Abstract
Background
The addition of opioids to local anesthetics contributes to the quality of spinal anesthesia and postoperative analgesia.
Objective
In our prospective, randomized, double-blind, controlled study, our aim was to compare the effect of low-dose sufentanil plus levobupivacaine or a fentanyl plus levobupivacaine mixture on anesthesia quality, block characteristics, newborn and mother well-being, surgeon satisfaction, and duration of postoperative analgesia.
Methods
Ninety-three patients were randomized into 3 groups (n = 31). Patients in Group C received 0.5% levobupivacaine (2.2 ± 0.2 mL), Group S received 2.5 µg sufentanil plus 0.5% levobupivacaine (2.2 ± 0.2 mL), and Group F received 10 µg fentanyl plus 0.5% levobupivacaine (2.2 ± 0.2 mL) intrathecally completed to a volume of 3 mL with the addition of saline in all groups. Patients’ demographics, sensory and motor block characteristics, hemodynamics, Apgar scores, umbilical blood gas values, maternal side effects, surgeon satisfaction score, time to first analgesia requirement, and additional analgesic use within 24 hours were recorded.
Results
In Group S and Group F, target levels of sensory and motor block were achieved more rapidly (P < 0.001). The hemodynamic values were lower (P < 0.05), and the duration of sensory blockade and the time of first analgesic requirement were longer (P < 0.001) in Group S. Additional analgesic requirement during first 24-hour period was lowest in Group S, and highest in Group C (P < 0.001). Apgar scores and umbilical blood gas samples were similar between groups. Postoperative pruritus was more frequent in Group S (P < 0.001) and surgeon satisfaction score was significantly lower in Group C (P = 0.003).
Conclusions
We suggest that the addition of sufentanil and fentanyl to intrathecal levobupivacaine during caesarean section surgery is more effective than the administration of levobupivacaine alone. The addition of sufentanil to levobupivacaine allowed rapid onset time for sensory and motor block levels. It also extended the duration of postoperative analgesia and led to a decrease in total analgesic requirement. ClinicalTrials.gov identifier: NCT01858090.
Key Words: caesarean section, fentanyl, levobupivacaine, local anesthetics, spinal anesthesia, sufentanil
Introduction
Spinal anesthesia is a preferred method in elective and emergency caesarean section surgeries.1,2 Levobupivacaine is a frequently used local anesthetic (LA) due to its longer sensory block, lower cardiac toxicity, and shorter motor block properties.3–5 The addition of opioids to LA spinal anesthesia increases anesthesia quality and ensures effective analgesia during intraoperative and early postoperative periods.6–9 For this reason, the strongly lipophilic drugs sufentanil and fentanyl are preferred during caesarean section surgeries.7,8,10 However, these agents may cause dose-dependent side effects on fetal heart rate in newborns such as bradycardia, as well as various side effects in the mother such as maternal hypotension, pruritus, nausea, vomiting, and respiratory depression.7,11,12 The doses of fentanyl and sufentanil that would provide effective analgesia and minimum side effects were reported to be 10 to 25 µg and 2.5 to 5 µg, respectively.6–8,10,12–15 Furthermore, the equivalent dose range between fentanyl and sufentanil during intrathecal administration was identified as 3.4 to 4.5:1.11,12
Although there are studies in the literature comparing the effectiveness of fentanyl and sufentanil added as adjuvants to 0.5% levobupivacaine during spinal anesthesia, there is no consensus regarding which agents would be more effective. In our prospective, randomized, double-blind, controlled study, our aim was to compare the effect of adding low-dose fentanyl (10 µg) or sufentanil (2.5 µg) to 0.5% levobupivacaine (2.2 ± 0.2 mL) on anesthesia quality, block characteristics, side effects, duration of postoperative analgesia, and surgeon satisfaction score.
Patients and Methods
Following the approval by the Baskent University Ethics Committee (No. KA08/48) and receipt of written informed consents from patients, 93 pregnant women older than age 18 years without fetal distress/anomaly (ie, gestational pregnancy age ≥36 weeks, height ≥155 cm, weight ≤110 kg, and fetal weight ≥2500 g) and with American Society of Anesthesiologists physical status of I to II who were undergoing elective caesarean section were enrolled in the study. Patients with allergies to any local anesthetic, with a history of hypersensitivity and anaphylactic reactions, who were taken to emergency surgery, and who had pre-eclampsia were excluded.
Patients were randomized by a computer into 3 groups. The demographic data and gestational age of patients were recorded. No premedications were administered. Following monitoring with noninvasive blood pressure, electrocardiogram, and pulse oxymeter in all patients, Ringer’s lactate solution (15 mL/kg) was administered for 10 to 15 minutes. Spinal anesthesia was performed in the sitting position with midline approach at the L3–L4 intervertebral space by an anesthesiologist blinded to the drug injected. The drug syringes were prepared before injection by another anesthesiologist who was not involved in the study. Intraoperative and postoperative assessments were performed by an anesthesiologist blinded to patient allocations and study drugs. The study drugs included 2.2 mL (11 mg) levobupivacaine (Chirocaine; Abbott Laboratories, Abbott Park, Illinois) administered to patients with height <163 cm, whereas 2.4 mL (12 mg) levobupivacaine was administered to patients with height ≥163 cm. In addition, 2.2 ± 0.2 mL 0.5% levobupivacaine plus 10 µg fentanyl (Fentanyl citrate ampule 50 µg/mL; Johnson & Johnson, New Brunswick, New Jersey) was administered to Group F and 2.2 ± 0.2 mL levobupivacaine plus 2.5 µg sufentanil (Sufenta; Johnson & Johnson, New Brunswick, New Jersey) was administered to Group S at rate of 3 mL/30 sec and completed to a volume of 3 mL with the addition of saline in all groups. Following intrathecal administration, patients were placed in the supine position and their heads were slightly elevated. Patients were then directed to a left lateral position with a 15˚ to 20° angle to prevent aortocaval compression, and oxygen (at 2–4 L/min) was provided with a facemask.
Sensory block was evaluated every 2 minutes with a pinprick test; motor block was evaluated with the Bromage scale (0 = no motor loss, 1 = inability to flex the hip, 2 = inability to flex the knee, and 3 = inability to flex the ankle). Onset times for sensory and motor blocks were recorded. Surgical intervention was initiated when block reached the T5 level. If sensory block did not achieve the T5 level within 20 minutes, general anesthesia was administered.
Onset time of sensory block at the L1-T10 level, the highest level of sensory block, duration of sensory block, time for 2-segment regression of sensory block through the T10 level, and the onset and duration of motor block were recorded.
Systolic blood pressure (SBP) and diastolic blood pressure (DBP), heart rate (HR), and oxygen saturation were evaluated every 3 minutes during the first 15 minutes, and every 5 minutes afterward. If SBP values decreased >20% with respect to the baseline values, or decreased to <100 mm Hg, fluid loading and ephedrine (5 mg) were administered. A decrease in heart rate to <55 beats/min was considered bradycardia and atropine (0.5 mg) was administered. A decrease in the respiratory rate to <10/min, and a decrease in oxygen saturation to <90% were considered indications of respiratory depression. In such cases, support was provided with facemask ventilation.
Apgar scores were evaluated at first and fifth minutes by a pediatrician with no information regarding the groups. For blood gas measurements, samples were obtained from the umbilical cord of newborns.
Intraoperative pain evaluation was performed with the visual analog scale (VAS) (0 = no pain and 10 = worst pain possible) while performing surgical incision, uterine incision, and skin closure. In cases where VAS >3, IV fentanyl (50 µg) was administered. Sedation levels were monitored, and propofol (up to 0.5 mg/kg) was administered if patients had still discomfort. When patients required >50 µg fentanyl and/or 0.5 mg/kg propofol, the block was considered unsuccessful and general anesthesia was administered. Intraoperative and postoperative nausea, vomiting, pruritus, respiratory depression, and other side effects were recorded at the first, second, sixth, and 12th hours. Metoclopramide (10 mg IV) was administered for nausea, and diphenhydramine (25 mg) was administered for severe pruritus.
Surgeries were performed by the same surgeon with no knowledge regarding the group allocation. Surgeon satisfaction score was evaluated according to the sufficiency of muscle relaxation and the provision of adequate surgical conditions (0 = poor, 1 = fair, 2 = good, and 3 = excellent).
The time of first analgesic requirement was recorded for the patients, and pethidine hydrochloride (50 mg IM) was administered as the first analgesic choice when VAS score was >3 and additional analgesic requirements were satisfied with diclofenac sodium (75 mg IM). The total amount of diclofenac sodium required during the first 24 hours was also recorded.
Statistical Analysis
Data analysis was performed using SPSS (version 17.0, IBM-SPSS Inc, Armonk, New York). The primary outcome of this study was the duration of sensory block. The mean duration of sensory block was assumed to be 140 ±34.4 minutes based on a previous study,14 in which a group of patients received fentanyl. A power analysis indicated that 25 patients per group were required to detect an increase in duration of sensory block by 20%. We allowed for 6 more patients in each group to compensate for drop-outs during the study period. Categorical measurements were recorded as numbers and percentages, continuous measurements as mean (SD), and also the median (minimum–maximum) where necessary. In the comparison of categorical variables, the χ2 test or the Fisher exact test were used. The ANOVA test was used for distributions in the comparison of continuous measurements between the groups, whereas the Kruskal-Wallis test was used for parameters without normal distribution. Hemodynamic data were analyzed with repeated measures analysis. The α error was set at 0.05, type II error was set at 0.20, and a P value <0.05 was considered significant for all comparisons.
Results
Ninety-three patients scheduled for caesarean sections were enrolled in the study. Figure 1 presents the allocation of patients into the study groups. No significant intergroup differences were identified with regard to individual characteristics, the duration of surgery, and total fluid use (Table I). All groups had similar baseline SBP, DBP, and HR values. All groups had a significant SBP and DBP decrease with respect to the baseline values (P = 0.001 and P = 0.029, respectively) during the third minute, with the highest decrease in Group S, and the lowest decrease in Group C (Figure 2). The number of patients requiring ephedrine, and the administered ephedrine doses were similar between all groups (Table I). In Group S, 4 patients were treated with atropine due to bradycardia (P < 0.05), and HR values were significantly higher than the basal values at the sixth, ninth, and 12th minutes (P = 0.007, P = 0.007, and P = 0.004, respectively). HR values did not differ in comparison to baseline values in Group F and Group C. Oxygen saturation values were similar between groups and above 90% at all times.
Figure 1.
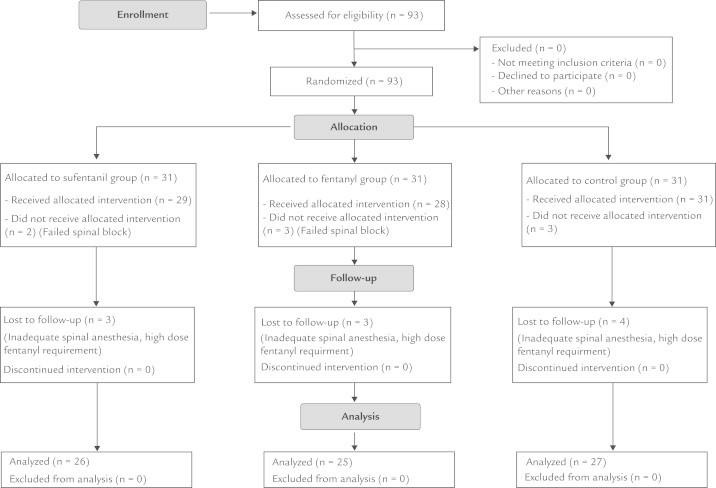
Study flow chart.
Table I.
Demographic, surgical, and perioperative characteristics.
| Group S | Group F | Group C | P | |
|---|---|---|---|---|
| Age, mean y (SD) | 30.38 (5.60) | 28.84 (5.13) | 29.26 (5.13) | 0.56 |
| Height, mean cm (SD) | 162.96 (4.54) | 163.36 (5.20) | 162.44 (4.59) | 0.06 |
| Weight, mean kg (SD) | 75.65 (9.77) | 76.48 (7.84) | 74.37 (6.47) | 0.09 |
| Gestational age, mean wk (SD) | 38.53 (0.65) | 38.41 (0.64) | 38.28 (0.83) | 0.46 |
| Nulliparous, n | 14 | 14 | 13 | 0.84 |
| Multiparous, n | 12 | 11 | 14 | |
| Duration of surgery, mean min (SD) | 43.19 (9.87) | 41.6 (8.22) | 43.11 (5.70) | 0.732 |
| Total fluid, mean mL (SD) | 2170.38 (371.058) | 2126.00 (411.582) | 2138.89 (311.428) | 0.905 |
| Ephedrine requirement, n (%) | 16 (61.5) | 17 (68.0) | 13 (51.9) | 0.487 |
| Dose of ephedrine, median mg (range) | 7.5 (0–30) | 5.0 (0–20) | 5.0 (0–15) | 0.301 |
| Atropine requirement, n (%) | 4 (15.4)* | 0 (0.0) | 0 (0.0) | 0.015 |
P = 0.015 versus Group F and Group C.
Figure 2.
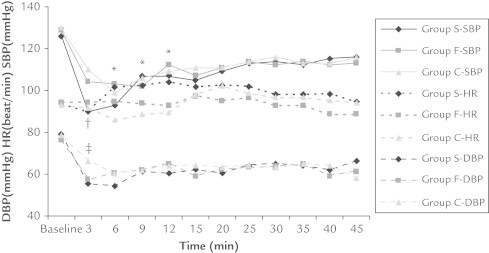
Hemodynamic differences among groups. *Significant differences at 6, 9, and 12 minutes in heart rate (HR) (P = 0.007, P = 0.007, and P = 0.004, respectively, Group F-HR vs Group C-HR). †Significant difference at third minute in systolic blood pressure (SBP) (P = 0.001 vs Group F-SBP and Group C-SBP). ‡Significant difference at the third minute in diastolic blood pressure (DBP) (P = 0.029 vs Group S-DBP and Group F-DBP).
The onset time of sensory block, the onset time of the block reached the T10 level—the highest sensory block level—and the onset time of motor block were significantly longer in Group C (P < 0.001), whereas no significant differences were identified between Group S and Group F. The highest block level (T4) and the duration of motor block was similar in all 3 groups, and no significant difference was observed between groups with regard to the time of 2-segment regression of the sensory block. The time of regression of the sensory block to the T10 level and the duration of sensory block was significantly longer for Group S (P = 0.003 and P < 0.001, respectively) (Table II).
Table II.
Spinal block characteristics and analgesic requirement.*
| Group S | Group F | Group C | P | |
|---|---|---|---|---|
| Time of onset of sensory block, min | 1.50 (1–8) | 1.50 (1–8) | 8.00 (3–12)† | 0.000 |
| Time to T10 sensory block, min | 3.00 (1–10) | 2.50 (1–10) | 11.00 (6–15)† | 0.000 |
| Highest sensory block level | T4 (3–5) | T4 (3–5) | T4 (4–5) | 0.219 |
| Time to highest sensory block level, min | 8.54 (3.52) | 7.52 (2.18) | 12.70 (3.74)† | 0.000 |
| Time of onset of motor block, min | 3.75 (1.5–20) | 3.0 (1.5–20) | 10.0 (7–15)† | 0.000 |
| Time for 2-segment regression, min | 107.85 (34.62) | 96.48 (24.46) | 93.70 (18.84) | 0.135 |
| Regression time to T10, min | 148.9 (40.62) | 115.71 (23.67) | 101.53 (15.12) | 0.003 |
| Duration of sensory block, min | 211.73 (51.88)‡ | 179.0 (38.83) | 155.26 (23.09) | 0.000 |
| Time for complete motor recovery, min | 142.22 (25.71) | 152.24 (35.87) | 129.73 (35.08) | 0.052 |
| Time to first analgesic request, min | 218.96 (52.76)‡ | 174.72 (25.16) | 141.11 (26.17) | 0.000 |
| Analgesic requirement in the first 24 h, mg | 115.38 (52.95)‡ | 171.05 (59.37) | 208.31 (70.02) | 0.000 |
Values are presented as mean (SD) or median (range).
P < 0.001 versus Group S and Group C.
P < 0.001 versus Group F and Group C.
VAS values for surgical incision, uterus incision, and skin closure, as well as intravenous fentanyl demand and sedation requirement were significantly higher in Group C (P < 0.05 for each comparisons) (Table III). The time to first analgesic requirement was significantly longer and the analgesic demand during the first 24 hours was significantly lower in Group S (P < 0.001) (Table II).
Table III.
Visual analog scale (VAS) and sedation scores, requiring fentanyl and propofol.
| Group S | Group F | Group C | P | |
|---|---|---|---|---|
| Skin incision VAS, median (range) | 0 (0–5) | 0 (0–3) | 1 (0–5)* | 0.000 |
| Uterus incision VAS, median (range) | 0 (0–4) | 0 (0–3) | 1 (0–5)* | 0.032 |
| End of surgery VAS, median (range) | 0 (0–0) | 0 (0–0) | 1 (0–3)* | 0.032 |
| No. of patients requiring fentanyl, n (%) | 5 (19.2) | 0 (0.0)† | 6 (22.2) | 0.037 |
| Skin incision sedation score, n | 0.143 | |||
| 0 | 20 | 17 | 23 | |
| 1 | 4 | 8 | 4 | |
| 2 | 2 | 0 | 0 | |
| 3 | 0 | 0 | 0 | |
| Uterus incision sedation score, n | 0.204 | |||
| 0 | 18 | 15 | 18 | |
| 1 | 4 | 10 | 5 | |
| 2 | 4 | 0 | 2 | |
| 3 | 0 | 0 | 2 | |
| End of surgery sedation score, n | 0.196 | |||
| 0 | 14 | 14 | 15 | |
| 1 | 7 | 11 | 11 | |
| 2 | 5 | 0 | 1 | |
| 3 | 0 | 0 | 0 | |
| No. of patients requiring propofol, n (%) | 18 (69.2) | 11 (44.3) | 21 (77.8)* | 0.018 |
P < 0.05 versus Group S and Group F.
P < 0.05 versus Group S and Group C.
Apgar scores and umbilical venous blood gas values (pH, oxygen partial pressure, carbon dioxide partial pressure, and base excess) were similar between groups and within normal ranges (Table IV).
Table IV.
Apgar scores and umbilical vein blood gas analyses.*
| Group S (n = 26) | Group F (n = 25) | Group C (n = 27) | P | |
|---|---|---|---|---|
| Apgar score at 1 min | 8 (7–10) | 8 (8–9) | 8 (7–9) | 0.432 |
| Apgar score at 5 min | 10 (9–10) | 10 (9–10) | 10 (9–10) | 0.905 |
| pH | 7.31 (7–7) | 7.30 (7–7) | 7.32 (7–7) | 0.671 |
| Oxygen partial pressure, mm Hg | 19.79 (10–43) | 20.40 (11–44) | 25.20 (11–36) | 0.156 |
| Carbon dioxide partial pressure, mm Hg | 49.85 (40–88) | 53.0 (33–69) | 50.50 (31–69) | 0.731 |
| Base excess, mmol/L | −2.20 (2.0) | −2.10 (3.1) | −1.9 (2.9) | 0.538 |
Values are presented as median (range) or mean (SD).
During the intraoperative period, all groups were similar with regard to the frequency of complications. Postoperatively, the frequency of pruritus was significantly higher in Group S for the first 2 hours (P < 0.001). One patient in Group S had pruritus and eruption on the legs, whereas 1 patient in Group C developed lumbar pain. However, no further complications were observed in the patient follow-ups (Table V).
Table V.
Intraoperative and postoperative side effects.
| Group S (n = 26) | Group F (n = 25) | Group C (n = 27) | P | |
|---|---|---|---|---|
| Nausea, n | ||||
| Intraoperative | 11 | 11 | 11 | 0.96 |
| Postoperative | 1 | 6 | 3 | 0.93 |
| Vomiting, n | ||||
| Intraoperative | 1 | 1 | 1 | 0.99 |
| Postoperative | 0 | 4 | 3 | 0.12 |
| Shivering, n | ||||
| Intraoperative | 6 | 5 | 8 | 0.79 |
| Postoperative | 1 | 3 | 0 | 0.13 |
| Pruritus, n | ||||
| Intraoperative | 1 | 0 | 0 | 0.36 |
| Postoperative | 13* | 8† | 0 | 0.000 |
| Respiratory depression, n | ||||
| Intraoperative | 2 | 1 | 3 | 0.63 |
| Postoperative | 0 | 0 | 0 | 0.16 |
P < 0.001 versus Group F and Group C.
P < 0.05 versus Group C.
Surgeon satisfaction score was statistically lower in Group C in comparison to the other groups (P = 0.003) (Figure 3).
Figure 3.
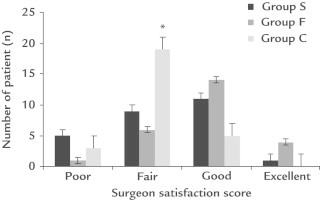
Surgeon satisfaction score between groups. *P = 0.003 versus Group S and Group F.
Discussion
Spinal anesthesia is preferred over epidural anesthesia due to its rapid onset, the higher level of muscle relaxation, and the lower dose requirement of LA during caesarean section. It ensures reliable and good quality of block for both the mother and the newborn.1
In our study, the duration of sensory block and first analgesic requirement were longer and analgesic requirement during the 24-hour period was lower in Group S, and sufficient intraoperative anesthesia could not be ensured. The frequency of perioperative pruritus was also higher in the sufentanil group. Surgeon satisfaction score was insufficient in all groups, being the lowest in the control group.
In caesarean section surgeries performed under spinal anesthesia, it has been reported that the administration of LA alone has a short duration of effect, that it is insufficient for preventing visceral pain and nausea especially during uterus manipulation and peritoneum closure, and that it leads to postoperative analgesic requirement at an earlier stage.15–17 The most frequently used agent among intrathecal opioids is fentanyl, which was demonstrated to be effective for 180 to 240 minutes when administered at doses of 10 to 25 µg.7 Sufentanil is also a lipophilic opioid; it has been reported that its onset of effect is more rapid,16,18–20 and that its duration of effect is 25% longer than fentanyl.21 Due to the rapid onset of its effects, as well as the effective postoperative analgesia that it ensures, sufentanil is an alternative to fentanyl and other opioids, and its use is becoming increasingly more common in caesarean section surgeries. In our study, we also aimed to determine the effectiveness of sufentanil.
The selection of different combinations and suitable doses when using opioids with LAs requires a careful consideration of factors such as the formation of sensory and motor block, the quality and duration of postoperative analgesia, and the side effects that might be observed in the mother and the newborn.2,14 In previous studies, the fact that different results were reported in normal individuals despite the use of similar dosages is associated with the differing distribution of the drugs during pregnancy.1,12,14,17,22 For caesarean section surgeries, the minimum intrathecal dose was reported as 10.58 mg levobupivacaine,8 6.25 μg fentanyl,23 and 1.5 to 2.5 μg sufentanil.11 We have also used a low-dose LA and adjuvant opioid in our study.
In Vercauteren et al22 an intrathecally administered 2 mL 0.125% levobupivacaine plus 1.5 µg sufentanil plus 2.5 µg epinephrine combination was used for delivery analgesia, and reported onset times for sensory block (4.4 minutes) were longer than the times obtained for the sufentanil and fentanyl groups in our study. This can be explained with the higher sufentanil concentrations and doses in our study. With a combination of 8 mg levobupivacaine plus 2.5 µg sufentanil, Gautier et al24 reported that the mean time to the highest level of sensory block was 17 minutes. In our study, this time was determined as 8.54 minutes with the levobupivacaine plus sufentanil combination, 7.52 minutes with the levobupivacaine plus fentanyl combination, and 12.7 minutes with the levobupivacaine only group. The shorter times in our study might be associated with the higher levobupivacaine doses.
In studies where intrathecal levobupivacaine was used alone, motor block onset times were reported as 10.0 minutes by Glaser et al25 and as 15 minutes by Burke et al.26 In our study, the addition of opioids to LA decreased onset times of motor block for levobupivacaine (Group S 3.75 minutes, Group F 3.0 minutes, and Group C 10.0 minutes). In a study comparing the effects of fentanyl addition (10, 15, or 25 μg) to intrathecal levobupivacaine (5, 7.5, or 10 mg) on sensory and motor blocks, the time to maximum motor block was reported as being shorter in the 10 mg levobupivacaine plus fentanyl group.27 Similarly in our study, motor and sensory block levels increased more rapidly in comparison to the control group; this effect was probably associated with the lipophilic character of sufentanil and fentanyl.
In studies comparing sufentanil with fentanyl, longer spinal anesthesia durations have been reported with sufentanil.13–15,21,28 In the study by Dahlgren et al,14 where 2.5 or 5 µg sufentanil and10 µg fentanyl was added to bupivacaine, and in the study by Meininger et al,13 where 2.5 or 5 µg sufentanil and10 µg fentanyl was added to 60 mg 2% mepivacaine, effective spinal analgesia durations were found to be higher in sufentanil groups in comparison to fentanyl and placebo groups. In our study, the duration of sensory block was, in agreement with the literature, significantly longer in the sufentanil group. The fact that this duration was shorter in the fentanyl group compared with the sufentanil group (179 minutes vs 211 minutes) is also consistent with other studies in which intrathecal 10 µg fentanyl was added to treatment.13–15,28
In our study, the durations of motor block were found to be shorter than the duration of the sensory block. These results are consistent with other studies in the literature.13,14,21,28
Continuation of postoperative analgesia despite an early end to the motor block significantly extended the time to the first analgesic requirement in the sufentanil group. For spinal anesthesia performed during caesarean sections, Gautier et al24 reported the time to first analgesic requirement as 136 minutes for a 8 mg levobupivacaine plus 2.5 µg sufentanil combination. This duration was longer in our study, and was identified as 219 minutes for the sufentanil group. This difference might be due to the higher dose of levobupivacaine used in our study.
In a study where fentanyl and sufentanil were added to bupivacaine, Dahlgren et al14 reported intraoperative intravenous fentanyl requirement for 1 patient in the 5 µg sufentanil group. In their study where 20 µg fentanyl, 2.5 µg sufentanil, or saline was added to 1.5 to 2.4 mL 0.5% bupivacaine, Lee et al28 reported that intraoperative intravenous fentanyl administration was only required by 22 of 24 patients in the control group. Intraoperative pain and discomfort caused by separation of the rectus muscle and stretching of the peritoneum also necessitated the use of additional analgesia and sedation in our study. During uterus incision and skin closure, 22% of control patients required intravenous fentanyl use. Despite higher VAS values in the sufentanil group that were not statistically significant, 19% of patients required intravenous fentanyl. Intraoperative analgesia was best ensured in the group with fentanyl added to levobupivacaine, with none of the patients requiring any IV fentanyl for intraoperative analgesia. In our study, the requirement for fentanyl and sedation affected anesthesia quality. When fentanyl and propofol use were considered, it was determined that the quality of anesthesia was lowest in the control group, and was highest in the fentanyl group. This is in agreement with the characteristics of the blocks determined in our study, with inadequate block levels in the control group leading to an increase in both fentanyl and propofol use. This also suggests that the intraoperative administration of intravenous fentanyl may have contributed to the sensory block duration and effective postoperative analgesia in the sufentanil group.
Incidence of hypotension in spinal anesthesia procedures during caesarean section surgeries is 45%.12,29 Methods such as fluid loading, having the patient assume a left lateral position, and using vasoconstrictor agents have been recommended for its prevention.12,29,30 Significant levels of hypotension compared with the basal values was observed in all groups at the third minute following the block; such cases were corrected with rapid administration of fluid loading and ephedrine. In their study comparing addition of 5 µg sufentanil and 10 and 20 µg fentanyl to10 mg levobupivacaine and10 mg bupivacaine during spinal anesthesia, Bremeric et al15 reported that the hemodynamic data of both groups were similar, with no differences regarding side effects. Gunusen et al27 demonstrated that the addition of 10 µg fentanyl to 10 mg levobupivacaine increased the incidence of hypotension, whereas lower doses of levobupivacaine led to insufficient anesthesia and analgesia.
In our study, no intergroup differences were observed with regard to intraoperative side effects. Pruritus was significantly more frequent in the sufentanil group during the postoperative period, whereas pruritus did not develop in any control group patients. This demonstrates that the addition of opioids had an effect on pruritus. Incidence of pruritus among pregnant women with various opioids (especially lipophilic opioids) administered intrathecally was reported at between 30% and 95%, although the pruritus was generally transient and mild.11,12,31 In their study performed with varying intrathecal fentanyl doses (5–45 µg), Palmer et al32 described that pruritus was not dose-dependent, and that it was observed in all patients. In certain studies, intrathecal sufentanil increased the incidence of pruritus in a dose-dependent fashion.10,11,14,27,28 In their study adding placebo, 1.5, 2.5, or 5 µg sufentanil to 0.5% hyperbaric bupivacaine during elective caesarean section surgery, Demiraran et al11 reported that the lowest incidence of pruritus was observed in the group with 1.5 µg sufentanil added to their LA. Similarly in our study, the frequency of pruritus increased in the group with 2.5 µg sufentanil combined with their treatment. However, the cases of pruritus were transient, and only 1 patient required treatment.
Best postoperative analgesia in our study was provided by the addition of sufentanil to levobupivacaine, which affected the use of additional analgesics within the postoperative first 24 hours. It has been reported in the literature that intrathecal doses of 2.5 to 5 µg sufentanil reduced the postoperative requirement for analgesia, especially during the first 6 hours.12 Dahlgren et al14 determined in their study a significantly lower use of additional analgesia in the sufentanil group during the first 24 hours (especially during the first 6 hours). Although the additional use of analgesics during the postoperative first 24 hours in our study was significantly lower in the sufentanil group, the use of additional analgesics was highest in the control group.
The addition of intrathecal fentanyl or other opioids to LA administration during caesarean sections did not affect the Apgar scores and newborn blood gas values.10,14,27 There was no direct relationship between the likelihood of hypotension and the Apgar scores. It was emphasized that the depth and duration of hypotension could have a considerable influence on hypotension’s effect on newborns.31 No differences were observed between the Apgar scores and the gas values of the umbilical blood, and fetal acidosis was not seen in any of the groups. This might be associated with the transient nature of the changes in blood pressure, and also its rapid correction.
Surgeon satisfaction score in our study was significantly lower in the control group. However, muscle relaxation at surgery site was generally moderate in the other groups, and considered excellent by only 5 patients. Sufficient muscle relaxation was not achieved in some patients; this adversely affected the quality of anesthesia and surgeon satisfaction. A study published in recent years reported a 50% effective dose of 6.2 mg for levobupivacaine, and a 95% effective dose of 12.9 mg.33 The study described that the addition of 2.5 µg sufentanil to the 95% effective dose did not affect hemodynamics, that sufficient block and effective anesthesia and analgesia were achieved, and that lower doses resulted were not effective. Our results suggest that the administered levobupivacaine doses could have been insufficient.
The limitation of our study is the lack of assesment of patients’ satisfaction levels. This could have let us evaluate the compatibility of patients’ and surgeons’ satisfaction scores about the anesthetic technique.
Conclusions
Our study demonstrated that the addition of sufentanil and fentanyl to intrathecal levobupivacaine during caesarean section surgery is more effective than the administration of levobupivacaine alone. The addition of sufentanil to levobupivacaine allowed rapid onset time for sensory and motor block levels. It also extended the duration of postoperative analgesia, and led to a decrease in total analgesic requirement. However, physicians should keep in mind that it may be insufficient in terms of intraoperative anesthesia quality and sufentanil might invoke perioperative pruritus.
Acknowledgments
Drs. Ozyilkan, Kocum, Sener, Caliskan, Tarim, Ergenoglu, and Aribogan all made significant contributions to the study design, data collection, data interpretation, figure creation, and writing of the manuscript.
Conflicts of interest
The authors have indicated that they have no conflicts of interest regarding the content of this article.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Gogarten W. Spinal anaesthesia for obstetrics. Anaesthesiology. 2003;17:377–392. doi: 10.1016/s1521-6896(02)00116-7. [DOI] [PubMed] [Google Scholar]
- 2.Vercauteren M. Obstetric spinal analgesia and anesthesia. Curr Opin Anaesthesiol. 2003;16:503–507. doi: 10.1097/00001503-200310000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Foster R.H., Markham A. Levobupivacaine: a review of its pharmacology and use as a local anaesthetic. Drugs. 2000;59:551–579. doi: 10.2165/00003495-200059030-00013. [DOI] [PubMed] [Google Scholar]
- 4.Bardsley H., Gristwood R., Baker H. A comparison of the cardiovascular effects of levobupivacaine and rac-bupivacaine following intravenous administration to healthy volunteers. Br J Clin Pharmacol. 1998;46:245–249. doi: 10.1046/j.1365-2125.1998.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morrison S.G., Dominguez J.J., Frascarolo P. A comparison of the electrocardiographic cardiotoxic effects of racemic bupivacaine, levobupivacaine, and ropivacaine in anesthetized swine. Anesth Analg. 2000;90:1308–1314. doi: 10.1097/00000539-200006000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Lee Y.Y., Muchhal K., Chan K. Levobupivacaine and fentanyl for spinal anaesthesia: a randomized trial. Eur J Anaesthesiol. 2005;22:889–903. doi: 10.1017/S0265021505001523. [DOI] [PubMed] [Google Scholar]
- 7.Dahl J.B., Jeppesen I.S., Jørgensen H. Intraoperative and postoperative analgesic efficacy and adverse effects of intrathecal opioids in patients undergoing cesarean section with spinal anesthesia: a qualitative and quantitative systematic review of randomized controlled trials. Anesthesiology. 1999;91:1919–1927. doi: 10.1097/00000542-199912000-00045. [DOI] [PubMed] [Google Scholar]
- 8.Hunt C.O., Naulty J.S., Bader A.M. Perioperative analgesia with subarachnoid fentanyl-bupivacaine for cesarean delivery. Anesthesiology. 1989;71:535–540. doi: 10.1097/00000542-198910000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Milligan K.R. Recent advances in local anaesthetics for spinal anaesthesia. Eur J Anaesthesiol. 2004;21:837–847. doi: 10.1017/s0265021504000158. [DOI] [PubMed] [Google Scholar]
- 10.Karaman S., Kocabas S., Uyar M. The effects of sufentanil or morphine added to hyperbaric bupivacaine in spinal anaesthesia for caesarean section. Eur J Anaesthesiol. 2006;23:285–291. doi: 10.1017/S0265021505001869. [DOI] [PubMed] [Google Scholar]
- 11.Demiraran Y., Ozdemir I., Kocaman B. Intrathecal sufentanil (1.5 microg) added to hyperbaric bupivacaine (0.5%) for elective cesarean section provides adequate analgesia without need for pruritus therapy. J Anesth. 2006;20:274–278. doi: 10.1007/s00540-006-0437-2. [DOI] [PubMed] [Google Scholar]
- 12.Belzarena S.D. Clinical effects of intrathecally administered fentanyl in patients undergoing cesarean section. Anesth Analg. 1992;74:653–657. doi: 10.1213/00000539-199205000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Meininger D., Byhahn C., Kesler P. Intrathechal fentanyl, sufentanil, or plasebo combined with hyperbaric mepivacaine 2% for parturients undergoing elective cesarean delivery. Anesth Analg. 2003;96:852–858. doi: 10.1213/01.ANE.0000049685.38809.7E. [DOI] [PubMed] [Google Scholar]
- 14.Dahlgren G., Hultstrand C., Jakobsson J. Intrathecal sufentanil, fentanyl, or placebo added to bupivacaine for cesarean section. Anesth Analg. 1997;85:1288–1293. doi: 10.1097/00000539-199712000-00020. [DOI] [PubMed] [Google Scholar]
- 15.Bremerich D.H., Fetsch N., Zwissler B.C. Comparison of intrathecal bupivacaine and levobupivacaine combined with opioids for Caesarean section. Curr Med Res Opin. 2007;23:3047–3054. doi: 10.1185/030079907X242764. [DOI] [PubMed] [Google Scholar]
- 16.Hamber E.A., Viscomi C.M. Intrathecal lipophilic opioids as adjuncts to surgical spinal anesthesia. Reg Anesth Pain Med. 1999;24:255–263. doi: 10.1016/s1098-7339(99)90139-6. [DOI] [PubMed] [Google Scholar]
- 17.Bogra J., Arora N., Srivastava P. Synergistic effect of intrathecal fentanyl and bupivacaine in spinal anesthesia for cesarean section. BMC Anesthesiol. 2005;5:5. doi: 10.1186/1471-2253-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coda B.A. Opioids. In: Barash P.G., Cullen B.F., Stoelting R.K., editors. Clinic Anesthesia. 4th ed. Lippincott Williams & Wilkins; Philadelphia, Pa: 2001. pp. 345–375. [Google Scholar]
- 19.Ummenhofer W.C., Arends R.H., Shen D.D. Comparative spinal distribution and clearance kinetics of intrathecally administered morphine, fentanyl, alfentanil, and sufentanil. Anesthesiology. 2000;92:739–753. doi: 10.1097/00000542-200003000-00018. [DOI] [PubMed] [Google Scholar]
- 20.Wang L.Z., Chang X.Y., Liu X. Comparison of bupivacaine, ropivacaine and levobupivacaine with sufentanil for patient-controlled epidural analgesia during labor: arandomized clinical trial. Chin Med J. 2010;123:178–183. [PubMed] [Google Scholar]
- 21.Nelson K.E., Rauch T., Terebuh V. A comparison of intrathecal fentanyl and sufentanil for labor analgesia. Anesthesiology. 2002;96:1070–1073. doi: 10.1097/00000542-200205000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Vercauteren M.P., Hans G., Decker K.D. Levobupivacaine combined with sufentanil and epinephirine racemic bupivacaine. Anesth Analg. 2001;93:996–1000. doi: 10.1097/00000539-200110000-00040. [DOI] [PubMed] [Google Scholar]
- 23.Parpaglioni R., Frigo M.G., Lemma A. Minimum local anaesthetic dose (MLAD) of intrathecal levobupivacaine and ropivacaine for Caesarean section. Anaesthesia. 2006;61:110–115. doi: 10.1111/j.1365-2044.2005.04380.x. [DOI] [PubMed] [Google Scholar]
- 24.Gautier P., De Kock M., Huberty L. Comparison of the effects of intrathecal ropivacaine, levobupivacaine, and bupivacaine for Caesarean section. Br J Anaesth. 2003;91:684–689. doi: 10.1093/bja/aeg251. [DOI] [PubMed] [Google Scholar]
- 25.Glaser C., Marhofer P., Zimpfer G. Levobupivacaine versus racemic bupivacaine for spinal anesthesia. Anesth Analg. 2002;94:194–198. doi: 10.1097/00000539-200201000-00037. [DOI] [PubMed] [Google Scholar]
- 26.Burke D., Kennedy S., Bannister J. Spinal anestehesia with 0.5 %5 S(-) bupivacaine for elective lower limb surgery. Reg Anesth Pain Medicine. 1999;24:519–523. doi: 10.1016/s1098-7339(99)90042-1. [DOI] [PubMed] [Google Scholar]
- 27.Gunusen I., Karaman S., Sargin A. A randomized comparison of different doses of intrathecal levobupivacaine combined with fentanyl for elective cesarean section: prospective, double-blinded study. J Anesth. 2011;25:205–212. doi: 10.1007/s00540-011-1097-4. [DOI] [PubMed] [Google Scholar]
- 28.Lee J.H., Chung K.H., Lee J.Y. Comparison of fentanyl and sufentanil added to 0.5% hyperbaric bupivacaine for spinal anesthesia ain patients undergoing cesarean sectin. Korean J Anesthesiol. 2011;60:103–108. doi: 10.4097/kjae.2011.60.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mercier F.J., Riley E.T., Frederickson W.L. Phenylephrine added to prophylactic ephedrine infusion during spinal anesthesia for elective cesarean section. Anesthesiology. 2001;95:668–674. doi: 10.1097/00000542-200109000-00020. [DOI] [PubMed] [Google Scholar]
- 30.Carpenter R.L., Caplan R.A., Brown D.L. Incidence and risk factors for side effects of spinal anesthesia. Anesthesiology. 1992;76:906–916. doi: 10.1097/00000542-199206000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Akay S., Ayoglu H., Yurtlu B.S. The effects of intrathecal levobupivacaine and opioid combinations in caesarean operations. Turk J Anaesth Reanim. 2009;37:25–34. [Google Scholar]
- 32.Palmer C.M., Cork R.C., Hays R. The dose-response relation of intrathecal fentanyl for labor analgesia. Anesthesiology. 1998;88:355–361. doi: 10.1097/00000542-199802000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Bouvet L., Da-Col X., Chassard D. ED50 and ED95 of intrathecal levobupivacaine with opioids for caesarean delivery. Br J Anaesth. 2011;106:215–220. doi: 10.1093/bja/aeq296. [DOI] [PubMed] [Google Scholar]


