Abstract
Background
Tumor resistance to platinum-based drugs has been an obstacle to the treatment of ovarian cancer. Extract of the plant Rauwolfia vomitoria has long been used by cancer patients. However, there have not been systematic studies of its anticancer activity.
Objective
In an effort to enhance the effectiveness of platinum-based drugs, we investigated the anticancer effect of a Rauwolfia vomitoria extract (Rau), both alone and in combination with carboplatin (Cp).
Methods
In vitro cytotoxicity and colony formation were evaluated in several ovarian cancer cell lines. In vivo effects were evaluated in an intraperitoneal ovarian cancer mouse model. The combination of Rau and Cp was assessed using Chou-Talalay’s constant ratio design and median effect analysis based on the isobologram principle to determine the combination index values.
Results
Rau decreased cell growth in all 3 tested ovarian cancer cell lines dose dependently and completely inhibited formation of colonies in soft agar. Apoptosis was induced in a time- and dose-dependent manner and was the predominant form of Rau-induced cell death. Synergy of Rau with Cp was detected, with combination index values <1 and dose reduction index values for Cp ranging from 1.7- to 7-fold. Tumor growth in mice was significantly suppressed by 36% or 66% with Rau treatment alone at a low (20 mg/kg) or a high dose (50 mg/kg), respectively, an effect comparable to that of Cp alone. The volume of ascitic fluid and the number of nonblood cells in ascites were also significantly decreased. Combining Rau with Cp remarkably enhanced the effect of Cp and reduced tumor burden by 87% to 90% and ascites volume by 89% to 97%.
Conclusions
Rau has potent antitumor activity and in combination significantly enhances the effect of Cp against ovarian cancer.
Key words: carboplatin, ovarian cancer, plant extract, Rauwolfia vomitoria, synergy
Introduction
With an estimated 22,280 new cases and 15,500 deaths in the United States in 2012, ovarian cancer causes more death than any other cancer of the female reproductive system.1 Due to the lack of significant symptoms in the early stages and the absence of effective biomarkers for early detection, ovarian cancer is usually diagnosed in patients at a late stage of the disease.2–4 As a result, these patients have a poor prognosis and severely impaired quality of life. Although current primary therapy can improve the 5-year survival rate, it has not increased the overall rate of cure.1,4 This is because >70% of patients experience a relapse and develop a resistance to platinum- and taxane-based treatments.4,5 Malignant ascites resistant to conventional chemotherapy affect 28% of ovarian cancer patients in their last period of life.6 Effective and novel treatment strategies for advanced ovarian cancer are urgently needed.
Numerous studies have attempted to improve the efficacy of standard platinum-based therapy by incorporating newer cytotoxic agents. A promising strategy is to use natural products with anticancer effects in combination with platinum-based drugs. One of the advantages of some natural products is their low toxicity compared with conventional chemotherapy drugs. Combinations of natural compounds with standard chemotherapy drugs may exert additive or synergistic effects on killing cancer cells, thereby allowing lower and safer doses of the more toxic drug to be used.
Herbal preparations of Rauwolfia vomitoria, a tropical shrub in the family of Apocynaceae, have been used in traditional folk medicine in Africa to treat a variety of ailments including fever, general weakness, gastrointestinal diseases, liver diseases, psychosis, pain, and cancers.7–14 Extracts from this plant are enriched in β-carboline alkaloids and indole alkaloids.15,16 Many of these alkaloids have been isolated from the stem, leave, and root of Rauwolfia vomitoria. From the root alone, there are mainly 5 types of >20 alkaloids identified.15,17–19 Table I listed the main alkaloids isolated from the Rauwolfia vomitoria root. Reserpine, a drug to control high blood pressure and relieve psychotic symptoms, was isolated from the root bark of Rauwolfia vomitoria.20–22 Other reported activities of the isolated compounds mainly affect the neurological and cardiovascular systems, with many of them not studied for their bioactivities.
Table I.
Major alkaloids and their bioactivities isolated from the root of Rauwolfia vomitoria.
| Type | Alkaloids Detected | Activities Reported | Reference |
| Yohimbine | α-Yohimbine | Pre- and postsynaptic α2-adrenoceptor inhibitor, stimulant and aphrodisiac effect, antidiabetic | 41,42 |
| 18-Hydroxy-yohimbine | Reserpine | Antipsychotic, antihypertension, antidepression, anticancer, carcinogenesis | 43–48 |
| Rescinnamine | Antihypertension | 49 | |
| Heteroyohimbine | Aricine | Unknown | |
| Reserpiline | Antipsychotic, antigastric secretion, antihypertension | 50–53 | |
| Isoreserpiline | Antipsychotic | 8,53 | |
| Dihydroindole | Sarpagine | Unknown | |
| Ajmaline | Antiarrhythmia, antifibrillation, cardiac and liver toxicity | 54,55 | |
| Sandwicine | Unknown | ||
| Iso-sandwicine | Unknown | ||
| Mitoridine | Unknown | ||
| Rauvomitine | Unknown | ||
| N-demethyl-rauvomitine | Unknown | ||
| Purpeline | Unknown | ||
| Seredamine | Unknown | ||
| Suaveoline | Unknown | ||
| Tetraphyllicine | Myocardial excitation | 56 | |
| Vomalidine | Unknown | ||
| Serpentine | Antioxidant, possible anticancer | 23,40 | |
| Alstonine | Antipsychotic, possible anticancer | 23 | |
| Ψ-Indoxyl | Isoreserpiline- Ψ-indoxyl | Unknown | |
| Oxindole | Carapanaubine | Unknown | |
| Rauvoxine | Unknown | ||
The anticancer activities of these components have barely been studied. One early study in 1986 suggested anti–lymphoma ascites cells effects of 3 alkaloids, alstonine, serpentine, and sempervirine, in specific conditions.23 Another study in 2006 reported on the anti–prostate cancer activity of Rauwolfia vomitoria, but the active anticancer component in the extract was not known.24 Apart from the investigations of its components, the extract as a whole is widely used and actively studied. The extract of this medical herb as a whole mixture has been a traditional medicine for >2000 years in Africa for the treatment of hypertension and mental disorders. The effectiveness has been confirmed in more recent studies to be mainly as antipsychotic, antihypertensive, anti-inflammatory and improving blood chemistry. A Rauwolfia-citrus tea is in an early phase clinical trial in Denmark for its antidiabetic effect.7 In this study, we evaluated an important but not yet well-understood aspect of the effect of an extract from the root of Rauwolfia vomitoria, enriched with alkaloids and with reserpine removed, in the treatment of ovarian cancer, used alone and in combination with carboplatin (Cp).
Materials and Methods
Experimental materials, cell lines, and cell viability assay
Human ovarian cancer cell lines OVCAR-5 and OVCAR-8 were obtained from the American Type Culture Collection (Manassas, Virginia), SHIN-3 was donated by Dr. Perter Eck at the National Institutes of Health.25 Immortalized human lung epithelial cells MRC-5 were provided by Dr. Sittampalam at the University of Kansas Medical Center and were compared with cancer cells. All the cells were cultured at 37°C in 5% CO2/95% air in recommended growth media containing 10% fetal calf serum.
Rauwolfia vomitoria extract (Rau) was provided by Natural Source International (New York, New York). The extract was prepared from air-dried clean roots of Rauwolfia vomitoria, first powdered with an electric blender and then extracted twice with 80% ethanol, according to a reported method.26 From the extract, reserpine was removed by chloroform extraction conducted efficiently in columns. Quality control was ensured by HPLC. All the experiments used the extract from a single lot. Rau and Cp (Sigma, St. Louis, Missouri) were prepared in sterile water and stored at −20°C.
Cells were tested for viability by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay at 48 hours of treatment. Cells in the exponential growth phase were exposed to serial dilutions of Rau, Cp, or a combination of the two for 48 hours. The incubation time was determined by pre-experiments to obtain the optimal cell death effect. Then cells were changed to fresh media containing MTT and incubated for 4 hours. The colorimetric MTT assay assessed relative proliferation, based on the ability of living, but not dead, cells to reduce MTT to formazan.27,28 Cells did not reach the plateau phase during the incubation period. IC50 was defined as the concentration of drug that inhibited cell growth by 50% relative to the untreated control.
Anchorage-independent colony formation assay
Anchorage-independent colony formation assays in soft agar were used to determine long time survival of tumorigenic cancer cells after treatments. In 6-well plates, SHIN-3 cells (5000 cells per well) were seeded in the upper layer containing 0.5% agar, Dulbecco’s modified Eagle medium, and 10% fetal bovine serum, with or without 400 μg/mL Rau. The solid agar base (lower layer) contained 0.75% agar and complete growth medium, with or without 400 μg/mL Rau, respectively. After 20 days of incubation, colonies were visualized by crystal violet staining, and the number of colonies was counted.
Apoptosis detection by flow cytometry
Cells were exposed to various concentrations of Rau for 48 hours. Cells were washed in phosphate-buffered saline, resuspended in binding buffer, and subjected to fluorescein isothiocyanate–conjugated annexin V and propidium iodide (PI) staining according to the manufacturer’s protocol (BD Biosciences, San Jose, California). Cells were analyzed by flow cytometry. Annexin V–positive and annexin V-PI double–positive cells were identified as apoptotic cells, whereas PI single–positive cells were identified as necrotic cells.
Western blot
Forty micrograms of protein were loaded for SDS-PAGE. Primary and secondary antibodies were from Cell Signaling Technology Inc. (Danvers, Massachusetts): rabbit anti–poly (ADP-ribose) polymerase (PARP) (1:2000), rabbit anti-caspase-3 (1:1000), rabbit anti-capase-8 (1:1000), mouse anti-β-actin (1:1000), and goat anti-rabbit or anti-mouse immunoglobulin G (1:5000). Blots were developed using immobilon chemiluminescent substrate (Thermo Scientific, Waltham, Massachusetts).
Intraperitoneal ovarian cancer mouse model
Nude mice were injected with SHIN-3 cells by intraperitoneal injection (2.6 × 106/mouse). Seven days after tumor cell inoculation, treatment began with intraperitoneal injection of Cp (15 mg/kg weekly), Rau (20 or 50 mg/kg/d), the respective combination of Cp and Rau, and saline solution as control. After 23 days of treatment, mice were euthanized. All tumor lesions in the peritoneal cavity were collected and weighed; ascites were collected, and nonblood cells were counted in ascitic fluids as an index of tumor cells in the fluids. Major organs such as the liver, kidney, and spleen were fixed in formaldehyde and subjected to histological analysis for any damage caused by potential drug toxicity.
Data analysis
MTT data were normalized to their corresponding untreated controls for each condition (drug, cell type) and were expressed as a percentage of viability. Dose-reduction index (DRI) values for Cp were calculated by the equation DRIICx = (DCp/DCp+Rau), where DCp is the dose of Cp alone required to produce an ICx level of cytotoxicity, and DCp+Rau is the dose of Cp needed to produce the same ICx level of cytotoxicity when it is combined with Rau (at a given molar ratio). DRICp is defined with respect to Cp. Combination index (CI) values were calculated by the equation CIICx = (DCpCombo ICx/DCp ICx) + (DRauCombo ICx/DRau ICx) + α[(DCpComboICx)(DRauComboICx)/(DCp ICx)(DRau ICx)], where D is the dose of Cp and Rau, either alone or in combination, at a given constant ratio required to produce an ICx level of cytotoxicity.29–31 The more conservative assumption of mutual exclusivity was adopted (α = 0). SPSS version 15.0 (SPSS Inc., Chicago, Illinois) was used for additional statistical analysis.
Results
Inhibition of ovarian cancer cell viability and colony formation by Rau
Human ovarian cancer cell lines (SHIN-3, OVCAR-5, and OVCAR-8) were subjected to Rau treatment to test for sensitivity at concentrations from 0 to 800 µg/mL. An immortalized nontumorigenic epithelial cell line (MRC-5) was subjected to the same treatment for comparison. The dose-response curves showed that all cancer cells were susceptible to Rau treatment with IC50 values of ∼300 µg/mL, whereas the immortalized noncancerous cells MRC-5 was less sensitive with IC50 = 566 µg/mL, a significant difference compared with cancer cells (Figure 1A). At a concentration of 400 µg/mL, Rau was able to reduce cell viability by 60% to 80% in all 3 tested ovarian cancer cells and only decreased viability in MRC-5 cells by 28%.
Fig. 1.
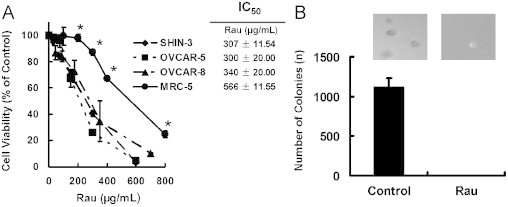
Cytotoxicity of Rauwolfia vomitoria extract (Rau) in normal cells and ovarian cancer cells. (A) Dose-response curves of normal and ovarian cancer cells. Ovarian cancer cells SHIN-3, OVCAR-5, and OVCAR-8 were exposed to serial concentrations of Rau for 48 hours, and cell viabilities were detected by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. A noncancerous epithelial cell, MCR-5, was subjected to the same treatment. IC50 was defined as the concentration of drug that inhibited cell growth by 50% relative to the untreated control. (*P < 0.05 relative to any of the cancer cells). (B) Colony formation of SHIN-3 cells in soft agar with and without Rau treatment. Five thousand SHIN-3 cells per well in 6-well plates were either treated with 400 μg/mL Rau or untreated (Control). All values are expressed as mean ± SD of 3 independent experiments.
Colony formation in soft agar was used to assess the drug effect on tumor cell survival for a longer time, as well as to assess the survival of tumorigenic cells, which are important for in vivo tumor formation.32,33 Untreated SHIN-3 cells formed colonies in soft agar at a rate of 23%. Rau at 400 µg/mL completely inhibited formation of colonies of SHIN-3 cells in soft agar (Figure 1B), indicating no survival of tumorigenic cancer cells with this treatment.
Induction of apoptosis in ovarian cancer cells by Rau
To assess the Rau-induced death pathway, annexin V/PI straining was performed to detect apoptosis versus necrosis in SHIN-3 cells treated with Rau. Data from flow cytometry demonstrated that predominant apoptosis was induced by Rau treatment. With 0, 50, 100, and 400 μg/mL Rau treatment, the percentage of positive cells with annexin V and PI straining were 5.7%, 33.6%, 49.6%, and 85.3% respectively (Figure 2A). The induction of apoptosis was clearly dependent on the concentration of Rau with a logarithmic relationship (Figure 2A) and was the major form of cell death induced by Rau. Necrosis (cells that were PI positive only) contributed only 6% to 14% of total cell death all across the Rau concentrations.
Fig. 2.
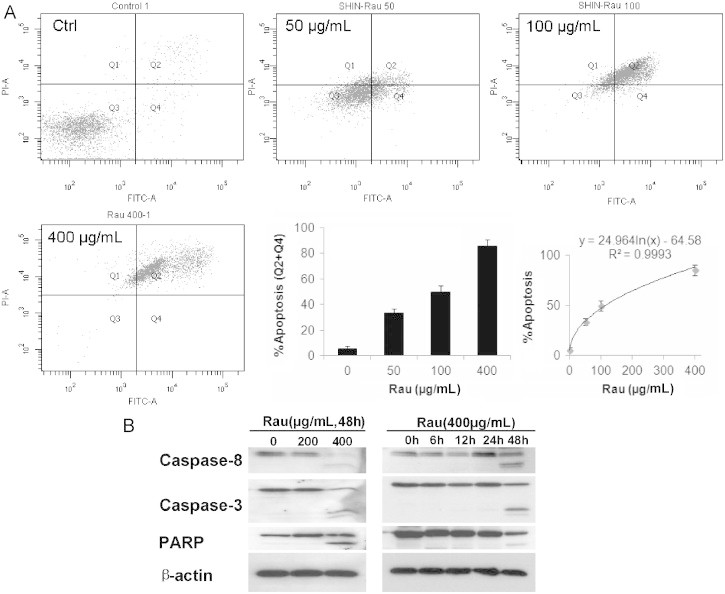
Apoptosis in SHIN-3 cells induced by Rauwolfia vomitoria extract (Rau). (A) Flow-cytometry detection of apoptotic cells. SHIN-3 cells were treated with 400 μg/mL of Rau for 48 hours and then underwent double staining with fluorescein isothiocyanate–conjugated annexin V and propidium iodide. The rate of apoptosis was represented by the percentage of cells in the Q2 and Q4 quadrants. A logarithmic relationship between the apoptosis rate and concentration of Rau was shown. (B) Cleavage of caspases and poly(ADP-ribose) polymerase (PARP) in SHIN-3 cells treated with Rau. Cells were treated with Rau at the indicated concentrations and time. The dose-dependent and time-dependent cleavage of caspase-8, caspase-3, and PARP were detected by Western blot. β-Actin acted as a loading control.
Consistent with this finding, Western blot analysis detected extensive cleavage of caspase-8, caspase-3, and PARP in Rau-treated SHIN-3 cells in a dose-dependent and time-dependent manner (Figure 2B).
Synergy of Rau in combination with Cp against ovarian cancer cells
As platinum-based treatment is a current primary care for ovarian cancer patients, the effect of the combination of Rau and Cp was examined using cultured ovarian cancer cells. After determining the dose-response relationships for Rau (Figure 1A), the dose-response relationships for Cp cytotoxicity were established in SHIN-3, OVCAR-5, and OVCAR-8 cells (Figure 3A, dotted lines). A constant ratio design was used to systematically examine combination dose-response relationships between Cp and Rau. The ratio of Cp to Rau was chosen as IC50Cp:IC50Rau. Combination data were presented in terms of Cp concentration. Despite the inherent sensitivity to Cp, results clearly showed that when Rau was added to Cp, decrease in cell viability was dramatically enhanced in all tested cells compared with Cp as a single agent (Figure 3A).
Fig. 3.
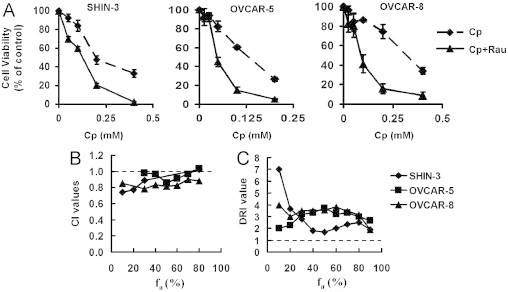
Combination effect of Rauwolfia vomitoria extract (Rau) and carboplatin (Cp) in ovarian cancer cells. (A) Dose-response curves. Ovarian cancer cells were treated with Cp (dotted line) and the combination of Cp and Rau (Cp + Rau, solid line) for 48 hours. The molar ratio of the combination was IC50Rau:IC50Cp. (B) Combination index (CI) across the fraction affected (fa). The molar ratio of Rau:Cp was IC50Rau:IC50Cp. A CI value >1 indicates antagonism, a CI value of 1 indicates additive effect, and a CI value <1 indicates synergy. (C) The dose-reduction index (DRI) across the fa for Cp when Rau was combined.
The CI value for each cell type was calculated relying on Chou-Talalay’s isobologram principle to determine whether drug combinations were synergistic (CI <1), additive (CI = 1), or antagonistic (CI >1).28–30 In addition, DRI value for Cp was calculated as described in the Material and Methods section. DRI values >1 indicate a favorable combination, whereas a DRI value <1 would be interpreted as an antagonistic combination. In all cell lines tested, CI values were ≤1 (Figure 2B), and DRI values for Cp were >1 (Figure 2C) across the desired levels of effect (fa, fraction affected). This indicated an additive to synergistic effect when Rau was combined with Cp. Reduction in Cp doses ranged from 1.7- to 7-fold when Rau was combined with Cp, depending on cell lines and the aimed level of effect. These data unequivocally support the conclusion that the concentration of Cp can be decreased to produce an equitoxic effect on ovarian cancer cells when Rau is combined with Cp.
In vivo tumor inhibitory effect of Rau alone or in combination with Cp
An intraperitoneally implanted SHIN-3 mouse xenograft model was used to evaluate the effect of Rau and the combination of Cp and Rau (Cp + Rau) treatment. Compared with the subcutaneous implantation model, the intraperitoneal model better mimics the clinical condition of advanced human ovarian cancer, especially in peritoneal metastasis and ascites formation. Tumor-bearing mice were treated for 23 days, and necropsy was performed at euthanasia. All tumor lesions in the peritoneal cavity were collected and examined, as was ascitic fluid.
As shown in Figure 4A, Rau treatment alone decreased total tumor weight by 36% and 66% at the daily dose of 20 mg/kg or 50 mg/kg, respectively, compared with saline solution–treated control. Cp at the dose used decreased tumor weight by 51%. By combining Rau with Cp, the tumor inhibitory effect was dramatically enhanced. Tumor weight decreased 87% (Cp + 20 mg/kg Rau) and 90% (Cp + 50 mg/kg Rau) relative to control. The enhancement was significant compared with either Rau or Cp single-agent treatment.
Fig. 4.
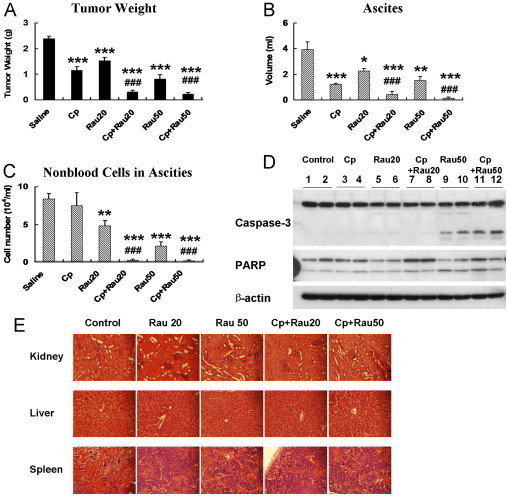
Effect of Rauwolfia vomitoria extract (Rau) and the combination of Rau and carboplatin (Cp) against SHIN-3 ovarian cancer cells in an intraperitoneal mouse model. Cells (2.6 × 106) were intraperitoneally injected into nude mice. After 7 days of tumor inoculation, treatment commenced with an intraperitoneal injection of Rau at 20 mg/kg/d (Rau20) or 50 mg/kg/d (Rau50), Cp at 15 mg/kg/wk, and respective combinations of Rau and Cp (Cp+Rau20, and Cp+Rau50). Treatment lasted for 23 days, and tumor weight, volume of ascites, and number of cells in ascites were assessed at the end of treatment. (A) Total tumor weight. (B) Volume of ascitic fluids. (C) Number of nonblood cells in the ascitic fluids. (D) Cleavage of capase-3 and poly(ADP-ribose) polymerase (PARP) in tumor samples from different treatment groups. (E) Hematoxylin and eosin staining of major organs from different treatment group (×400 magnification). Kidney, liver, and spleen were collected from each treatment group and fixed in 4% formaldehyde and later underwent histological analysis. (*P < 0.05, **P < 0.01, ***P < 0.001 relative to control group; #P < 0.05, ##P< 0.01, ###P < 0.001 relative to Cp-treated group.)
Excessive volumes of ascites were formed in saline solution–treated control mice at the endpoint of the experiment (4 mL of ascites/mouse in average). Rau treatment alone significantly decreased the volume of ascitic fluid to an average of 2.3 mL per mouse at the dose of 20 mg/kg and to 1.5 mL per mouse at the dose of 50 mg/kg (P < 0.05). The degree of reduction is comparable to that of Cp (Figure 4B). By combining Rau with Cp (20 or 50 mg/kg Rau), the combination treatments decreased ascites volume by 89% and 97% compared with control to a minimal amount of 0.44 mL per mouse and 0.13 mL per mouse (Figure 4B).
Nonblood cells in ascitic fluid were counted as an estimation of tumor cells in the ascites. The results were shown as the number of cells per milliliters of ascites. Although Cp at the doses used did not reduce the number of cells in ascites, Rau showed a strong effect in decreasing the number of cells per milliliters of ascites by 43% and 75%. Moreover, when combined with Cp, Rau at either of the doses used decreased the number of cells per milliliters of ascites by ~98% (Figure 4C).
Proteins were isolated from tumor samples of the treated and control mice. Western blot analysis showed cleavage of caspase-3 and PARP in Rau and Cp + Rau treatment groups at either high or low doses of Rau (Figure 4D), whereas Cp treatment alone did not cause cleavage in caspases and PARP. These results confirmed the in vitro data that Rau induced apoptosis in tumor cells.
None of the mice demonstrated observable toxicity associated with the treatments. At the end of the experiments, major organs (kidney, liver, and spleen) were underwent hematoxylin and eosin staining and histological analysis. No tissue damage was detected in the treatment groups, and there were no significant differences between control group and treated groups (Figure 4E).
Discussion
Inherent or acquired drug resistance has been a serious problem in ovarian cancer therapy. Combining platinum-based chemotherapy with other anticancer drugs has been an effective way to improve therapeutic outcome.34–36 Rauwolfia vomitoria has been a folk medicine for centuries, and its extract has been shown to provide antioxidant and antiproliferative effects in different systems based on its inhibition of diverse cellular events associated with tumor pathogenesis.23,24,37 Our data demonstrated that Rau substantially inhibited ovarian cancer cell growth, both in vitro and in vivo. Despite the inherent Cp sensitivity of the cell lines, Rau increased chemosensitivity of all tested ovarian cancer cells and synergized with Cp in the inhibition of ovarian cancer.
Importantly, Rau inhibited the tumor growth in a mouse model with intraperitoneal metastasis and massive ascites formation, either alone or in combination with Cp. With Rau and Cp combination treatment, a remarkable tumor inhibition of >90% was achieved, and the ascites were nearly eradicated. This effect was not achieved by Cp treatment alone. It is possible that different degrees of additive/synergistic effects will be achieved if conditions of combination are different, such as the doses of both drugs to be combined, the time and order of administration, and the intervals of treatment. The study here provides a basis for further investigation in refining the combination regimen to achieve optimal effects, even possible complete tumor inhibition. As the prognosis and survival rate are poor for ovarian cancer patients who have intraperitoneal metastasis,1 these results provided a basis for further investigation for Rau as an adjuvant treatment for ovarian cancer patients.
Moreover, our data showed that Rau had relatively low toxicity on normal cells. This was evident in mice treated with Rau in which major organ toxicities were absent. As many studies have reported psychological and cardiovascular activities of Rau, the anticipated toxic side effects could be hypotension, cardiac toxicity, psychological effects, and gastrointestinal disturbance. However, in our extract, the compound reserpine was removed, which is the main contributor to the hypotensive effect and part of the psychological effects. Therefore, low toxicity with our extract could be expected. Consistent with this hypothesis, no abnormalities or toxic signs were observed in the mice. In summary, the antitumor activities of Rau synergizing with Cp suggest that this herbal preparation could potentially offer therapeutic benefit in the management of ovarian cancer.
Although potential benefit was suggested by our study, the mechanism(s) of Rau that induced an anticancer effect warrant further study. A previous study reported DNA damage and cell cycle inhibition induced by Rau in prostate cancer cells.24 Activation of these pathways could lead to apoptosis, which is a powerful tumor-suppressive pathway preventing the uncontrolled proliferation of cancerous cells and potentially depleting stemlike and progenitor cancer cell pools.38,39 Consistent with these reports, our study showed that Rau mainly induced apoptosis in vitro and in vivo, as shown by flow cytometry and the cleavage of caspase-8, caspase-3, and PARP. The in vitro tumorigenic capacity of ovarian cancer cells was completely inhibited by Rau treatment. However, because this plant preparation contains a complex mixture of natural compounds, there is potential to affect multiple molecular targets and pathways.
Bioactive compounds are often found in medicinal plant and herbal mixtures, making them a superb source for the discovery of novel drug leads. The β-carboline– and indole alkaloid–enriched Rau appears to contain components that possess potent anticancer activity. Our study demonstrated for the first time the anticancer and chemo-potentiation activity of the mixture of Rau in ovarian cancer, with novelty and significance. Because its anticancer components are largely unknown, it is possible to trace the anticancer activity to single compound, such as alstonine and/or serpentine.23,40 On the other hand, there is also the possibility that several components work in concert to exhibit optimal anticancer activity and lower toxicity, such as that seen in many herbal medicines and traditional Chinese medicines. Also, a nonlinear or U-shape dose-response in vivo has been reported by other investigators, further suggesting that different components in the extract were working together.24 Although further studies are needed to investigate these interesting questions, the data presented here provide a basis for identifying the anticancer activity of Rau. Active components could be isolated and developed for optimizing the efficacy, toxicity, and other profiles that could lead to anticancer drug development.
Conflicts of Interest
The authors have indicated that they have no conflicts of interest regarding the content of this article.
Acknowledgments
This study was supported by a grant from the Beljanski Foundation, and the plant extract was provided by Natural Source International Ltd. The authors have no affiliation or financial relationship with the sponsors, and neither the Beljanski Foundation nor Natural Source International Ltd. played a role in the design and conduct of the study. Drs. Chen and Yu designed the study, analyzed the data, and wrote the manuscript. Dr. Yu conducted all the in-vitro experiments. Drs. Yu and Ma conducted the animal experiments. Dr. Drisko participated in study design and data interpretation.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.American Cancer Society. Cancer Facts and Figures 2012. http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-031941.pdf. Accessed January 18, 2012.
- 2.Chen H., Hardy T.M., Tollefsbol T.O. Epigenomics of ovarian cancer and its chemoprevention. Front Genet. 2011;2:67. doi: 10.3389/fgene.2011.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buys S.S., Partridge E., Greene M.H. Ovarian cancer screening in the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial: findings from the initial screen of a randomized trial. Am J Obstet Gynecol. 2005;193:1630–1639. doi: 10.1016/j.ajog.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Beller U., Quinn M.A., Benedet J.L. Carcinoma of the vulva. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S7–S27. doi: 10.1016/S0020-7292(06)60028-3. [DOI] [PubMed] [Google Scholar]
- 5.Monk B.J., Coleman R.L. Changing the paradigm in the treatment of platinum-sensitive recurrent ovarian cancer: from platinum doublets to nonplatinum doublets and adding antiangiogenesis compounds. Int J Gynecol Cancer. 2009;19(Suppl 2):S63–S67. doi: 10.1111/IGC.0b013e3181c104fa. [DOI] [PubMed] [Google Scholar]
- 6.Bellati F., Napoletano C., Ruscito I. Complete remission of ovarian cancer induced intractable malignant ascites with intraperitoneal bevacizumab. Immunological observations and a literature review. Invest New Drugs. 2010;28:887–894. doi: 10.1007/s10637-009-9351-4. [DOI] [PubMed] [Google Scholar]
- 7.Campbell-Tofte J.I., Molgaard P., Josefsen K. Randomized and double-blinded pilot clinical study of the safety and anti-diabetic efficacy of the Rauvolfia-Citrus tea, as used in Nigerian traditional medicine. J Ethnopharmacol. 2011;133:402–411. doi: 10.1016/j.jep.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Bisong S.A., Brown R., Osim E.E. Comparative effects of Rauwolfia vomitoria and chlorpromazine on locomotor behaviour and anxiety in mice. J Ethnopharmacol. 2010;132:334–339. doi: 10.1016/j.jep.2010.08.045. [DOI] [PubMed] [Google Scholar]
- 9.Bisong S., Brown R., Osim E. Comparative effects of Rauwolfia vomitoria and chlorpromazine on social behaviour and pain. N Am J Med Sci. 2011;3:48–54. doi: 10.4297/najms.2011.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bisong S.A., Brown R.E., Osim E.E. Comparative extrapyramidal effects of Rauwolfia vomitoria, chlorpromazine and reserpine in mice. J Nat Med. 2013;67:107–112. doi: 10.1007/s11418-012-0657-8. [DOI] [PubMed] [Google Scholar]
- 11.Pesewu G.A., Cutler R.R., Humber D.P. Antibacterial activity of plants used in traditional medicines of Ghana with particular reference to MRSA. J Ethnopharmacol. 2008;116:102–111. doi: 10.1016/j.jep.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Kweifio-Okai G. Antiinflammatory activity of a Ghanaian antiarthritic herbal preparation: I. J Ethnopharmacol. 1991;33:263–267. doi: 10.1016/0378-8741(91)90087-t. [DOI] [PubMed] [Google Scholar]
- 13.La Barre J., Castiau J. [Effect of reserpine-free Rauwolfia vomitoria extract on gastric motility in dogs] C R Seances Soc Biol Fil. 1957;151:2222–2224. [PubMed] [Google Scholar]
- 14.La Barre J. Hypotensive effects of the completely dereserpinised extract of Rauwolfia vomitoria. Arzneimittelforschung. 1973;23:600–605. [PubMed] [Google Scholar]
- 15.Iwu M.M., Court W.E. Root alkaloids of Rauwolfia vomitoria afz. Planta Med. 1977;32:88–99. doi: 10.1055/s-0028-1097565. [DOI] [PubMed] [Google Scholar]
- 16.Poisson J. [Research on the alkaloids of Rauwolfia vomitoria Afz. roots (Apocynacea)] Trav Lab Matiere Med Pharm Galenique Fac Pharm Paris. 1959;44:2–118. [PubMed] [Google Scholar]
- 17.Plummer A.J., Earl A., Schneider J.A. Pharmacology of Rauwolfia alkaloids, including reserpine. Ann N Y Acad Sci. 1954;59:8–21. doi: 10.1111/j.1749-6632.1954.tb45914.x. [DOI] [PubMed] [Google Scholar]
- 18.Schlittler E., Macphillamy H.B., Dorfman L. Chemistry of Rauwolfia alkaloids, including reserpine. Ann N Y Acad Sci. 1954;59:1–7. doi: 10.1111/j.1749-6632.1954.tb45913.x. [DOI] [PubMed] [Google Scholar]
- 19.Wilkins R.W. Clinical usage of Rauwolfia alkaloids, including reserpine (serpasil) Ann N Y Acad Sci. 1954;59:36–44. doi: 10.1111/j.1749-6632.1954.tb45916.x. [DOI] [PubMed] [Google Scholar]
- 20.Poisson J., Le Hir A., Goutarel R., Janot M.M. [Isolation of reserpine from roots of Rauwolfia vomitoria Afz] C R Hebd Seances Acad Sci. 1954;238:1607–1609. [PubMed] [Google Scholar]
- 21.Hariga M. [Preliminary trial of therapeutic use of reserpiline, a hypotensive extract of Rauwolfia vomitoria] Brux Med. 1959;39:451–455. [PubMed] [Google Scholar]
- 22.Shamon S.D., Perez M.I. Blood pressure lowering efficacy of reserpine for primary hypertension. Cochrane Database Syst Rev. 2009:CD007655. doi: 10.1002/14651858.CD007655.pub2. [DOI] [PubMed] [Google Scholar]
- 23.Beljanski M., Beljanski M.S. Three alkaloids as selective destroyers of cancer cells in mice. Synergy with classic anticancer drugs. Oncology. 1986;43:198–203. doi: 10.1159/000226363. [DOI] [PubMed] [Google Scholar]
- 24.Bemis D.L., Capodice J.L., Gorroochurn P. Anti-prostate cancer activity of a beta-carboline alkaloid enriched extract from Rauwolfia vomitoria. Int J Oncol. 2006;29:1065–1073. [PubMed] [Google Scholar]
- 25.Imai S., Kiyozuka Y., Maeda H. Establishment and characterization of a human ovarian serous cystadenocarcinoma cell line that produces the tumor markers CA-125 and tissue polypeptide antigen. Oncology. 1990;47:177–184. doi: 10.1159/000226813. [DOI] [PubMed] [Google Scholar]
- 26.Isaiah A.M., Olawale O., Effiong E.E. Vitamin e supplementation with rauwolfia vomitoria root bark extract improves hematological indices. N Am J Med Sci. 2012;4:86–89. doi: 10.4103/1947-2714.93383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denizot F., Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 28.Cole S.P. Rapid chemosensitivity testing of human lung tumor cells using the MTT assay. Cancer Chemother Pharmacol. 1986;17:259–263. doi: 10.1007/BF00256695. [DOI] [PubMed] [Google Scholar]
- 29.Chou T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 30.Chou T., Rideout D., Chou J., Bertino J. Chemotherapeutic synergism, potentiation and antagonism. Encyclopedia Hum Biol. 1991;2:371–379. [Google Scholar]
- 31.Chou T., Talalay P. Applications of the median-effect principle for the assessment of low-dose risk of carcinogens and for the quantitation of synergism and antagonism of chemotherapeutic agents. New Avenues Dev Cancer Chemother. 1987;8:37–64. [Google Scholar]
- 32.Eagle H., Foley G.E., Koprowski H. Growth characteristics of virus-transformed cells. Maximum population density, inhibition by normal cells, serum requirement, growth in soft agar, and xenogeneic transplantability. J Exp Med. 1970;131:863–879. doi: 10.1084/jem.131.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeng G., Cai S., Liu Y., Wu G.J. METCAM/MUC18 augments migration, invasion, and tumorigenicity of human breast cancer SK-BR-3 cells. Gene. 2012;492:229–238. doi: 10.1016/j.gene.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 34.Fu S., Hennessy B.T., Ng C.S. Perifosine plus docetaxel in patients with platinum and taxane resistant or refractory high-grade epithelial ovarian cancer. Gynecol Oncol. 2012;126:47–53. doi: 10.1016/j.ygyno.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weroha S.J., Oberg A.L., Ziegler K.L. Phase II trial of lapatinib and topotecan (LapTop) in patients with platinum-refractory/resistant ovarian and primary peritoneal carcinoma. Gynecol Oncol. 2011;122:116–120. doi: 10.1016/j.ygyno.2011.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bell-McGuinn K.M., Matthews C.M., Ho S.N. A phase II, single-arm study of the anti-alpha5beta1 integrin antibody volociximab as monotherapy in patients with platinum-resistant advanced epithelial ovarian or primary peritoneal cancer. Gynecol Oncol. 2011;121:273–279. doi: 10.1016/j.ygyno.2010.12.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beljanski M., Beljanski M.S. Selective inhibition of in vitro synthesis of cancer DNA by alkaloids of beta-carboline class. Exp Cell Biol. 1982;50:79–87. doi: 10.1159/000163131. [DOI] [PubMed] [Google Scholar]
- 38.Chen F. JNK-induced apoptosis, compensatory growth, and cancer stem cells. Cancer Res. 2012;72:379–386. doi: 10.1158/0008-5472.CAN-11-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chao M.P., Majeti R., Weissman I.L. Programmed cell removal: a new obstacle in the road to developing cancer. Nat Rev Cancer. 2012;12:58–67. doi: 10.1038/nrc3171. [DOI] [PubMed] [Google Scholar]
- 40.Dutta S., Chowdhury A.R., Srivastava S.K. Evidence for Serpentine as a novel antioxidant by a redox sensitive HABP1 overexpressing cell line by inhibiting its nuclear translocation of NF-kappaB. Free Radic Res. 2011;45:1279–1288. doi: 10.3109/10715762.2011.610794. [DOI] [PubMed] [Google Scholar]
- 41.Rosengren A.H., Jokubka R., Tojjar D. Overexpression of alpha2A-adrenergic receptors contributes to type 2 diabetes. Science. 2010;327:217–220. doi: 10.1126/science.1176827. [DOI] [PubMed] [Google Scholar]
- 42.NCCAM, Yohimbe. 2012. http://nccam.nih.gov/health/yohimbe. Accessed April 8, 2013.
- 43.Davies D.L., Shepherd M. Reserpine in the treatment of anxious and depressed patients. Lancet. 1955;269:117–120. doi: 10.1016/s0140-6736(55)92118-8. [DOI] [PubMed] [Google Scholar]
- 44.Wilkins R.W., Judson W.E., Stone R.W. Reserpine in the treatment of hypertension; a note on the relative dosage and effects. N Engl J Med. 1954;250:477–478. doi: 10.1056/NEJM195403182501107. [DOI] [PubMed] [Google Scholar]
- 45.Chen G., Ensor C.R., Bohner B. A facilitation action of reserpine on the central nervous system. Proc Soc Exp Biol Med. 1954;86:507–510. doi: 10.3181/00379727-86-21149. [DOI] [PubMed] [Google Scholar]
- 46.Belkin M., Hardy W.G. Effect of reserpine and chlorpromazine on sarcoma 37. Science. 1957;125:233–234. doi: 10.1126/science.125.3241.233. [DOI] [PubMed] [Google Scholar]
- 47.Burton R.M., Goldin A., Humphreys S.R., Venditti J.M. Antileukemic action of reserpine. Science. 1957;125:156–157. doi: 10.1126/science.125.3239.156-a. [DOI] [PubMed] [Google Scholar]
- 48.National Toxicology Program, HHS, 12th Report on Carcinogens, 2011. http://ntp.niehs.nih.gov/ntp/roc/twelfth/profiles/Reserpine.pdf. Accessed April 8, 2013.
- 49.Smirk F.H., Mc Q.E. Comparison of rescinnamine and reserpine as hypotensive agents. Lancet. 1955;269:115–116. doi: 10.1016/s0140-6736(55)92116-4. [DOI] [PubMed] [Google Scholar]
- 50.Gupta S., Khanna V.K., Maurya A. Bioactivity guided isolation of antipsychotic constituents from the leaves of Rauwolfia tetraphylla L. Fitoterapia. 2012;83:1092–1099. doi: 10.1016/j.fitote.2012.04.029. [DOI] [PubMed] [Google Scholar]
- 51.La Barre J., Hans M.J., Desmarez J.J. [Reserpiline, an hypotensive and non-tranquilizing alkaloid] C R Seances Soc Biol Fil. 1958;152:533–534. [PubMed] [Google Scholar]
- 52.La Barre J., Lieber C.S., Castiau J. [Effect of reserpiline on gastric secretion and motility] C R Seances Soc Biol Fil. 1958;152:532–533. [PubMed] [Google Scholar]
- 53.Maurya A., Gupta S., Srivastava S.K. Large-scale separation of antipsychotic alkaloids from Rauwolfia tetraphylla L. by pH-zone-refining fast centrifugal partition chromatography. J Sep Sci. 2013;36:407–413. doi: 10.1002/jssc.201200273. [DOI] [PubMed] [Google Scholar]
- 54.Chatterjee M.L., De M.S. Pharmacological action of ajmaline, the possible mechanism of its antiarrhythmic action, and its therapeutic possibilities. Nature. 1963;200:1067–1068. doi: 10.1038/2001067a0. [DOI] [PubMed] [Google Scholar]
- 55.Lenox H., Dick H., Mc C.E. Clinical pharmacologic observations of the effects of ajmaline in chronic atrial fibrillation. Clin Pharmacol Ther. 1963;4:315–320. doi: 10.1002/cpt196343315. [DOI] [PubMed] [Google Scholar]
- 56.Duncan R.J., Nash C.B. Effects of the rauwolfia alkaloids, ajmaline, tetraphyllicine, and serpentine, on myocardial excitability. Arch Int Pharmacodyn Ther. 1970;184:355–361. [PubMed] [Google Scholar]


