google Scholar]
- 23.Kaludjerovic J, Ward WE. Neonatal administration of isoflavones attenuates deterioration of bone tissue in female but not male mice. J Nutr. 2010;140(4):766–72. doi: 10.3945/jn.109.116343. [DOI] [PubMed] [Google Scholar]
- 24.Kaludjerovic J, Franke AA, Ward WE. Circulating isoflavonoid levels in CD-1 mice: effect of oral versus subcutaneous delivery and frequency of administration. J Nutr Biochem. 2011 Jun 8; doi: 10.1016/j.jnutbio.2011.01.008. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 25.Hamidi M, Boucher BA, Cheung AM, Beyene J, Shah PS. Fruits and vegetable intake and bone health in women aged 45 years and over: a systematic review. Osteoporosis Intl. 2011;22:1681–1693. doi: 10.1007/s00198-010-1510-0. [DOI] [PubMed] [Google Scholar]
- 26.Mühlbauer RC, Lozano A, Reinli A, Wetli H. Various selected vegetables, fruits, mushrooms, and red wine residue inhibit bone resorption in rats. J Nutr. 2003;133:3592–3597. doi: 10.1093/jn/133.11.3592. [DOI] [PubMed] [Google Scholar]
- 27.Mühlbauer RC, Lozano A, Reinli A. Onion and a mixture of vegetables, salads, and herbs affect bone resorption in the rat by a mechanism independent of their base excess. J Bone Miner Res. 2002;17:1230–1236. doi: 10.1359/jbmr.2002.17.7.1230. [DOI] [PubMed] [Google Scholar]
- 28.Fenton TR, Tough SC, Lyon AW, Eliaziw M, Hanley DA. Casual assessment of dietary acid load and bone disease: A systematic review & meta-analysis applying Hill's epidemiological criteria for causality. Nutr J. 2011;10:41. doi: 10.1186/1475-2891-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rafferty K, Davies MK, Heaney RP. Potassium intake and the calcium economy. J Am Coll Nutr. 2005;24:999–106. doi: 10.1080/07315724.2005.10719450. [DOI] [PubMed] [Google Scholar]
- 30.Booth SL, Dallal G, Shea MK, Gunderberg C, Peterson JW, Dawson-Hughes B. Effect of vitamin K supplementation on bone loss in elderly men and women. J Clin Endocrin Metab. 2008;93:1217–1223. doi: 10.1210/jc.2007-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheung AM, Tile L, Lee Y, Tomlinson G, Hawker G, Scher J, Hu H, Vieth R, Thompson L, Jamal S, Josse R. Vitamin K supplementation in postmenopausal women with osteopenia (ECKO trial): a randomized controlled trial. PLOS Med. 2008;14(10):e196. doi: 10.1371/journal.pmed.0050196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang T-H, Mühlbauer RC, Tang C-H, Che H-I, Chang G-L, Huang Y-W, Lai Y-T, Lin H-S, Yang W-T, Yang R-S. Onion decreases the ovariectomy-induced osteopenia in young adult rats. Bone. 2008;42:1154–1163. doi: 10.1016/j.bone.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 33.Wetli HA, Brenneisen R, Tschudi I, Langos M, Bigler P, Sprang T, Schurch S, Mühlbauer RC. A Γ-glutamyl peptide isolated from onion (Allium cepa L.) by bioassay-guided fractionation inhibits resorption activity of osteoclasts. J Agric Food Chem. 2005;53:3408–3414. doi: 10.1021/jf040457i. [DOI] [PubMed] [Google Scholar]
- 34.Bu SY, Lucas EA, Franklin M, Marlow D, Brachett DJ, Boldrin EA, Devareddy L, Arjmandi BH, Smith BJ. Comparison of dried plum supplementation and intermittent PTH in restoring bone in osteopenic orchidectomized rats. Osteopor Intl. 2007;189:931–942. doi: 10.1007/s00198-007-0335-y. [DOI] [PubMed] [Google Scholar]
- 35.Holloran BP, Wronski TJ, Van Herzen DC, Chu V, Xia X, Pinzel JE, Williams AA, Smith BJ. Dietary dried plum increases bone mass in adult and aged male mice. J Nutr. 2010:1781–1787. doi: 10.3945/jn.110.124198. [DOI] [PubMed] [Google Scholar]
- 36.Hooshmand S, Arjmandi BH. Viewpoint: dried plum, an emerging functional food that may effectively improve bone health. Ageing Res Rev. 2009;8:122–7. doi: 10.1016/j.arr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Stacewicz-Saputzakis M, Bowen PE, Hussain EA, Damayanti-Wood BI, Farnsworth NR. Chemical composition and potential health effects of prunes: A functional food? Crit Rev Food Sci Nutr. 2001;41:25–286. doi: 10.1080/20014091091814. 2001. [DOI] [PubMed] [Google Scholar]
- 38.Caltagirone S, Ranelletti FO, Rinelli A, Muggiano N, Colasante A, Musiani P, Aiello FB, Piantelli M. Interactions with type II estrogen-binding sites and antiproliferative activity of tamoxifen and quercetin in human non-small cell lung cancer. Am J Respir Cell Mol Bio. 1997;17:51–59. doi: 10.1165/ajrcmb.17.1.2728. [DOI] [PubMed] [Google Scholar]
- 39.Devareddy L, Hooshmand S, Collins JK, Lucas EA, Chai SC, Arjmandi BH. Blueberry prevents bone loss in ovariectomized rat model of postmenopausal osteoporosis. J Nutr Biochem. 2008;19(10):694–699. doi: 10.1016/j.jnutbio.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Morrow R, Deyhim F, Patil BS, Stoeker BJ. Feeding orange pulp improved bone quality in a rat model of male osteoporosis. J Med Food. 2009;12:298–303. doi: 10.1089/jmf.2008.0145. [DOI] [PubMed] [Google Scholar]
- 41.Horcajada MN, Habauzit V, Trzecialkiewicz A, Morand C, Gil-Izquierdo A, Mardon J, Lebecque P, Davicco MJ, Chee WSS, Coxam V, Offord E. Hesperidine inhibits ovariectomized-induced osteopenia and shows differential effects on bone mass and strength in young adult rats. J Appl Physiol. 2008;104:648–654. doi: 10.1152/japplphysiol.00441.2007. [DOI] [PubMed] [Google Scholar]
- 42.Su JL, Yang M, Zhao M, Kuo ML, Yen ML. Forkhead proteins are critical for bone morphogenetic protein 2-regulation and anti-tumor activity of resveratrol. J Biol Chem. 2007;282:19385–19398. doi: 10.1074/jbc.M702452200. [DOI] [PubMed] [Google Scholar]
- 43.Kim T-H, Jung JW, Ha BG, Hong JM, Park EK, Kim H-J, Kim S-Y. The effects of luteolin on osteoclast differentiation, function in in vitro and ovariectomy-induced bone loss. J Nutr Biochem. 2011;22:8–15. doi: 10.1016/j.jnutbio.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 44.Kim SW, Kim HG, Lee BE, Hwang HH, Baek DH, Ko SY. Effects of mushroom, Pleurotus ergngii, extracts on bone metabolism. Clin Nutr. 2006;25:166–170. doi: 10.1016/j.clnu.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 45.Shimizu K, Yamanaka M, Gyokusen M, Kaneko S, Tsutsui M, Sato J, Sato I, Kondo R. Estrogen-like activity and prevention effect of bone loss in calcium deficient ovariectomized rate by the extract of Pleurotus ergngii. Phytotherapy Res. 2006;20:659–664. doi: 10.1002/ptr.1927. [DOI] [PubMed] [Google Scholar]
- 46.Mundy GR. Osteoporosis and inflammation. Nutr Rev. 2007;65:S147–S151. doi: 10.1111/j.1753-4887.2007.tb00353.x. [DOI] [PubMed] [Google Scholar]
- 47.Chen J-R, Lazarenko OP, Haley RL, Blackburn ML, Badger TM, Ronis MJJ. Ethanol impairs estrogen receptor signaling and activates senescence pathways in osteoblasts. Protection by estradiol. J Bone Min Res. 2009;24:221–230. doi: 10.1359/jbmr.081011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen J-R, Badger TM, Nagaragian S, Ronis MJJ. Inhibition of reactive oxygen species generation and downstream activation of the ERK/STAT3/RANKL-signaling cascade to osteoblasts accounts for the protective effect of estradiol on ethanol-induced bone loss. J Pharmacol Exp Ter. 2008;324:50–59. doi: 10.1124/jpet.107.130351. [DOI] [PubMed] [Google Scholar]
- 49.Sylvester FA, Wyzga N, Hyams JS, Davis PM, Lerer T, Vance K, Hawker G, Griffiths AM. Natural history of bone metabolism and bone mineral density in children with inflammatory bowel disease. Inflamm Bowel Dis. 2007 Jan;13(1):42–50. doi: 10.1002/ibd.20006. [DOI] [PubMed] [Google Scholar]
- 50.Turk N, Cukovic-Cavka S, Korsic M, Turk Z, Vucelic B. Proinflammatory cytokines and receptor activator of nuclear factor kappaB-ligand/osteoprotegerin associated with bone deterioration in patients with Crohn's disease. Eur J Gastroenterol Hepatol. 2009;21(2):159–166. doi: 10.1097/MEG.0b013e3283200032. [DOI] [PubMed] [Google Scholar]
- 51.Dai SM, Nishioka K, Yudoh K. Interleukin (IL) 18 stimulates osteoclast formation through synovial T cells in rheumatoid arthritis: comparison with IL1 beta and tumour necrosis factor alpha. Ann Rheum Dis. 2004;63(11):1379–1386. doi: 10.1136/ard.2003.018481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao Y, Grassi F, Ryan MR, Terauchi M, Page K, Yang X, Weitzmann MN, Pacifici R. IFN-gamma stimulates osteoclast formation and bone loss in vivo via antigen-driven T cell activation. J Clin Invest. 2007;117(1):122–132. doi: 10.1172/JCI30074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kotake S, Nanke Y, Mogi M, Kawamoto M, Furuya T, Yago T, Kobashigawa T, Toqari A, Kamatani N. IFN-gamma-producing human T cells directly induce osteoclastogenesis from human monocytes via the expression of RANKL. Eur J Immunol. 2005;35(11):3353–3363. doi: 10.1002/eji.200526141. [DOI] [PubMed] [Google Scholar]
- 54.Tsuboi M, Kawakami A, Nakashima T, Matsuoka N, Urayama S, Kawabe Y, Fujiyama K, Kiriyama T, Aoyaqi T, Maeda K, Equchi K. Tumor necrosis factor-alpha and interleukin-1beta increase the Fas-mediated apoptosis of human osteoblasts. J Lab Clin Med. 1999;134(3):222–231. doi: 10.1016/s0022-2143(99)90201-9. [DOI] [PubMed] [Google Scholar]
- 55.Gilbert L, He X, Farmer P, Boden S, Kozlowski M, Rubin J, Nanes MS. Inhibition of osteoblast differentiation by tumor necrosis factor-alpha. Endocrinology. 2000;141(11):3956–3964. doi: 10.1210/endo.141.11.7739. [DOI] [PubMed] [Google Scholar]
- 56.Tang X, Zhu X, Liu S, Wang S, Ni X. Isoflavones suppress cyclic adenoise 3',5'-monophoaphate regulatory element-mediated transcription in osteoblastic cell lines. J Nutr Biochem. 2011;22(9):865–73. doi: 10.1016/j.jnutbio.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 57.Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart S, Robertson PK, Kousteni S, O'Brien CA, Bellido T, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem. 2007;282:27285–27297. doi: 10.1074/jbc.M702810200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen JR, Singhal R, Lazarenko O, Liu X, Hogue W, Badger T, Ronis M. Short term effects on bone quality associated with consumption of soy protein isolate and other dietary protein sources in rapidly growing female rats. Exp Biol Med. 2008;233:1348–1358. doi: 10.3181/0802-RM-63. [DOI] [PubMed] [Google Scholar]
- 59.Chen J-R, Lazarenko OP, Blackburn ML, Badeaux J, Badger TM, Ronis MJJ. Infant formula promotes bone growth in neonatal piglets by enhancing osteoblastogenesis through bone morphogenic protein signaling. J Nutr. 2009;139:1839–1847. doi: 10.3945/jn.109.109041. [DOI] [PubMed] [Google Scholar]
- 60.Trzeciakiewicz A, Habauzit V, Mercier S, Barron D, Urpi-Sarda M, Manach C, Offord E, Horcajada M-N. Molecular mechanism of hesperetin-7-O-glucuronide, the main circulating metabolite of hesperidin, involved in osteoblast differentiation. J Agri Food Chem. 2010;58:686–675. doi: 10.1021/jf902680n. [DOI] [PubMed] [Google Scholar]
- 61.Chen J-R, Lazarenko OP, Wu X, Kang J, Blackburn ML, Shankar K, badger TM, Ronis MJ. Dietary-induced serum phenolic acids promote bone growth via p38 MAPK/β-catenin canonical wnt signaling. J Bone Min Res. 2010;25:2399–2412. doi: 10.1002/jbmr.137. [DOI] [PubMed] [Google Scholar]
- 62.Zhang J, Lazarenko O, Blackburn ML, Shankar K, Badger TM, Ronis MJ, Chen J-R. Feeding blueberry diets in early life prevent senescence of osteoblasts and bone loss in ovariectomzed adult female rats. PluS ONE. 2011;6(9):e24486. doi: 10.1371/journal.pone.0024486. doi:10.13711 journal. pone. O024486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bu SY, Hunt TS, Smith BJ. Dried plum polyphenols attenuate the detrimental effect of TNFa on osteoblast function coincident with up-regulation of Runx2, Osterix and IGF-1. J Nutr Biochem. 2009;120:35–44. doi: 10.1016/j.jnutbio.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 64.Bu SY, Lerner M, Stoecker BJ, Boldrin E, Brackett DJ, Lucas EA, Smith BJ. Dried plum polyphenols inhibit osteoclastogenesis by downregulating NFATc1 and inflammatory mediatros. Calcif Tissue Int. 2008;82:475–488. doi: 10.1007/s00223-008-9139-0. [DOI] [PubMed] [Google Scholar]
- 65.He X, Andersson G, Lindgren U, Li Y. Resveratrol prevents RANKL-induced osteoclast differentiation of murine osteoclast progenitor RAW 265. 7 cells through inhibition of ROS production. Biochem Biophys Res Commun. 2010;401(3):356–362. doi: 10.1016/j.bbrc.2010.09.053. [DOI] [PubMed] [Google Scholar]
- 66.Tseng P-C, Hou S-M, Chen R-J, Peng H-W, Hsieh C-F, Kuo M-L, Yen M-L. Resveratrol promotes osteogenesis of human mesenchymal stem cells by upregulating Runx2 gene expression via the SIRT1/FOX03A Axis. J Bone Miner Res. 2011;26:2552–2563. doi: 10.1002/jbmr.460. [DOI] [PubMed] [Google Scholar]
- 67.Shen CL, Yeh JK, Cao JJ, Chyu MC, Wang JS. Green tea and bone health: evidence from laboratory studies. Pharmacol Res. 2011;64(2):155–161. doi: 10.1016/j.phrs.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee W-H, Wastney ME, Jackson GS, Martin BR, Weaver CM. Interpretation of 41Ca data using compartmental modeling in post-menopausal women. Anal Bioanal Chem. 2011;399:1613–1622. doi: 10.1007/s00216-010-4454-5. [DOI] [PMC free article] [PubMed] [Google Scholar]