Abstract
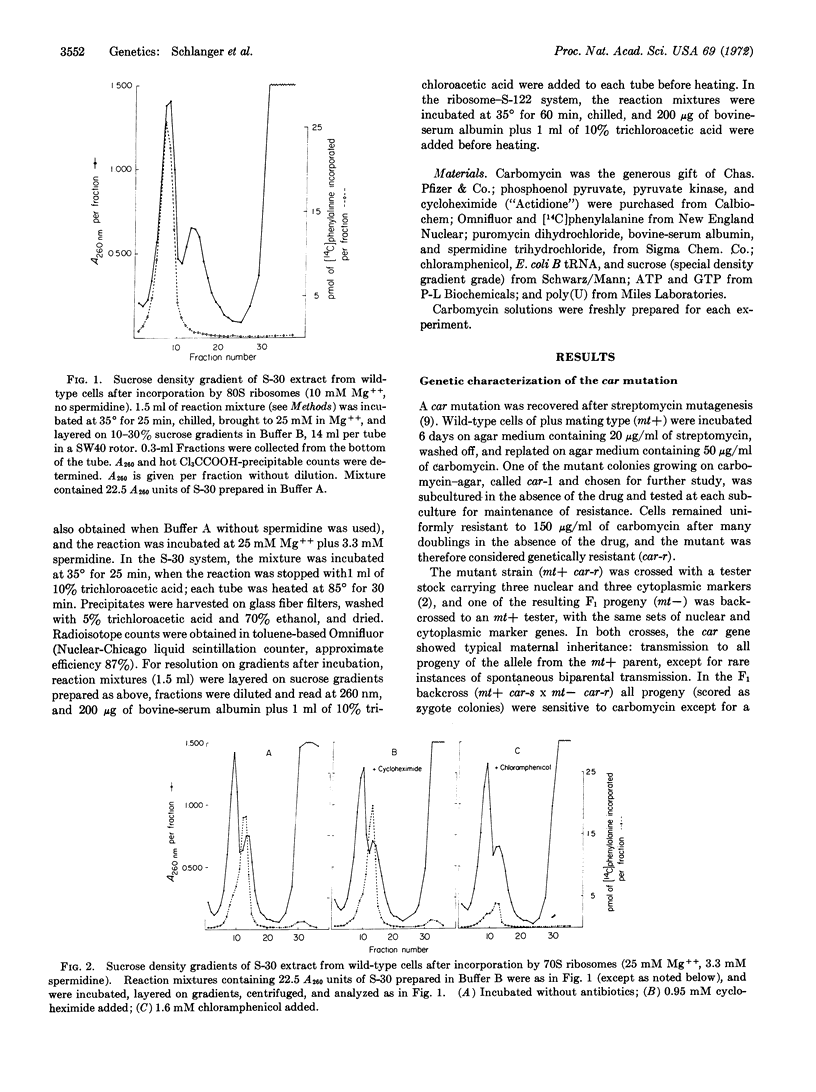
A mutation, car, determining resistance to several macrolide antibiotics, including carbomycin, has been identified in the alga Chlamydomonas as cytoplasmic, and mapped in the known cytoplasmic linkage group close to genes determining resistance to other antibiotics, including streptomycin, erythromycin, and spectinomycin. The effect of the car mutation on chloroplast ribosome function was demonstrated with an in vitro system incorporating amino acids especially developed to assess activity of 70S chloroplast ribosomes. In an S-30 extract containing both 70S chloroplast and 80S cytoplasmic ribosomes, low concentrations of Mg++ and spermidine favored 80S ribosome activity, and high concentrations activated 70S ribosomes and reversibly inactivated the 80S component. Under conditions favoring chloroplast ribosome activity, carbomycin inhibited incorporation by an S-30 extract, and by purified 70S ribosomes from wild-type but not from car cells. These results show that cytoplasmic genes are directly involved in chloroplast ribosome function and they suggest that the car gene product is a ribosomal protein; the results further strengthen the evidence that the cytoplasmic linkage group is located in chloroplast DNA.
Keywords: chloroplast ribosome, in vitro protein synthesis, carbomycin resistance
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boardman N. K., Francki R. I., Wildman S. G. Protein synthesis by cell-free extracts of tobacco leaves. 3. Comparison of the physical properties and protein synthesizing activities of 70 s chloroplast and 80 s cytoplasmic ribosomes. J Mol Biol. 1966 Jun;17(2):470–487. doi: 10.1016/s0022-2836(66)80157-2. [DOI] [PubMed] [Google Scholar]
- CHUN E. H., VAUGHAN M. H., Jr, RICH A. THE ISOLATION AND CHARACTERIZATION OF DNA ASSOCIATED WITH CHLOROPLAST PREPARATIONS. J Mol Biol. 1963 Aug;7:130–141. doi: 10.1016/s0022-2836(63)80042-x. [DOI] [PubMed] [Google Scholar]
- Cohen Y. Association, profession, adaptation. Singapore Med J. 1971 Jun;12(3):121–126. [PubMed] [Google Scholar]
- GILBERT W. Polypeptide synthesis in Escherichia coli. II. The polypeptide chain and S-RNA. J Mol Biol. 1963 May;6:389–403. doi: 10.1016/s0022-2836(63)80051-0. [DOI] [PubMed] [Google Scholar]
- Gillham N. W., Boynton J. E., Burkholder B. Mutations altering chloroplast ribosome phenotype in Chlamydomonas. I. Non-mendelian mutations. Proc Natl Acad Sci U S A. 1970 Oct;67(2):1026–1033. doi: 10.1073/pnas.67.2.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldthwaite C., Smith I. Genetic mapping of aminoglycoside and fusidic acid resistant mutations in Bacillus subtilis. Mol Gen Genet. 1972;114(3):181–189. doi: 10.1007/BF01788887. [DOI] [PubMed] [Google Scholar]
- Grivell L. A., Groot G. S.P. Spinach chloroplast ribosomes active in protein synthesis. FEBS Lett. 1972 Sep 1;25(1):21–24. doi: 10.1016/0014-5793(72)80444-7. [DOI] [PubMed] [Google Scholar]
- Grivell L. A., Reijnders L., Borst P. Isolation of yeast mitochondrial ribosomes highly active in protein synthesis. Biochim Biophys Acta. 1971 Sep 30;247(1):91–103. doi: 10.1016/0005-2787(71)90811-2. [DOI] [PubMed] [Google Scholar]
- Hoober J. K., Blobel G. Characterization of the chloroplastic and cytoplasmic ribosomes of Chlamydomonas reinhardi. J Mol Biol. 1969 Apr 14;41(1):121–138. doi: 10.1016/0022-2836(69)90130-2. [DOI] [PubMed] [Google Scholar]
- Mao J. C., Wiegand R. G. Mode of action of macrolides. Biochim Biophys Acta. 1968 Apr 22;157(2):404–413. doi: 10.1016/0005-2787(68)90094-4. [DOI] [PubMed] [Google Scholar]
- Mets L. J., Bogorad L. Mendelian and uniparental alterations in erythromycin binding by plastid ribosomes. Science. 1971 Nov 12;174(4010):707–709. doi: 10.1126/science.174.4010.707. [DOI] [PubMed] [Google Scholar]
- Pestka S. Inhibitors of ribosome functions. Annu Rev Microbiol. 1971;25:487–562. doi: 10.1146/annurev.mi.25.100171.002415. [DOI] [PubMed] [Google Scholar]
- SAGER R., GRANICK S. Nutritional studies with Chlamydomonas reinhardi. Ann N Y Acad Sci. 1953 Oct 14;56(5):831–838. doi: 10.1111/j.1749-6632.1953.tb30261.x. [DOI] [PubMed] [Google Scholar]
- SAGER R., ISHIDA M. R. CHLOROPLAST DNA IN CHLAMYDOMONAS. Proc Natl Acad Sci U S A. 1963 Oct;50:725–730. doi: 10.1073/pnas.50.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAGER R. Streptomycin as a mutagen for nonchromosomal genes. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2018–2026. doi: 10.1073/pnas.48.12.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAGER R., WEINSTEIN I. B., ASHKENAZI Y. Coding ambiguity in cell-free extracts of chlamydomonas. Science. 1963 Apr 19;140(3564):304–306. doi: 10.1126/science.140.3564.304. [DOI] [PubMed] [Google Scholar]
- Sager R., Hamilton M. G. Cytoplasmic and chloroplast ribosomes of Chlamydomonas: ultracentrifugal characterization. Science. 1967 Aug 11;157(3789):709–711. doi: 10.1126/science.157.3789.709. [DOI] [PubMed] [Google Scholar]
- Sager R., Lane D. Molecular basis of maternal inheritance. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2410–2413. doi: 10.1073/pnas.69.9.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager R., Ramanis Z. A genetic map of non-Mandelian genes in Chlamydomonas. Proc Natl Acad Sci U S A. 1970 Mar;65(3):593–600. doi: 10.1073/pnas.65.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager R., Ramanis Z. Biparental inheritance of nonchromosomal genes induced by ultraviolet irradiation. Proc Natl Acad Sci U S A. 1967 Sep;58(3):931–937. doi: 10.1073/pnas.58.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager R., Ramanis Z. Genetic studies of cloroplast DNA in Chlamydomonas. Symp Soc Exp Biol. 1970;24:401–417. [PubMed] [Google Scholar]
- Scragg A. H., Morimoto H., Villa V., Nekhorocheff J., Halvorson H. O. Cell-free protein synthesizing system from yeast mitochondria. Science. 1971 Mar 5;171(3974):908–910. doi: 10.1126/science.171.3974.908. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Tamaki M., Itoh T., Otaka E., Osawa S. Ribosomes from spiramycin resistant mutants of Escherichia coli Q13. Mol Gen Genet. 1972;114(1):23–30. doi: 10.1007/BF00268743. [DOI] [PubMed] [Google Scholar]


