Abstract
Background
Diabetic nephropathy (DN) is one of the most serious microvascular complications of diabetes and the leading cause of end-stage renal failure. However, the treatment of DN is still a problem in the world. Inflammatory process plays a critical role in the development of DN. Therefore, anti-inflammatory treatment of DN is worth exploring now and in the future.
Objective
The study aimed to evaluate the impact of ursolic acid (UA) on renal function in streptozotocin-induced diabetes.
Methods
Rats with streptozotocin-induced diabetes were treated with UA for 16 weeks. After 16 weeks, urine albumin excretion, serum creatinine, and blood urea nitrogen were measured. In addition, renal oxidative stress level, nuclear factor kappa-B (NF-κB) activity, P-selectin expression, and kidney histopathologic changes were evaluated.
Results
Sixteen weeks following streptozotocin injection, the rats produced significant alteration in renal function and increased oxidative stress, NF-κB activity, and P-selectin expression in the kidneys. Interestingly, UA significantly prevented biochemical and histopathologic changes in the kidneys associated with diabetes. Compared with untreated diabetic rats, UA treatment lowered urine albumin excretion, renal oxidative stress level, NF-κB activity, and P-selectin expression. Moreover, UA treatment also improved renal histopathologic changes in rats with diabetes.
Conclusions
UA treatment exhibited a protective effect on kidneys in diabetic rats, implying that UA could be a potential treatment for diabetic nephropathy.
Key Words: diabetic nephropathy, nuclear factor kappa-B, oxidative stress, ursolic acid
Introduction
Diabetic nephropathy (DN), 1 of the most serious microvascular complications of diabetes, is the leading cause of end-stage renal failure. The pathologic changes of DN are characterized by early glomerular hypertrophy and later glomerulosclerosis and tubulointerstitial fibrosis, which is caused by the accumulation of extracellular matrix components in the glomerular mesangium and tubulointerstitium. However, the intimate mechanisms leading to the development and progression of renal injury in diabetes have not been fully elucidated. Growing evidence demonstrates that the inflammatory process plays a critical role in the development of DN.1–3 Different inflammatory molecules, including chemokines, adhesion molecules, and proinflammatory cytokines, may be critical factors involved in DN.
The ubiquitous transcription factor nuclear factor-κ-B (NF-κB) is 1 of the cross-talk points of multiple signal transduction pathways that plays a key role in the regulation of transcription and the expression of many genes involved in inflammatory responses.4,5 An increase in the nuclear translocation of NF-κB and subsequently coordinated expression of gene products may play an important role in the pathogenesis of DN.6 Therefore, inhibition of NF-κB activation may be beneficial for the treatment of DN.
Ursolic acid (UA) is a pentacyclic triterpenoid found in many herbs and spices like rosemary and thyme, but also in fruits, including apples, cranberries, and blueberries. UA has been primarily studied as an anticancer and anti-inflammatory compound, a series of evidences indicated UA was a potent inhibitor of NF-κB.7,8 In addition, dietary supplementation with UA was found to improve glycemic control and lipid profiles, to decrease lipid accumulation in the liver, and increase antioxidant enzyme activity in rodent models of metabolic disease.9 Therefore, we sought to clarify that UA prevents the progression of DN in a rat model. Involvement of inflammatory mediators through the NF-κB pathway in the pathologic effects was also analyzed.
Materials and Methods
Animals and the model for diabetes
The animals used were male Wistar rats (weighing 160 [14] g). Diabetes was induced according intraperitoneal injections of streptozotocin (STZ) (55 mg/kg in citrate buffer; pH 4.2). Three days later, STZ-treated rats in which blood glucose concentration was <16.7 mmol/L were excluded from the experiment.
Design of the study
The animals were randomized into 3 groups: the control group, untreated diabetic group, and UA-treated diabetic group. Control group rats and untreated diabetic rats were fed standard rat chow diets, UA-treated diabetic rats were given diets supplemented with 0.2% UA. Sixteen weeks later, all rats were put to death. The day before anesthetizing, a 24-hour urine collected was done to determine urine albumin excretion. During anesthetizing, blood was taken by puncture of the right heart to determine blood glucose and serum total cholesterol, triglyceride, creatinine, and blood urea nitrogen levels. Both the kidneys were isolated; the left kidney was deep frozen till further enzymatic analysis, whereas the right kidney was stored in 10% formalin for histologic sectioning.
Laboratory determinations
Blood glucose was measured using a Bayer glucometer (Bayer HealthCare Pharmaceuticals, Montville, NJ). Urine albumin was measured by a competitive ELISA assay according to the manufacturer’s instruction (Exocell, Philadelphia, Pa). Serum total cholesterol, triglyceride, creatinine, and urea nitrogen were determined with a Technicon RA-XT biochemical analyzer (Technicon Corporation, Tarrytown, NY).
Measurement of 8-hydroxy-2’-deoxyguanosine (8-OhdG) levels by immunohistochemistry
Immunostaining analysis of 8-OHdG was performed to evaluate oxidative DNA injury of kidneys. The sections were incubated with anti-8-OHdG mouse monoclonal antibody, probed with antimouse immunoglobulin G antibody labeled with peroxidase. The sites of peroxidase were visualized with diaminobenzidine.
Electrophoretic mobility shift assays (EMSA) for NF-κB activity
To determine NF-κB activation, we performed EMSA. The specificity of binding was examined by competition with an unlabeled oligonucleotide. For supershift assays, nuclear extracts prepared from kidney tissue were analyzed by EMSA. The NF-κB activation was assayed using an EMSA kit (Viagene Biotech, Ningbo, China) according to the manufacturer’s protocol. The consensus NF-κB oligonucleotides included in the kit were 5‘-AGT TGA GGG GAC TTT CCC AGG C-3’, The dried gels were visualized and analyzed with Labworks 4.5 software (UVP Inc, Upland, CA).
Reverse transcription-polymerase chain reaction for P-selectin mRNA expression
Total RNA was extracted using Trizol (Invitrogen) according to the manufacturer’s instructions. Three micrograms of total RNA was used to prepare cDNA by Superscript II RNase H reverse transcriptase (Life Technologies, Carlsbad, Calif). cDNA was amplified by polymerase chain reaction using Taq DNA polymerase (MBI Fermentas, Waltham, MA) and the following gene-specific primers (synthesized by Shanghai Bioasia Biotechnology Co, Shanghai, China): sense 5’– TCC AGT AAC ACA GACAGT GC – 3’, and antisense 5’– GAG GTG ATG TCC AGG AAG AA – 3’; β-actin (as an internal standard), sense 5’– ATC TGG CAC CAC ACC TTC TA’, and antisense 5’– CTCCTTAAT GTC ACG CAC GA – 3’. Amplification was carried out using the Biometra PCR System (Goettingen, Germany) as follows: polymerase chain reaction mixture was incubated at 95°C for 5 minutes, followed by 28 cycles at 94°C for 30 seconds, 58°C for 30 seconds, and 72°C for 1 minute, with a final extension at 72°C for 10 minutes. Then aliquots were run on 2% agarose gels containing ethidiumn bromide. The density of the bands photographed under ultraviolet illumination was analyzed by computer with Gel-Pro Analyzer 4.0 (Media Cybernetics Co., Rockville, MD). The corrected relative value for the gene was expressed as a ratio of P-selectin to β-actin.
Western blot for P-selectin protein expression
Thirty micrograms protein from kidney were denatured in boiling water for 15 minutes, separated by SDS-PAGE gel and then transferred onto nitrocellulose membranes. The blots were blocked with 5% nonfat dry milk, followed by incubation for 1 hour with rabbit anti-P-selectin at a dilution of 1:200. After being washed with Tris-buffered saline with Tween, the blots were then incubated with horseradish peroxidase conjugated secondary antibody (1:2000 dilution) for 1 hour. The membranes were visualized with an enhanced chemiluminescent system (KPL, Gaithersburg, MD) after 3 washes using Tris-buffered saline with Tween.
Histopathologic examination
The right kidney was isolated immediately after sacrificing each animal and washed with ice-cold saline. It was then fixed in a 10% neutral buffered formalin solution, embedded in paraffin, and used for histopathologic examination. Three- micrometers-thick sections were cut, deparaffinized, hydrated, and stained with periodic acid-Schiff. The renal sections were examined in a blinded fashion by pathologists in our hospital.
Statistical analysis
All data were presented as means [SD]. Comparisons were performed using a 1-way ANOVA, followed when appropriate by between-group comparisons using the Student-Neuman-Keuls multiple comparison test. P < 0.05 was considered statistically significant.
Results
The results of laboratory determinations
To examine if dietary supplementation of UA attenuates renal injury in diabetic rats, we fed STZ-treated rats a diet supplemented with 0.2% UA for16 weeks. Comparing with untreated diabetic rats, UA treatment lowered blood glucose levels and resulted in a decrease in plasma lipid profiles to different extents, although these levels were still higher than those in the normal control group. When compared with the control group, urine albumin excretion in untreated diabetic rats was significantly elevated and UA therapy dramatically decreased urine albumin excretion in diabetic rats. The serum creatinine and blood urea nitrogen concentrations did not differ significantly throughout the experimental period among three groups. (Table)
Table.
Laboratory determinations of control rats (Group C), untreated diabetic rats (Group D), and diabetic rats treated with ursolic acid (Group D+UA) (n = 6).
| Determinant |
Group C |
Group D |
Group D+UA |
|---|---|---|---|
| Mean [SD] | |||
| Glucose (mmol/L) | 4.8 [0.5] | 18.3 [3.6]* | 15.9 [3.6]* |
| Total cholesterol (mmol/L) | 1.92 [0.18] | 2.83 [0.15] | 2.62 [0.27] |
| Triglycerides (mmol/L) | 0.73 [0.07] | 1.02 [0.17]* | 0.97 [0.18]* |
| Serum creatinine (μmol/L) | 61.73 [10.52] | 86.33 [10.22] | 69.80 [12.86] |
| Blood urea nitrogen (mmol/L) | 5.22 [0.93] | 7.23 [0.52] | 6.81 [0.79] |
| Urine albumin excretion (μg/min) | 22.33 [10.09] | 68.96 [14.1]* | 34.90 [8.19]† |
P < 0.05 versus group C.
P < 0.05 versus group D.
Effect of UA on oxidative DNA injury in kidneys of diabetic rats
To examine if dietary UA affect oxidative stress in kidneys of diabetic rats, we detected renal 8-OHdG level. Immunohistochemistry analysis revealed that rats receiving dietary UA showed significantly reduced 8-OHdG formation both in glomerular and in tubulointerstitial area, as shown in Figure 1.
Figure 1.
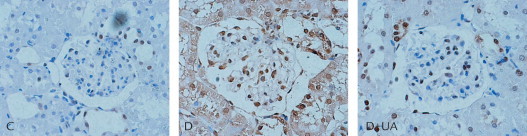
Renal renal 8-hydroxy-2’-deoxyguanosine level in rats of each group. Ursolic acid (UA) treatment showed significantly reduced 8-hydroxy-2’-deoxyguanosine formation both in glomerular and in tubulointerstitial area. C = normal kidney sections; D = kidney sections of untreated diabetic rat; D+UA = kidney sections of UA-treated diabetic rat.
Effect of UA on NF-κB activity and P-selectin expression in kidneys of diabetic rats
As shown in Figure 2, renal NF-κB activity in untreated diabetic rats was significantly increased compared with the control group, UA administration resulted in a significant decrease in renal NF-κB activity after 16 weeks of therapy. Because NF-κB played a key role in the regulation of transcription and the expression of many inflammatory factors, the decrease in renal NF-κB activity would be accompanied by a reduction of inflammatory factors expression. Therefore, we examined if dietary UA affects P-selectin expression. As shown in Figure 3, UA supplementation resulted in a reduction of P-selectin mRNA and protein expression. In summary, UA protected diabetic rats against the development of kidney lesions, possibly by inhibiting NF-κB activation and then decreasing inflammation factor expression.
Figure 2.
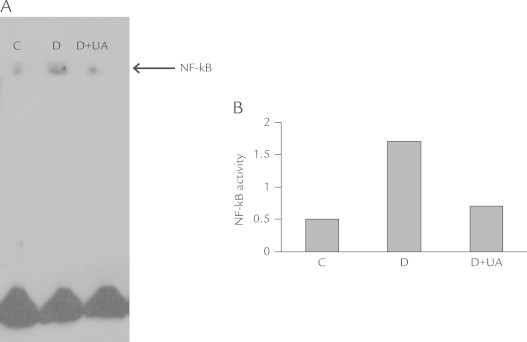
The nuclear factor-κB (NF-κB) activity of rat kidneys in each group. Ursolic acid (UA) administration resulted in a significant decrease in renal NF-κB activity after 16 weeks of the therapy compared with untreated diabetic rats. C = normal control group; D = diabetic group; D+UA = UA-treated diabetic group. (A) Source image. (B) The result of quantitative analysis.
Figure 3.
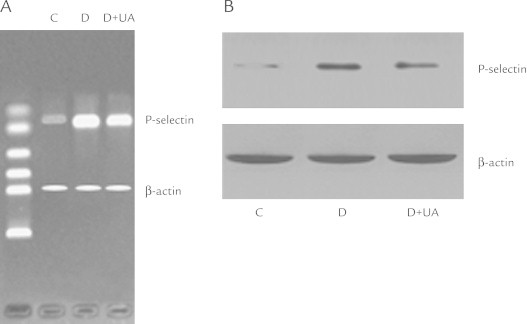
(A) P-selectin mRNA expression of rat kidney in each group. (B) P-selectin protein expression of rat kidney in each group. Ursolic acid (UA) supplement resulted in a reduction of P-selectin mRNA and protein expression. C = normal control group; D= diabetic group; D+UA = UA-treated diabetic group.
Effect of UA on renal pathologic morphology
Examined under a microscope, the structure of glomerulus and tubulointerstitium in kidney sections from the control rats appeared in order. In contrast, the glomerular and tubular volume from the diabetes group was increased, with diffuse matrix expansion. The periodic acid-Schiff-positive area within glomeruli was noticeably augmented. Treatment with UA showed the ability to lower the increase in glomerular volume and the periodic acid-Schiff-positive area seen in the rats with diabetes (Figure 4).
Figure 4.
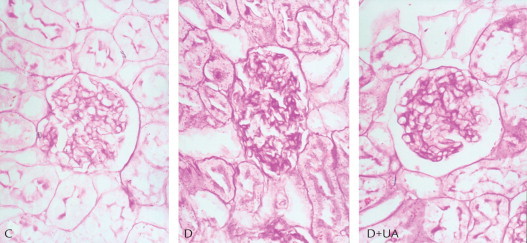
Periodic acid-Schiff-stained sections of rat kidneys (× 400): Ursolic acid (UA) treatment improved renal histopathologic changes in diabetes. C = normal kidney sections; D = kidney sections of untreated diabetic rat; D+UA = kidney sections of UA-treated diabetic rat.
Discussion
In our study, we examined if dietary supplementation with UA protects kidney injury associated with diabetes. We show that UA has the ability to protect the kidneys in rats with diabetes by lowering urine albumin excretion and returning renal tissue impairment. It is not possible that glucose lowering or lipid lowering have contributed to renal protective effect in UA-treated diabetic rats because neither blood glucose level nor blood lipid level was reduced significantly by UA treatment. Instead, our data suggest anti-inflammation may be explained for the renal protection in diabetes by dietary supplementation with UA.
Traditionally, DN has been considered a nonimmune, degenerative disease. However, more recent reports have suggested that inflammation may underlie disease progression in DN. The activation of NF-κB-linked regulatory pathways generally underlies inflammatory processes, and an increase in the nuclear translocation of NF-κB has been demonstrated in human DN.10 Studies show that in the diabetic state, many factors, such as advanced glycation end products, shear stress, and oxidative stress, contribute to NF-κB activation.11 Activated NF-κB promotes the expression of a number of genes involved in inflammation, such as cytokines and adhesion molecules. All these products play a vital role in the pathogenesis of renal diseases.12 Therefore, inhibition of NF-κB activation may be benefit for the treatment of DN. In our study, we observed that UA significantly suppressed NF-κB signaling in kidneys of diabetic rats. The result was the same as that UA inhibits NF-κB activation in another disease model.13 This finding suggests that UA might be useful in the prevention and inhibition of nephrologic complication in diabetes by inhibiting NF-κB activation.
To better understand the benefit of NF-κB inhibition for DN, we investigated the expression of endothelial adhesion molecules in the downstream pathway of NF-κB such as P-selectin in diabetic rat kidneys. There is accumulating evidence that endothelial adhesion molecules play a pivotal role in the genesis of diabetic vascular complications.14,15 P-selectin, a member of adhesion molecular family, is a marker of platelet activation, and is also associated with endothelial dysfunction. The elevation of P-selectin level has been previous reported in patients with DN.16 In our study, the expression renal P-selectin in diabetic rats was higher than healthy control rats, and UA treatment markedly inhibited P-selectin expression. This result further shows an anti-inflammatory effect of UA.
Growing evidence indicates that oxidative stress is a critical pathologic state that leads to most of the complications seen in diabetes. We and others have reported that increased generation of reactive oxygen species and decreased activities of key antioxidant enzymes resulting in oxidative stress in kidneys of rats with STZ-induced diabetes. Here, we observed the influence of UA on the renal oxidative stress state in diabetes. The results showed that UA treatment decreased oxidative injury in the kidneys of diabetic rats, indicating the antioxidant effect of UA.
Conclusions
Treatment with UA in rats with STZ-induced diabetes brought about significantly lowered urine albumin excretion and improved kidney tissue injury. We have shown that UA treatment inhibits NF-κB activation and decreases the expression of P-selectin in the kidneys of diabetic rats. We also showed that UA had antioxidant effects. These findings suggest the renal protective effect of UA by anti-inflammation.
Conflicts of Interest
The authors have indicated that they have no conflicts of interest regarding the content of this article.
Acknowledgments
Dr. Ling contributed to the literature search, study design, data interpretation, and writing. Ms. Jinping and Dr. Xia provided data collection and analysis. Mr. Renyong provided consultation.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommerical-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Wolf G., Ruster C. The role of chemokines and chemokine receptors in diabetic nephropathy. Front Biosci. 2008;13:944–955. doi: 10.2741/2734. [DOI] [PubMed] [Google Scholar]
- 2.Fornoni A., Ijaz A., Tejada T. Role of inflammation in diabetic nephropathy. Curr Diabet Rev. 2008;4:10–17. doi: 10.2174/157339908783502361. [DOI] [PubMed] [Google Scholar]
- 3.Mesallamy H.E., Ahmed H.H., Bassyouni A.A. Clinical significance of inflammatory and fibrogenic cytokines in diabetic nephropathy. Clin Biochem. 2012;45:646–650. doi: 10.1016/j.clinbiochem.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 4.Nakajima K., Matsushita Y., Tohyama Y. Differential suppression of endotoxin-inducible inflammatory cytokines by nuclear factor kappa B (NFκB) inhibitor in rat microglia. Neurosci Lett. 2006;401:199–202. doi: 10.1016/j.neulet.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 5.Rial N.S., Choi K., Nguyen T. Nuclear factor kappa B (NF-κB): a novel cause for diabetes, coronary artery disease and cancer initiation and promotion? Med Hypoth. 2012;78:29–32. doi: 10.1016/j.mehy.2011.09.034. [DOI] [PubMed] [Google Scholar]
- 6.Chen L., Zhang J., Zhang Y. Improvement of inflammatory responses associated with NF-kB pathway in kidneys from diabetic rats. Inflamm Res. 2008;57:199–204. doi: 10.1007/s00011-006-6190-z. [DOI] [PubMed] [Google Scholar]
- 7.Li Y., Xing D., Chen Q., Chen W.R. Enhancement of chemotherapeutic agent-induced apoptosis by inhibition of NF-kappaB using ursolic acid. Int J Cancer. 2010;127:462–473. doi: 10.1002/ijc.25044. [DOI] [PubMed] [Google Scholar]
- 8.Manu K.A., Kuttan G. Ursolic acid induces apoptosis by activating p53 and caspase-3 gene expressions and suppressing NF-kappaB mediated activation of bcl-2 in B16F-10 melanoma cells. Int Immunopharmacol. 2008;8:974–981. doi: 10.1016/j.intimp.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Jayaprakasam B., Olson L.K., Schutzki R.E. Amelioration of obesity and glucose intolerance in high-fat-fed C57BL/6 mice by anthocyanins and ursolic acid in Cornelian cherry (Cornus mas) J Agric Food Chem. 2006;54:243–248. doi: 10.1021/jf0520342. [DOI] [PubMed] [Google Scholar]
- 10.Kuhad A., Chopra K. Attenuation of diabetic nephropathy by tocotrienol: involvement of NF-kB signaling pathway. Life Sci. 2009;84:296–301. doi: 10.1016/j.lfs.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 11.Edwards J.L., Vincent A.M., Cheng H.T. Diabetic neuropathy: mechanisms to management. Pharmacol Ther. 2008;120:1–34. doi: 10.1016/j.pharmthera.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu W.H., Zhang X.Y., Liu P.Q. Effects of berberine on matrix accumulation and NF-kappa B signal pathway in alloxan-induced diabetic mice with renal injury. Eur J Pharmacol. 2010;638:150–155. doi: 10.1016/j.ejphar.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 13.Shao J.W., Dai Y.C., Xue J.P. In vitro and in vivo anticancer activity evaluation of ursolic acid derivatives. Eur J Med Chem. 2011;46:2652–2661. doi: 10.1016/j.ejmech.2011.03.050. [DOI] [PubMed] [Google Scholar]
- 14.Tetsuya H.a.r.a., Tatsuro I.s.h.i.d.a., Husni M. Endothelial cell-selective adhesion molecule regulates albuminuria in diabetic nephropathy. Microvasc Res. 2009;77:348–355. doi: 10.1016/j.mvr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Lu J.X., Randell E., Han Y.C. Increased plasma methylglyoxal level, inflammation, and vascular endothelial dysfunction in diabetic nephropathy. Clin Biochem. 2011;44:307–311. doi: 10.1016/j.clinbiochem.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Wang F., Xing T., Wang N.S. Clinical significance of plasma CD146 and P-selectin in patients with type 2 diabetic nephropathy. Cytokine. 2012;57:127–129. doi: 10.1016/j.cyto.2011.10.010. [DOI] [PubMed] [Google Scholar]


