Abstract
Objective
The aim of our study is to compare the effect of ketamine–propofol mixture (ketofol) and propofol on the laryngeal tube-Suction II (LTS II) insertion conditions and hemodynamics.
Methods
Eighty American Society of Anesthesiologists class 1 and 2 patients were divided into 2 random groups to receive either 1 µg/kg remifentanil and propofol 2 mg/kg in Group P (n = 40), or 1 µg/kg remifentanil and ketofol (using a 1:1 single syringe mixture of 5 mg/mL ketamine and 5 mg/mL propofol) in Group K (n = 40) before induction of anesthesia. After induction, LTS II was inserted. Heart rate and noninvasive blood pressure were recorded before induction of anesthesia (t0); immediately following induction (t1); immediately after LTS II insertion (t2); and 3 minutes (t3), 5 minutes (t4), and 10 (t5) minutes after LTS II insertion. Conditions of insertion of LTS II were assessed and scored 1 to 3 using 6 variables as follows: mouth opening, swallowing, coughing, head and body movements, laryngospasm, and ease of LTS II insertion by the same experienced anesthesiologist who did not know the agents. LTS II insertion summed score was prepared depending upon these variables.
Results
In regard to LTS II insertion summed score, Group K was more favorable than Group P (P < 0.05). Apnea duration was longer in Group P (385.0 seconds [range = 195.0–840.0 seconds]) compared with Group K (325.50 seconds [range = 60.0–840.0 seconds]) but this was not statically significant. The heart rate values were significantly lower at all measurement intervals in both groups compared with the baseline values (P < 0.05). There was no difference in heart rate between Group P and Group K. The mean arterial pressure values were significantly lower at all measurement intervals in Group P compared with baseline values (P < 0.05). In Group K, the mean arterial pressure values were significantly lower at all measurement intervals compared with the baseline values, except t2 (P < 0.05). There was a significant difference between Group P and Group K in terms of mean arterial pressure at t3 (P < 0.05).
Conclusions
We found that ketofol provided better insertion summed score for LTS II than propofol, with minimal hemodynamic changes.
Key words: anesthesia, ketamine, laryngeal tube-Suction II, propofol
Introduction
Propofol is an agent that provides rapid induction and recovery, depresses airway reflexes, and is used for sedation and anesthesia. The fact that it causes dose-dependent hypotension and respiratory depression, limits the use of propofol.1,2 If it is used as a single agent to place laryngeal mask (LMA), it can cause unwanted responses such as coughing, hiccups, laryngospasm, and movements.3
In addition to its amnesic and analgesic properties, ketamine increases heart rate and blood pressure by activating the sympathetic nervous system.4 It was observed that a combination of ketamine and propofol reduced consumption of propofol and opioids and ensured better hemodynamic and respiratory stability in patients.5–7
Ketamine and propofol mixture (ketofol) is 5 mg/mL ketamine and 5 mg/mL propofol, 1:1 mixture in a 20-mL syringe. It is reported that if ketamine and propofol are mixed in a polypropylene syringe they are physically compatible and chemically stable, and that they can be stored at room temperature and under light.8 Ketofol has successfully been used in brief, painful interventions in emergency departments; for sedation in pediatric cases; for regional anesthesia; and in anesthesia applications in electroconvulsive therapy.9–12
Laryngeal tube-Suction II (LTS II) (VBM, Medizintechnik, Sulz, Germany) is a double-lumen silicone tube and it is the latest version of the laryngeal tube (LT), which is a supraglottic airway device. It is inserted blindly in the upper esophagus and hypopharynx by determining position with the distal tip. LTS II has 2 lumens: 1 for ventilation and the other for the passage of a gastric tube. It was originally designed for emergency airway management, including out-of-hospital use, but now it is used for indications where LMA is used during general anesthesia.13–15
There is no research on the use of ketofol for insertion of LTS II. The aim of this study is to compare the effect of ketofol and propofol on LTS II insertion conditions and hemodynamics.
Methods
Following approval of the ethics committee of Inonu University Faculty of Medicine, Malatya, Turkey (No. 2010/158, January 4, 2011), and provision of written informed consent from the patients, 80 American Society of Anesthesiologists class 1 and 2 patients, aged between 18 and 65 years, scheduled for elective surgery that would last <2 hours were included in the study between January 2011 and November 2011. Patients with increased aspiration risk, body mass index >30, allergy to any of the agents used in the study, predicted to have difficult airway (eg, Mallampati score >2 or mouth opening <3 cm), an open eye injury or other ophthalmologic disorder, psychiatric disease such as schizophrenia, a history of adverse reaction to ketamine, and vascular aneurysms were excluded from the study.
The patients were not premedicated. In the operating room standard anesthetic monitoring was applied with noninvasive blood pressure, ECG, and peripheric oxygen saturation. After providing intravenous access, Ringer’s lactate solution infusion was started. Patients were divided into 2 random groups: a propofol group (Group P; n = 40) and a ketofol group (Group K; n = 40) using a computer-generated random number table. All study medications were prepared in identical 20-mL polypropylene syringes. A 20-mL syringe of propofol 1% (10 mg/mL) was used for Group P. For Group K, a ketofol solution (total 20 mL) was prepared, with the agents in the same syringe, using ketamine 100 mg (Ketalar 50 mg/mL; Pfizer, New York, NY) and propofol 100 mg (1% propofol; Fresenius, Waltham, MA); the ketofol syringes contained ketamine 2 mL (100 mg), propofol 1% 10 mL (100 mg), and saline 8 mL. The concentrations of these drugs were thus 5 mg/mL ketamine and 5 mg/mL propofol, and there was no interaction between these drugs in the mixture.
After 3 minutes of preoxygenation, 1 µg/kg remifentanil was given in 60 seconds. Later, Group P was administered 0.2 mL/kg propofol and Group K was administered 0.2 mL/kg ketofol (1 mg/kg ketamine + 1 mg/kg propofol) approximately in 30 seconds. If required, further increments of drugs (ketofol or propofol), at 0.05 mL/kg, were given every 30 seconds until loss of consciousness and loss of eyelash reflex. Sixty seconds after induction, LTS II with deflated cuff was inserted by an anesthetist who was unaware of the agents given, using a water-soluble lubricant per guidelines of the manufacturer. A size-3 LTS II was used for patients shorter than 155 cm, a size 4 was used for patients between 155 and 180 cm, and a size 5 was used for patients taller than 180 cm. Cuffs were inflated with the proposed amount of air using the injector proposed by the manufacturing company. Effective ventilation was confirmed by capnography and chest expansion. With the cuff manometer (VBM, Sulz, Germany) maximum pressure was set to 60 cm water and cuff pressure was measured at certain times during operation. A gastric tube was inserted along the gastric channel of LTS II and correctness of the insertion was assessed by aspiration of the gastric fluid and auscultation of the injected air in the epigastrium.
A maximum of 3 attempts were allowed for the insertion of LTS II and scoring was done only for the first attempt. If LTS II could not be inserted in the 3 attempts, alternative airway devices were used. After successful LTS II insertion, anesthesia was maintained with sevoflurane 2%, 60% nitrous oxide, and 40% oxygen. Following insertion of LTS II, patients were ventilated manually until spontaneous respiration returned and this period was recorded as apnea time. Patients were ventilated with the synchronized intermittent mandatory ventilation mode until end of the operation.
Conditions of insertion of LTS II were assessed using 6 variables: mouth opening (1 = full, 2 = partial, and 3 = nil), gagging or coughing (1 = nil, 2 = slight, and 3 = gross), swallowing (1 = nil, 2 = slight, and 3 = gross), head or limb movements (1 = nil, 2 = slight, and 3 = gross), laryngospasm (1 = nil, 2 = partial, and 3 = complete), and ease of laryngeal tube insertion (1 = easy, 2 = difficult, and 3 = impossible). The insertion condition summed score for LTS II insertion was calculated by summing the insertion score for each patient and then totaling the score for all patients in the groups. Insertion time was recorded from the time of removal of the facemask to delivery of the first breath through the assigned airway device.
Systolic arterial pressure, mean arterial pressure (MAP), heart rate (HR), and peripheric oxygen saturation were measured before anesthesia induction (t0); immediately after induction (t1); immediately after insertion of LTS II (t2); and 3 minutes (t3), 5 minutes (t4), and 10 minutes (t5) after insertion of LTS II. The incidence of adverse events during anesthesia such as laryngospasm, bronchospasm, excessive secretion, hallucination, bradycardia, muscular rigidity, nausea, and vomiting were recorded.
At the end of the operation, LTS II was removed by deflating the cuff when patients were in deep anesthesia and had sufficient spontaneous respiration (tidal volume >5 mL/kg, number of breaths >12 breaths/min). After patients were taken to recovery unit, nausea, vomiting, hallucination, sore throat, hoarseness, and dysphagia were monitored within postoperative period of 1 to 24 hours.
A power analysis suggested a minimum 39 patients in each group with a mean (SD) change of 8 (13) mm Hg in MAP in Group P, and 8 (12) mm Hg in Group K (α = 0.05 and β = 0.20 [0.80 power]). We included 40 patients in both groups in the study. The SPSS version 16 software package (IBM SPSS Inc, Armonk, New York) was used in evaluation of the data. Normality of the distribution within the groups was measured by Shapiro-Wilk test. Differences between the groups were assessed by independent samples t test and Mann-Whitney U test. Repeating measurements of analysis of variance was performed in the comparison of the hemodynamic data within each group based on our hypothesis, and then Bonferroni test was carried out for multiple comparisons. Conditions of the insertion of LTS II were evaluated by Fisher exact χ2 test. Yates’ corrected χ2 test was used for ease of insertion of the laryngeal tube. P < 0.05 was considered to be statically significant.
Results
There were no differences in the demographic data of the groups (Table I).
Table I.
Demographic data, apnea time, and laryngeal tube-Suction II (LTS II) insertion time.
| Propofol group (n = 40) | Ketofol group (n = 40) | P | |
|---|---|---|---|
| Age (mean y [SD]) | 41.47 [12.86] | 37.75 [9.60] | 0.14 |
| Sex (women:men) | 18:22 | 23:17 | |
| Weight (mean kg [SD]) | 73.52 [12.61] | 70.35 [12.42] | 0.26 |
| Height (mean cm [SD]) | 168.15 [8.04] | 165.17 [7.83] | 0.09 |
| Apnea time (median sec [min-max]) | 385 (195-840) | 325.50 (60-840) | 0.07 |
| LTS II Insertion time (median sec [min-max]) | 20 (8-90) | 13.50 (8-45) | 0.11 |
Median (minimum-maximum) LTS II insertion time was similar for Group K (13.50 [8.0-45.0] seconds) and Group P (20.0 [8.0-90.0] seconds). Apnea duration was longer in Group P (385.0 [195.0-840.0] seconds) compared with Group K (325.50 [60.0-840.0] seconds), but this was not statically significant (Table I).
Number of attempts needed to insert LTS II, successful insertions, and correct position of LTS II was similar among the groups. However, when considering the LTS II insertion conditions summed scores, Group K was favourable compared with Group P (P < 0.05) (Table II).
Table II.
Insertion conditions of laryngeal tube-Suction II (LTS II).
| Propofol group (n = 40) | Ketofol group (n = 40) | |
|---|---|---|
| Attempts (n) | ||
| 1 | 37 | 38 |
| 2 | 3 | 1 |
| 3 | 0 | 1 |
| Mouth opening (n) | ||
| Full | 34 | 38 |
| Partial | 6 | 2 |
| Nil | 0 | 0 |
| Gagging or coughing (n) | ||
| Nil | 35 | 39 |
| Slight | 5 | 1 |
| Gross | 0 | 0 |
| Swallowing(n) | ||
| Nil | 34 | 38 |
| Slight | 6 | 2 |
| Gross | 0 | 0 |
| Movement (n) | ||
| Ni | 28 | 36 |
| Slight | 11 | 4 |
| Gross | 1 | 0 |
| Laryngospasm (n) | ||
| Nil | 40 | 36 |
| Partial | 0 | 4 |
| Complete | 0 | 0 |
| Ease of LTS II insertion (n) | ||
| Easy | 34 | 35 |
| Difficult | 6 | 4 |
| Impossible | 0 | 1 |
| Insertion condition summed score (median [range]) | 7 (6-10) | 6 (6-10)* |
P < 0.05 Group P compared with Group K.
The HR values were significantly lower at all measurement intervals in both groups compared with the baseline values (P < 0.05). There was no difference in HR between Group P and Group K (Figure 1).
Figure 1.
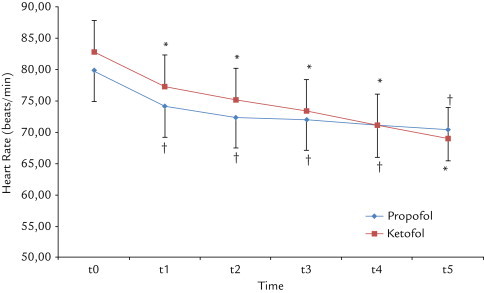
The changes in mean (SD) in heart rate with ketamine–propofol mixture (ketofol) and propofol use. t0 = before induction; t1 = immediately following induction; t2 = immediately following laryngeal tube-Suction II (LTS II) placement; t3 = 3 minutes after LTS II placement; t4 = 5 minutes after LTS II placement; t5 = 10 minutes after LTS II placement. *P < 0.01 compared with baseline values in Group P. †P < 0.001 compared with baseline values in Group K.
The MAP values were significantly lower at all measurement intervals in Group P compared with the baseline values (P < 0.05). In Group K, the MAP values were significantly lower at all measurement intervals compared with the baseline values except at t2 (P < 0.05).
There was a significant difference between Group P and Group K in terms of MAP values at t3 (P < 0.05) (Figure 2).
Figure 2.
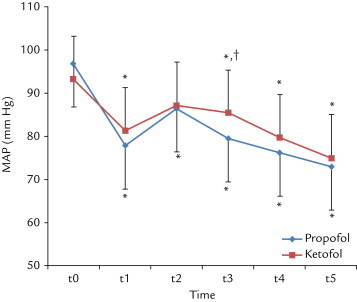
The changes in mean (SD) in mean arterial pressure (MAP) with ketamine–propofol mixture (ketofol) and propofol use. t0 = Before induction; t1 = immediately following induction; t2 = immediately following laryngeal tube-Suction II placement; t3 = 3 minutes after laryngeal tube-Suction II placement; t4 = 5 minutes after laryngeal tube-Suction II placement; t5 = 10 minutes after laryngeal tube-Suction II placement. *P < 0.01 compared with baseline values; †P < 0.05 between groups.
Adverse effects such as excessive secretion, hallucination, bradycardia, or muscular rigidity was not observed in any patients.
Nausea and vomiting were seen in 2 patients in Group P and 3 patients in Group K. Within the first 24 hours, sore throat was observed in 8 patients in Group P and 6 patients in Group K. Hoarseness was observed in 1 patient in Group P and 2 patients in Group K.
Discussion
In our study LTS II insertion condition summed score was better in the Group K compared with the Group P, and the hemodynamic changes were similar between the groups except for MAP values at t3.
LTS II is a supraglottic airway device used for spontaneous or controlled ventilation during general anesthesia.16 Success ratio, leakage pressure, number of times the position of the device was corrected, and ratio of insertion of the gastric tube were found to be similar in previous studies that compare LTS and LMA.17–19 Propofol is an induction agent used frequently to insert LT. Burlacu et al20 reported that the insertion of LT and LMA requires similar doses of propofol.
Sufficient anesthetic deepness and mouth openness is needed for correct insertion of supraglottic airway devices and prevent such complications as coughing, hiccups, swallowing, head, and extremity movements and laryngospasm.20,21 When propofol is used alone it cannot provide LMA insertion conditions, or high-dose propofol is needed to improve the insertion conditions. On the other hand, high doses of propofol cause cardiorespiratory depression.21,23 Addition of lidocaine, opioids, ketamine, or myorelaxants to propofol reduced the dose of propofol used and increased the success ratio of LMA insertion.22–24 Bein et al14 administered 2 mg/kg propofol and 0.1 mg/kg remifentanil for LTS insertion in the their study and reported that the success ratio was 100%. Likewise, in our study, success ratio of LTS II insertion was 100% in Group P. Goh et al24 reported success rate was 86.7% when ketamine was added to propofol. We found our LTS II insertion success ratio to be 97.5% in Group K. We think use of both ketamine and remifentanil in Group K was effective in this result.
Ketamine increases heart rate and arterial blood pressure by activation of the sympathetic nervous system. Clinical effects of propofol and ketamine seem to be complementary. When propofol and ketamine are administered in combination, doses of both agents decrease and unwanted effects are minimized.25 It was shown in studies of adult and pediatric patients that ketamine applied before propofol induction for LMA insertion preserved hemodynamic stability.24,26,27 Erdogan et al28 reported that ketofol provided Proseal LMA insertion conditions similar to those for propofol with a decreased ephedrine requirement in elderly patients. In our study, MAP maintained a higher level in Group K compared with Group P at all measurement times; however, this reached a statistically significant level at 3 minutes after insertion of LTS II.
Tosun et al29 reported that hemodynamic parameters were similar in both groups when 1 mg/kg ketamine or 1 µg/kg fentanil was added to propofol for sedation in children with burns. Likewise, in a study by Erden et al30 propofol-fentanil and propofol-fentanil-ketamine combinations provided similar hemodynamic stability in children. However, in our study hemodynamic stability was not maintained because we used a higher dose of propofol than was used in the previous study. Iwata et al31 could not achieve hemodynamic stability with 0.5 mg/kg or 1 mg/kg ketamine applied before 2 mg/kg propofol for anesthetic induction in the double-lumen tube application. Those authors pointed out that this might be due to use of fentanil and sevoflurane in both groups. Similarly, use of remifentanil before induction may have impaired the hemodynamic effects of ketofol in our study.
Akın et al32 showed that addition of ketamine to propofol protected respiration better than use of propofol alone for the purpose of sedation of children. Its depressive effect on the central respiratory system is minimal and it preserves response to carbon dioxide.33 In our study, apnea time was shorter in Group K than Group P, although the difference was not statistically significant.
Concerns regarding ketamine are increased secretion, delay of recovery, and emergence of reactions.34 We did not see these adverse effects in our study. Those adverse effects might be reduced by the presence of propofol.
There are some limitations to our study. First, we were unable to measure anesthetic depth, so LTS II insertion conditions may have been adversely affected and changes may have been observed in hemodynamic parameters. Second, use of remifentanil in both groups before induction may have impaired the hemodynamic effects of the agents.
Conclusions
In our study LTS II insertion condition summed score was better in Group K compared with Group P with minimal the hemodynamic changes. When these parameters are considered, ketofol might be used as an alternative to propofol for LTS II insertion in adults.
Conflicts of Interest
The authors have indicated that they have no conflicts of interest regarding the content of this article.
Acknowledgments
All authors contributed equally to the creation of this manuscript.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Claeys M.A., Gepts E., Camu F. Haemodynamic changes during anaesthesia induced and maintained with propofol. Br J Anaesth. 1988;60:3–9. doi: 10.1093/bja/60.1.3. [DOI] [PubMed] [Google Scholar]
- 2.Hug C.C., Jr, McLeskey C.H., Nahrwold M.L. Hemodynamic effects of propofol: data from over 25,000 patients. Anesth Analg. 1993;77(Suppl):S21–S29. [PubMed] [Google Scholar]
- 3.Scanlon P., Carey M., Power M., Kirby F. Patient response to laryngeal mask insertion after induction of anaesthesia with propofol or thiopentone. Can J Anaesth. 1993;40:816–818. doi: 10.1007/BF03009250. [DOI] [PubMed] [Google Scholar]
- 4.Arora S. Combining ketamine and propofol ("ketofol") for emergency department procedural sedation and analgesia: a review. West J Emerg Med. 2008;9:20–23. [PMC free article] [PubMed] [Google Scholar]
- 5.Guit J.B., Koning H.M., Coster M.L. Ketamine as analgesic for total intravenous anaesthesia with propofol. Anaesthesia. 1991;46:24–27. doi: 10.1111/j.1365-2044.1991.tb09308.x. [DOI] [PubMed] [Google Scholar]
- 6.Gray C., Swinhoe C.F., Myint Y., Mason D. Target controlled infusion of ketamine as analgesia for TIVA with propofol. Can J Anaesth. 1999;46:957–961. doi: 10.1007/BF03013131. [DOI] [PubMed] [Google Scholar]
- 7.Morse Z., Sano K., Kanri T. Effects of a propofol—ketamine admixture in human volunteers. Pac Health Dialog. 2003;10:51–54. [PubMed] [Google Scholar]
- 8.Trissel L.A., Gilbert D.L., Martinez J.F. Compatibility of propofol injectable emulsion with selected drugs during simulated Y-site administration. Am J Health Syst Pharm. 1997;54:1287–1292. doi: 10.1093/ajhp/54.11.1287. [DOI] [PubMed] [Google Scholar]
- 9.Andolfatto G., Willman E. A prospective case series of single-syringe ketamine-propofol (ketofol) for emergency department procedural sedation and analgesia in adults. Acad Emerg Med. 2011;18:237–245. doi: 10.1111/j.1553-2712.2011.01010.x. [DOI] [PubMed] [Google Scholar]
- 10.Weatherall A., Venclovas R. Experience with a propofol-ketamine mixture for sedation during pediatric orthopedic surgery. Paediatr Anaesth. 2010;20:1009–1016. doi: 10.1111/j.1460-9592.2010.03420.x. [DOI] [PubMed] [Google Scholar]
- 11.Erdogan Kayhan G., Yucel A., Colak Y.Z. Ketofol (mixture of ketamine and propofol) administration in electroconvulsive therapy. Anaesth Intensive Care. 2012;40:305–310. doi: 10.1177/0310057X1204000214. [DOI] [PubMed] [Google Scholar]
- 12.Rapeport D.A., Martyr J.W., Wang L.P. The use of "ketofol" (ketamine-propofol admixture) infusion in conjunction with regional anaesthesia. Anaesth Intensive Care. 2009;37:121–123. doi: 10.1177/0310057X0903700108. [DOI] [PubMed] [Google Scholar]
- 13.Mihai R., Knottenbelt G., Cook T.M. Evaluation of the revised laryngeal tube suction: the laryngeal tube-Suction II in 100 patients. Br J Anaesth. 2007;99:734–739. doi: 10.1093/bja/aem260. [DOI] [PubMed] [Google Scholar]
- 14.Bein B., Carstensen S., Gleim M. A comparison of the proseal laryngeal mask airway, the laryngeal tube S and the oesophageal-tracheal combitube during routine surgical procedures. Eur J Anaesthesiol. 2005;22:341–346. doi: 10.1017/s026502150500058x. [DOI] [PubMed] [Google Scholar]
- 15.Asai T., Shingu K., Cook T. Use of the laryngeal tube in 100 patients. Acta Anaesthesiol Scand. 2003;47:828–832. doi: 10.1034/j.1399-6576.2003.00183.x. [DOI] [PubMed] [Google Scholar]
- 16.Asai T., Shingu K. The laryngeal tube. Br J Anaesth. 2005;95:729–736. doi: 10.1093/bja/aei269. [DOI] [PubMed] [Google Scholar]
- 17.Gaitini L.A., Vaida S.J., Somri M. A randomized controlled trial comparing the ProSeal Laryngeal Mask Airway with the Laryngeal Tube Suction in mechanically ventilated patients. Anesthesiology. 2004;101:316–320. doi: 10.1097/00000542-200408000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Esa K., Azarinah I., Muhammad M. A comparison between Laryngeal Tube Suction II Airway and Proseal Laryngeal Mask Airway in laparascopic surgery. Med J Malaysia. 2011;66:182–186. [PubMed] [Google Scholar]
- 19.Roth H., Genzwuerker H.V., Rothhaas A. The ProSeal laryngeal mask airway and the laryngeal tube suction for ventilation in gynaecological patients undergoing laparoscopic surgery. Eur J Anaesthesiol. 2005;22:117–122. doi: 10.1017/s0265021505000220. [DOI] [PubMed] [Google Scholar]
- 20.Burlacu C.L., Gaskin P., Fernandes A. A comparison of the insertion characteristics of the laryngeal tube and the Laryngeal Mask Airway: a study of the ED50 propofol requirements. Anaesthesia. 2006;61:229–233. doi: 10.1111/j.1365-2044.2005.04442.x. [DOI] [PubMed] [Google Scholar]
- 21.Singh R., Arora M., Vajifdar H. Randomized double-blind comparison of ketamine-propofol and fentanyl-propofol for the insertion of laryngeal mask airway in children. J Anaesthesiol Clin Pharmacol. 2011;27:91–96. [PMC free article] [PubMed] [Google Scholar]
- 22.Stoneham M.D., Bree S.E., Sneyd J.R. Facilitation of laryngeal mask insertion. Effects of lignocaine given intravenously before induction with propofol. Anaesthesia. 1995;50:464–466. doi: 10.1111/j.1365-2044.1995.tb06007.x. [DOI] [PubMed] [Google Scholar]
- 23.Cheam E.W., Chui P.T. Randomised double-blind comparison of fentanyl, mivacurium or placebo to facilitate laryngeal mask airway insertion. Anaesthesia. 2000;55:32332–32336. doi: 10.1046/j.1365-2044.2000.01214.x. [DOI] [PubMed] [Google Scholar]
- 24.Goh P.K., Chiu C.L., Wang C.Y. et tal. Randomized double-blind comparison of ketamine-propofol, fentanyl-propofol and propofol-saline on haemodynamics and laryngeal mask airway insertion conditions. Anaesth Intensive Care. 2005;33:223–228. doi: 10.1177/0310057X0503300211. [DOI] [PubMed] [Google Scholar]
- 25.Okuyama K., Inomata S., Okubo N., Watanabe I. Pretreatment with small-dose ketamine reduces predicted effect-site concentration of propofol required for loss of consciousness and laryngeal mask airway insertion in women. J Clin Anesth. 2011;23:113–118. doi: 10.1016/j.jclinane.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Gupta A., Kaur S., Attri J.P., Saini N. Comparative evaluation of ketamine-propofol, fentanyl-propofol and butorphanol-propofol on haemodynamics and laryngeal mask airway insertion conditions. J Anaesthesiol Clin Pharmacol. 2011;27:74–78. [PMC free article] [PubMed] [Google Scholar]
- 27.Begec Z., Demirbilek S., Onal D. Ketamine or alfentanil administration prior to propofol anaesthesia: the effects on ProSeal laryngeal mask airway insertion conditions and haemodynamic changes in children. Anaesthesia. 2009;64:282–286. doi: 10.1111/j.1365-2044.2008.05782.x. [DOI] [PubMed] [Google Scholar]
- 28.Erdogan M.A., Begec Z., Aydogan M.S. Comparison of effects of propofol and ketamine-propofol mixture (ketofol) on laryngeal mask airway insertion conditions and hemodynamics in elderly patients: a randomized, prospective, double-blind trial. J Anesth. 2013;27:12–17. doi: 10.1007/s00540-012-1484-5. [DOI] [PubMed] [Google Scholar]
- 29.Tosun Z., Esmaoglu A., Coruh A. Propofol-ketamine vs propofol-fentanyl combinations for deep sedation and analgesia in pediatric patients undergoing burn dressing changes. Paediatr Anaesth. 2008;18:43–47. doi: 10.1111/j.1460-9592.2007.02380.x. [DOI] [PubMed] [Google Scholar]
- 30.Erden I.A., Pamuk A.G., Akinci S.B. Comparison of propofol-fentanyl with propofol-fentanyl-ketamine combination in pediatric patients undergoing interventional radiology procedures. Paediatr Anaesth. 2009;19:500–506. doi: 10.1111/j.1460-9592.2009.02971.x. [DOI] [PubMed] [Google Scholar]
- 31.Iwata M., Inoue S., Kawaguchi M. Ketamine eliminates propofol pain but does not affect hemodynamics during induction with double-lumen tubes. J Anesth. 2010;24:31–37. doi: 10.1007/s00540-009-0833-5. [DOI] [PubMed] [Google Scholar]
- 32.Akin A., Esmaoglu A., Tosun Z. Comparison of propofol with propofol-ketamine combination in pediatric patients undergoing auditory brainstem response testing. Int J Pediatr Otorhinolaryngol. 2005;69:1541–1545. doi: 10.1016/j.ijporl.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 33.Frizelle H.P., Duranteau J., Samii K. A comparison of propofol with a propofol-ketamine combination for sedation during spinal anesthesia. Anesth Analg. 1997;84:1318–1322. doi: 10.1097/00000539-199706000-00026. [DOI] [PubMed] [Google Scholar]
- 34.Tomatir E., Atalay H., Gurses E. Effects of low dose ketamine before induction on propofol anesthesia for pediatric magnetic resonance imaging. Paediatr Anaesth. 2004;14:845–850. doi: 10.1111/j.1460-9592.2004.01303.x. [DOI] [PubMed] [Google Scholar]


