Abstract
Background
Iloprost, which has efficacy in the microvascular space, is shown to have beneficial effects on the kidney, which has an extensive microvascular network.
Objective
We aimed to evaluate the effect of iloprost treatment on kidney functions in patients with critical limb ischemia.
Methods
Forty-eight patients with critical limb ischemia who were not suitable for revascularization and who were treated with iloprost were evaluated prospectively in our clinic between September 2010 and December 2012. The patients were divided into 2 groups as patients with chronic renal dysfunction (Group I) and patients with normal renal function (Group II). Urine albumin:creatinine ratio and glomerular filtration rate (GFR) calculated using serum creatinine and serum cystatin C (GFRcyc) were used to establish the presence of renal dysfunction. The decrease analgesic requirement, walking distance, reduction in ulcer diameter, the increase in ankle-brachial index, and changes in The Society of Vascular Surgery/International Society of Cardiovascular Surgery criteria were used in the evaluation of treatment response.
Results
Opioid analgesic requirement and decubitus pain disappeared after treatment in 58.3% (n = 28) of subjects. Walking distance increased in 66.6% (n = 32). Iloprost treatment significantly increased ankle-brachial index (P < 0.01). In Group I the levels of serum urea, creatinine, and cystatin C significantly decreased (P < 0.05), whereas GFRcyc and GFR calculated using the equation of the Chronic Kidney Disease Epidemiology Collaboration (ie, GFR expressed for specified race, sex, and serum creatinine in milligrams per deciliter) was increased significantly compared with pretreatment levels (P < 0.05). No significant change was observed in urine albumin:creatinine ratio (P > 0.05).
Conclusions
The use of iloprost in critical limb ischemia can slow down the progress of early stage renal damage. GFRcyc and cystatin C, which are indicators of early stage chronic renal dysfunction, can be used for the evaluation of treatment response.
Key words: cystatin C, iloprost, peripheral arterial disease, renal function
Introduction
Critical limb ischemia (CLI) is the severest form of peripheral arterial disease, associated with major cardiovascular events such as extremity loss, myocardial infarction, stroke, and death. CLI presents clinically with rest pain in the extremity, trophic skin lesions, and tissue loss. According to Fontaine classification stages III and IV are accepted as CLI. The definition of CLI by Trans-Atlantic Inter-Society Consensus associates recurrent ischemic rest pain in the extremity (Fontaine stage III), ulceration, and gangrenous lesion (Fontaine stage IV) with systolic blood pressure ≤70 mm Hg in the knee and ≤50 mm Hg in the foot.1 Peripheral bypass surgery and peripheral transluminal angioplasty are used in the treatment of CLI, according to the level of localization and structure of the lesion.2
Revascularization cannot be carried out in patients with CLI because of diffuse distal arterial disease, recurrent postoperative graft occlusion, or recurrent stent thrombosis after peripheral transluminal angioplasty.3 Iloprost treatment is preferred in patients with CLI because of its advantages such as reducing rest pain, decreasing analgesic requirements, regressing trophic lesions, increasing walking distance, and decreasing the progress to extremity amputation in the long run.4 Iloprost shows its efficacy through platelet aggregation inhibition, vasodilation, fibrinolysis, anti-inflammatory effect, proangiogenic effect (vascular endothelial growth factor-mediated), and protective effect against ischemia-reperfusion injury.5,6 Iloprost is generally well tolerated despite the adverse effects limiting its use, such as hypotension, headache, flushing, nausea, vomiting, and diarrhea.7
Iloprost, which has efficacy in the microvascular space, is shown to have beneficial effects on the kidneys, which have an extensive microvascular network.8,9 The determination of glomerular filtration rate (GFR) is the most valuable criteria in the assessment of renal function.10 But usually serum urea and creatinine values are used in clinical practice. Cystatin C, which is a low-molecular weight protein, is under investigation for superiority over creatinine as a filtration marker and routine use.
The purpose of our study was to examine the effect of iloprost on renal functions in patients with CLI. For this purpose, serum levels of cystatin C, urea, creatinine, GFR calculated using the equation of the Chronic Kidney Disease Epidemiology Collaboration (ie, GFR expressed for specified race, sex, and serum creatinine in milligrams per deciliter) (CKD-EPI), calculated using serum cystatin C (GFRcyc), and albumin:creatinine ratio were measured before and after iloprost treatment in patients with CLI.
Patients and Methods
Study design
A prospective design was used in this study. Approval was obtained from the local ethics committee. Informed consent was obtained from patients.
Patients
Forty-two men (87.5%) and 6 women (12.5%) admitted to our clinic with severe chronic ischemia symptoms in the lower extremity, who were unsuitable for revascularization and were treated with intravenous iloprost* were included in the study. Mean age was 54.37 (10.76) years (range = 29–68 years). Medical history (eg, hypertension, diabetes mellitus, hyperlipidemia, and coronary artery disease) of patients was recorded. Serum biochemistry (eg, fasting blood glucose, lipid profile, urea, creatinine, cystatin C, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, lactic acid dehydrogenase, sodium, potassium, calcium, phosphorus, albumin, and thyroid function tests) and hematologic parameters (eg, complete blood count, erythrocyte sedimentation rate, C-reactive protein, and urine albumin:creatinine ratio) were checked. Only ejection fraction as cardiac function was assessed with echocardiography. Patients with ejection fraction <40% were excluded in the study.
Initial assessment for patients and exclusion criteria
Patients presenting with CLI symptoms (eg, ischemic rest pain, leg or foot ulcers, or gangrene) who were unsuitable for surgery or percutaneous revascularization were admitted for initial assessment. The patients conformed to stage III and IV according to Fontaine classification. Patients with a duration of ischemic symptoms <2 weeks were excluded from analysis on the basis of Trans-Atlantic Inter-Society Consensus definition.11 Acute myocardial infarction or cerebrovascular accident upto 6 months before the study, heart failure (New York Heart Association Class >I), ventricular arrhythmias, severe hypertension (sitting systolic blood pressure >180 mm Hg and sitting diastolic blood pressure >110 mm Hg), hemorrhagic diathesis, clinical situations in which iloprost can increase the risk of hemorrhage (eg, active-phase peptic ulcer, cerebral hemorrhage, thrombocytopenia, or thrombosis), contrast agent administration during the past 2 months, thyroid dysfunction, stage 3 or higher chronic renal dysfunction,12 severe hepatic failure, and active infection were other major causes of exclusion.
Study group
At the beginning of treatment, patients were divided into 2 groups according to renal function. Patients with chronic renal dysfunction were designated as Group I and patients with normal renal function were designated as Group II. According to National Kidney Foundation guidelines, stage 1 (renal damage with normal or mildly increased [GFR >90 mL/min]) and stage 2 (renal damage with mildly decreased GFR [60–89 mL/min]) patients were included in the study.12
Analysis of renal functions
Urine albumin:creatinine ratio and GFR calculated from serum creatinine and serum cystatin C levels were used in the determination of chronic renal dysfunction. Urine samples were collected after a first morning void. Blood samples were taken in the morning after 12 hours of fasting. After serum centrifugation, creatine was measured by colorimetric Jaffe method and urea by photometric urea method by using a Cobas 8000 modular analyzer (Roche Diagnostics GmbH, Mannheim, Germany). Serum cystatin C was studied by using N-Latex cystatin C kit (Dade Behring, Marburg, Germany) with particle-enhanced immunonefelometry in BN ProSpec plasma protein analyzer (Dade Behring). Urinary albumin was analyzed by turbidimetric method, and creatine by colorimetric Jaffe method in Cobas 6000 analyzer (Roche Diagnostics GmbH). GFR was calculated using GFR CKD-EPI (GFR CKD-EPI = 141 × min (Scr /κ, 1)α × max(Scr /κ, 1)-1.209 × 0.993Age × 1.018 [if woman] × 1.159 [if black], where Scr is serum creatinine in milligrams per deciliter, κ is 0.7 for women and 0.9 for men, α is –0.329 for women and –0.411 for men, min indicates the minimum of Scr /κ or 1, and max indicates the maximum of Scr/K or 1) and GFRcyc = 74.835/cystatin C1/0.75 formulas.13,14) Spot urine albumin:creatinine ratio >17 mg/g for men and >25 mg/g for women was accepted as the criterion of renal damage.12
Treatment procedure
The patients were administered iloprost infusion for 7 days. A dosage of 0.5 to 2 ng/kg/min was administered 6 hours a day for 7 consecutive days. Dose range was increased to the maximum dose that the patient could tolerate and continued as such. Nephrotoxic agents were avoided during the treatment. The first ankle-brachial index (ABI) measurement was done on the first day of treatment. The second measurement was done on the 10th day and the patients were discharged. Clinical assessment and assessment of changes in the status of the extremity were made on the basis of a modified scale of the Society of Vascular Surgery/International Society of Cardiovascular Surgery (SVS/ISCVS) classification combining a change in relatively subjective symptomatology.15 Upward or downward category shifts were recorded at the beginning of treatment and during the follow-up visit. ABI and analgesic requirements were also recorded on the first and 10th days of treatment. Tramadol hydrochloride 100 mg ampoules were given as an analgesic in saline 1 to 3 times a day by slow intravenous infusion. Serum albumin, creatinine, cystatin C, urine albumin, and creatinine levels were measured at the beginninng and at the end of the treatment. The calculated GFR CKD-EPI, GFRcyc, and urine albumin:creatinine ratios were compared.
Statistical analysis
The Statistical Package for Social Sciences for Windows 15.0 software (IBM SPSS Statistics, Armonk, New York) was used in the statistical analysis of the data. Student t test was used in the intergroup comparison of quantitative variables conforming to normal distribution, and Mann Whitney U test was used in the intergroup comparison of quantitative variables not conforming to normal distribution. Differences between categorical variables were analyzed using the χ2 test. Fisher exact test was used whenever conditions for χ2 test could not be established. Significance was assumed at P < 0.05.
Results
Demographic data
The demographic characteristics of the patients are presented in Table I. There was no statistically significant difference between the 2 groups regarding demographic data (P > 0.005). None of the bypass grafts were patent in the initial assessment. Two patients had undergone recent toe amputation and 2 had previously undergone major leg amputation. Thirty-two patients (66.6%) had lost walking skills because of ischemic pain (n = 28) and gangrene (n = 4).
Table I.
Baseline characteristics of study group
| Group I | Group II | Total | P | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Age (mean y [SD]) | 53.0 [10.5] | 55.5 [11.0] | 54.37 [10.76] | 0.42 | |||
| Sex | |||||||
| Woman | 4 | 20.6 | 2 | 7.6 | 6 | 12.5 | 0.28 |
| Man | 18 | 81.8 | 24 | 92.3 | 42 | 87.5 | |
| Hypertension | 14 | 63.6 | 18 | 69.2 | 32 | 66.6 | 0.69 |
| Diabetes mellitus | 12 | 54.5 | 10 | 38.4 | 22 | 45.8 | 0.27 |
| Hyperlipidemia | 8 | 36.3 | 6 | 23.0 | 14 | 29.1 | 0.32 |
| Chronic obstructive pulmonary disease | 6 | 27.2 | 8 | 30.7 | 14 | 29.1 | 0.79 |
| Smoking | 14 | 63.6 | 20 | 76.9 | 34 | 70.8 | 0.32 |
| Coronary artery disease | 7 | 31.8 | 12 | 46.1 | 19 | 39.5 | 0.50 |
| Ejection fraction (mean % [SD]) | 47.9 [9.1] | 46.1 [9.6] | 0.23 | ||||
| Peptic ulcer | 3 | 13.6 | 3 | 11.5 | 6 | 12.5 | 0.51 |
| Previous peripheral operation | 12 | 54.5 | 7 | 26.9 | 19 | 39.5 | 0.05 |
| Aortofemoral bypass | 3 | 13.6 | 1 | 3.8 | |||
| Femoropopliteal bypass | 5 | 22.7 | 3 | 11.5 | |||
| Popliteadistal bypass | 4 | 18.1 | 3 | 11.5 | |||
| Fontaine classification | |||||||
| Class III | 19 | 86.3 | 23 | 88.4 | 42 | 77.5 | 0.51 |
| Class IV | 3 | 13.6 | 3 | 11.5 | 6 | 12.5 | |
Treatment process
Iloprost was well tolerated throughout the study period. Headache and hypotension were seen in 1 patient as an adverse effect of iloprost and treatment was discontinued with the patient’s consent. Other adverse effects were nausea in 2 (4.1%) patients, abdominal cramps in 1 (2.1%) patient, and headache in 5 (10.4%) patients. Because these were short-term effects, they did not require treatment discontinuation. Forty-seven patients completed the study. No mortality was observed during the treatment period.
Clinical assessment of status of extremity after treatment
At the 10th-day follow-up visit, decubitus pain was decreased and analgesic requirement disappeared in 28 (58.3%) patients. Decubitus pain decreased but analgesic requirement persisted in 10 (20.8%) patients. Thirty-two (66.6%) patients began to walk or their walking distance improved. ABI showed significant improvement between Day 1 and Day 10 (P < 0.01). Clinical assessment and assessment of changes in the status of the extremity was made on the basis of a modified scale of SVS/ISCVS classification combining a change in relatively subjective symptomatology (Figure 1). According to the SVS/ISCVS grading scale, 12 (25%) patients demonstrated mild improvement, whereas 36 patients demonstrated moderate improvement. Mean improvement score was 1.166 (Table II).
Figure 1.
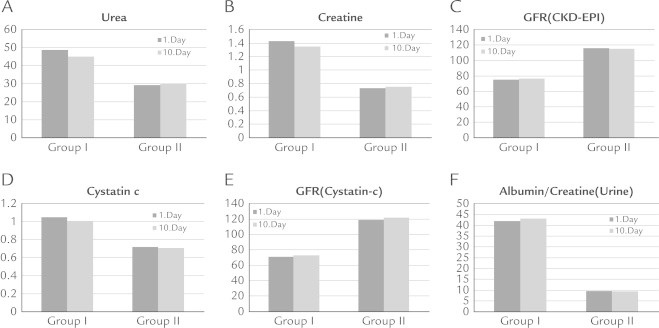
The results of iloprost therapy on renal markers, including (A) levels of urea, (B) creatine, (C) glomerular filtration rate Chronic Kidney Disease Epidemiology Collaboration (GFR [CKD-EPI]) (equation for estimating GFR expressed for specified race, sex, and serum creatinine in milligrams per deciliter), (D) cystatin C, (E) GFR (cystatin C) (GFR calculated by using serum cystatin C), and (F) albumin/creatine ratio (urine) on first and 10th day of treatment.
Table II.
Society of Vascular Surgery/International Society of Cardiovascular Surgery clinical status grading scale.
| +3 | Markedly improved: No ischemia symptoms, and any foot lesions completely healed; ankle-brachial index essentially normalized (ie, increased to >0.90) |
|---|---|
| +2 | Moderately improved: No open foot lesions; still symptomatic but only with exercise and improved by at least 1 category; ankle-brachial index not normalized but increased by >0.10 |
| +1 | Minimally improved: >0.10 increase in ankle brachial index* but no categorical improvement or vice versa (ie, upward categorical shift without an increase in ankle brachial index >0.10) |
| 0 | No change: No categorical shift and <0.10 change in ankle brachial index |
| −1 | Mildly worse: No categorical shift but ankle brachial index decreased >0.10, or downward categorical shift with ankle brachial index decrease <0.10 |
| −2 | Moderately worse: 1 category worse or unexpected minor amputation |
| −3 | Markedly worse: >1 category worse or unexpected major amputations |
⁎In cases where the ankle-brachial index cannot be accurately measured, an index based on toe pressure is used.
Renal function before and after treatment
In Group I, significant decrease (P < 0.05) in serum urea, creatinine, and cystatin C levels were observed compared with pretreatment levels, whereas GFRcyc and GFR CKD-EPI increased significantly (P < 0.05). No significant change was observed in urine albumin:creatinine ratio (P > 0.05) (Table III). The results of iloprost on renal function are summarized in Figure 1
Table III.
Clinical changes after the iloprost treatment
| Group I (n = 22) | Group II (n = 26) | P* | |
|---|---|---|---|
| Ankle-brachial index (mean [SD]) | |||
| Initial | 0.47 [0.14] | 0.44 [0.18] | 0.53 |
| 10th day | 0.53 [0.14] | 0.51 [0.17] | 0.66 |
| P† | 0.01 | 0.01 | |
| Analgesic requirement (n [%]) | |||
| Initial | 22 (100) | 26 (100) | 1.00 |
| 10th day | 8 (36.3) | 12 (46.1) | 0.56 |
| P† | <0.001 | <0.001 | |
| Society for Vascular Surgery and International Society for Cardiovascular Surgery grading scale | |||
| Initial | 0.00 [0.00] | 0.00 [0.00] | |
| 10th day | 1.19 [1.13] | 1.14 [0.98] | 0.09 |
| P† | <0.001 | <0.001 | |
| Improvement in walking distance on the 10th day (n [%]) | 14 (63.6) | 18 (69.2) | 0.76 |
Comparison of intergroup initial values and results of the treatment with iloprost on the 10th day.
Comparison of intragroup initial values and results of the treatment with iloprost on the 10th day.
Discussion
We present our experiences regarding use of iloprost treatment on kidney function in patients with CLI and early stage chronic renal dysfunction. Our results suggest that iloprost therapy has positive effects on renal function and may be preferred as a treatment option in patients with stage 1 and 2 chronic renal dysfunction and CLI. After treatment with iloprost compared with baseline, in Group 1 the levels of serum urea, creatinine, and cystatin C significantly decreased, the level of GFRcys and GFR CKD-EPI significantly increased. Urinary albumin:creatinine ratio did not change significantly.
Prostanoids are an important part of treatment in patients unsuitable for surgery or intravascular revascularization. Prostanoids act by the inhibition of platelet adhesion and activation, dilation in arteriols and venules, and inhibition of leukocyte adhesion and migration after endothelial injury. Moreover, they are contributory agents of revascularization. They facilitate neovascularization not only by increasing endothelial cell proliferation in vascular structures but also by increasing endothelial progenitor cell mobilization from bone marrow, which leads to reendothelization.5 Endothelial progenitor cell level is low in patients with CLI. Di Stefano et al6 determined that iloprost increases endothelial progenitor cell level in peripheral blood. There are numerous studies showing that 7-day 0.5 to 2.0 ng/kg/min application of iloprost, which is a prostacyclin analogue, is a safe and effective treatment in CLI by means of a decrease in rest pain, a reduction in the risk of amputation, and its positive effects on ulcer healing.4 In our study patients were administered a 7-day iloprost treatment for 6 hours each day. Iloprost is generally well tolerated despite adverse effects such as hypotension, headache, flushing, nausea, vomiting, and diarrhea, which limit its use.7 Treatment was discontinued during the second day in 1 patient in Group II due to hypotension and headache. Mildness of the side effects in other patients allowed continuation of the treatment.
The citeria for the evaluation of the treatment response in patients with CLI are reduction in analgesic requirement, commencement of walking, reduction in ulcer diameter, increase in ABI, and SVS/ISCVS criteria changes. The SVS/ISCVS grading scale provides objective information about the symptomatic improvement experienced by a patient. It combines clinical standards with objective, noninvasive tests. If a patient shifts at least 1 clinical category upward, he or she is considered to be clinically improved, except in patients for whom there is actual tissue loss (category 5). Category 5 patients are considered clinically improved when there is at least an upward shift of 2 clinical categories and the patient has reached at least the level of claudication. In this study, the increase in ABI was more than the required value. Mean improvement was considered to be minimal–moderate (mean score = 1.166, n = 48).
GFR is the most reliable marker for the assessment of renal function. In the clinical setting, clearance formulas are used in the calculation of GFR.10 GFR is obtained from the calculation of the urinary clearance of ideal filtration markers. The ideal marker should be found free in the circulation, should be freely filtered through the glomerular basal membrane, should not be secreted along the nephron, should not be reabsorbed, should be produced endogenously at a constant rate, and should be easily measured.10 Inulin is an exogenous polysaccharide that meets the criteria for the ideal marker and whose urinary clearance has been accepted as the standard method of GFR measurement. However, it is not used in clinical practice. Serum creatinine, which is the most widely used marker in clinical practice, is not sensitive in the detection of mild and moderate changes in GFR because of its nonlinear association with GFR. In our study, although serum creatinine levels decreased significantly after treatment in patients with impaired renal function, no statistically significant change was observed in GFR calculated using serum creatinine.
Cystatin C is a nonglycosylated, 122-amino-acid protein of low molecular weight (13kDa) that is a member of the cystatin super family of cystein proteinase inhibitors.16 There are numerous studies indicating that serum cystatin level is well correlated with GFR and cystatin C is accepted as an early marker of renal failure.17,18 Cystatin C is produced in all nucleated cells and its rate of production does not change in inflammatory states. Thyroid function has a major effect on cystatin C levels.19 In our study serum cystatin C level and GFRcyc, which give an earlier indication of renal dysfuntion, were used to assess the effects of iloprost on renal function, besides serum creatinine. At the end of treatment, no significant change was observed in cystatin C level or GFRcyc in Group II, composed of patients with normal renal function, whereas in Group I, composed of patients with stage 1 and 2 chronic renal dysfunction, cystatin C decreased and GFR increased significantly (Table IV).
Table IV.
Changes in renal function with iloprost use in patients with chronic renal dysfunction (Group I) and normal renal function (Group II)
| Parameters | Group I | Group II | ||||
|---|---|---|---|---|---|---|
| BI |
AI |
P | BI |
AI |
P | |
| Mean (SD) | Mean (SD) | |||||
| Urea | 48.7 (9.5) | 45.1 (8.8) | 0.037 | 29.1 (6.8) | 30.2 (4.0) | 0.292 |
| Creatinine | 1.43 (0.24) | 1.35 (0.18) | 0.017 | 0.73 (0.2) | 0.75 (0.10) | 0.534 |
| GFR CKD-EPI* | 75.5 (4.6) | 76.6 (4.1) | 0.032 | 116 (3.7) | 115 (3.8) | 0.29 |
| Cystatin C | 1.05 (0.27) | 1.01 (0.24) | 0.045 | 0.72 (0.06) | 0.71 (0.04) | 0.168 |
| GFR cystatin C† | 71 (8.9) | 73 (7.8) | 0.041 | 119 (12) | 122 (11) | 0.224 |
| Alb:Cr (urine) | 42.12 (8.7) | 43.1 (8.2) | 0.892 | 9.6 (4.3) | 9.5 (3.7) | 0.324 |
AI = after iloprost; Alb:Cr (urine) = albumin:creatinine ratio (urine); BI = before iloprost; CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration; GFR = glomerular filtration rate.
CKD-EPI equation for estimating glomerular filtration rate expressed for specified race, sex, and serum creatinine in milligrams per deciliter.
GFR calculated by using serum cystatin C.
There are many studies demonstrating the beneficial effects of iloprost on renal function. Spargias et al8 reported that iloprost was effective in the prevention of contrast-induced nephropathy through renal vasodilation. Canacankatan et al20 showed that in ischemia reperfusion studies iloprost has beneficial effects on apoptosis through its cytoprotective effect. Iloprost has beneficial effects on renal function by increasing intrarenal oxygenization in endotoxemic rats.21 The decrease in renal blood flow along with the progress of renal disease causes medullar ischemia and progressive renal dysfunction in patients with chronic renal failure. Prostacyclin increases renal blood flow via its vasodilator effect. Fujita et al22 stated that iloprost slows down the progress of renal dysfunction along with increasing renal blood flow. It was reported that, in patients with diabetic nephropathy, urinary albumin excretion and urinary albumin:creatinine ratio was reduced by 2 weeks of slow iloprost infusion (10 μg at a rate of 0.075 μg/kg/h) therapy. Conversely, in our study, although the parameters checked for the assessment of renal function in Group I (ie, serum urea, creatinine, cystatin C, and GFRcyc) improved significantly due to increased renal blood flow and vasodilation, they did not reach normal limits. Moreover, urine albumin:creatinine ratio, which is an indicator of renal damage, did not change significantly. In Group II, in which the renal function was normal at baseline, no deterioration was observed in renal function and all the parameters remained inside normal limits.
One of the 2 important limitations of this study is relatively small sample size. The second limitation is that patients received intravenous iloprost for 7 days and the markers of renal dysfunction improved significantly at the end of 10 days. However, these results are early results; mid- or long-term follow-up regarding renal functions of the patients after this 10-day period are not included in this report.
Conclusions
Iloprost, used in the treatment of a limited number of patients with CLI, can slow down the progress of early stage renal dysfunction. However, we think that a longer follow-up would be needed for a better assessment. GFRcyc and cystatin C, which are markers of early-stage chronic renal dysfunction, can be used in the evaluation of treatment efficacy.
Acknowledgments
All authors contributed throughout the manuscript in terms of writing and editing. Dr. Y. Ay carried out the study and had the main responsibility for writing the manuscript. Drs. Y. Ay, N.-K. Ay, and Gorur collected data, analyzed, interpreted data, and drafted the manuscript. Drs. Y. Ay, Aydin, and Kara contributed to concept, data analysis and study design. Drs. Findik, Kara, and Koksal provided important intellectual content to the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
The authors have indicated that they have no conflicts of interest regarding the content of this article.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Trademark: Ilomedin (Schering AG,Berlin, Germany).
References
- 1.Dormandy J.A., Rutherford R.B. Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter-Society Consensus (TASC) J Vasc Surg. 2000;31:1–296. [PubMed] [Google Scholar]
- 2.Dormandy J.A. Prostanoid drug therapy for peripheral arterial occlusive disease: the European experience. Vasc Med. 1996;1:155–158. doi: 10.1177/1358863X9600100212. [DOI] [PubMed] [Google Scholar]
- 3.O'Hare A.M., Bertenthal D., Sidawy A.N. Renal insufficiency and use of revascularization among a national cohort of men with advanced lower extremity peripheral arterial disease. Clin J Am Soc Nephrol. 2006;1:297–304. doi: 10.2215/CJN.01070905. [DOI] [PubMed] [Google Scholar]
- 4.Lessiani G., Vazzana N., Cuccurullo C. Inflammation, oxidative stress and platelet activation in aspirin-treated critical limb ischaemia: beneficial effects of iloprost. Thromb Haemost. 2011;105:321–328. doi: 10.1160/TH10-07-0499. [DOI] [PubMed] [Google Scholar]
- 5.Coppolino G., Buemi A., Bolignano D. Perioperative iloprost and endothelial progenitor cells in uremic patients with severe limb ischemia undergoing peripheral revascularization. J Surg Res. 2009;157:129–135. doi: 10.1016/j.jss.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 6.Di Stefano R., Barsotti M.C., Melillo E. The prostacyclin analogue iloprost increases circulating endothelial progenitor cells in patients with critical limb ischemia. Thromb Haemost. 2008;100:871–877. [PubMed] [Google Scholar]
- 7.Sert M., Soydas B., Aikimbaev K. Effects of iloprost (a prostacyclin analogue) on the endothelial dysfunction and foot ulcers in diabetic patients with peripheral arterial disease. Int J Diabetes Metab. 2008;16:7–11. [Google Scholar]
- 8.Spargias K., Adreanides E., Demerouti E. Iloprost prevents contrast-induced nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. Circulation. 2009;120:1793–1799. doi: 10.1161/CIRCULATIONAHA.109.863159. [DOI] [PubMed] [Google Scholar]
- 9.Sahsivar M.O., Narin C., Kiyici A. The effect of iloprost on renal dysfunction after renal I/R using cystatin C and beta2-microglobulin monitoring. Shock. 2009;32:498–502. doi: 10.1097/SHK.0b013e3181a1ba54. [DOI] [PubMed] [Google Scholar]
- 10.Manjunath G., Sarnak M.J., Levey A.S. Estimating the glomerular filtration rate. Postgrad Med. 2001;110:55–62. doi: 10.3810/pgm.2001.12.1065. [DOI] [PubMed] [Google Scholar]
- 11.Norgren L., Hiatt W.R., Dormandy J.A. Inter-society consensus for the management of peripheral arterial disease (TASC II) Eur J Vasc Endovasc Surg. 2007;33:1–75. doi: 10.1016/j.ejvs.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 12.Levey A.S., Coresh J., Balk E. National Kidney Foundation. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 13.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grubb A.O. Cystatin C for GFR. Adr Clin Chem. 2001;35:53–59. [Google Scholar]
- 15.Rutherford R.B., Baker J.D., Ernst C. Recommended standards for reports dealing with lower ex tremity ischemia: revised version. J Vasc Surg. 1997;26:517–538. doi: 10.1016/s0741-5214(97)70045-4. [DOI] [PubMed] [Google Scholar]
- 16.Coll E., Botey A., Alvarez L. Serum sistatin C as a new marker for non-invasive estimation of glomerular filtration rate and as a marker for early renal impairment. Am J Kidney Dis. 2000;36:29–34. doi: 10.1053/ajkd.2000.8237. [DOI] [PubMed] [Google Scholar]
- 17.Laterza O.F., Price C.P., Scott M.G. Sistatin C: an improved estimator of glomerular filtration rate? Clin Chem. 2002;48:699–707. [PubMed] [Google Scholar]
- 18.Filler G., Bökenkamp A., Hofmann W. Sistatin C as a marker of GFR- history, indications, and future research. Clin Biochem. 2005;38a:1–8. doi: 10.1016/j.clinbiochem.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 19.Fricker M., Wiesli P., Brandle M. İmpact of tyroid dysfunction on serum cystatin C. Kidney Int. 2003;63:1944–1947. doi: 10.1046/j.1523-1755.2003.00925.x. [DOI] [PubMed] [Google Scholar]
- 20.Canacankatan N., Sucu N., Aytacoglu B. Affirmative effects of ıloprost on apoptosis during ıschemia-reperfusion ınjury in kidney as a distant organ. Renal Fail. 2012;34:111–118. doi: 10.3109/0886022X.2011.633446. [DOI] [PubMed] [Google Scholar]
- 21.Johannes T., Ince C., Klingel K. Iloprost preserves renal oxygenation and restores kidney function in endotoxemia-related acute renal failure in the rat. Crit Care Med. 2009;37:1423–1432. doi: 10.1097/CCM.0b013e31819b5f4e. [DOI] [PubMed] [Google Scholar]
- 22.Fujita T., Fuke Y., Satomura A. PGl2 analogue mitigates the progression rate of renal dysfunction improving renal blood flow without glomerular hyperfiltration in patients with chronic renal insufficiency. Prostaglandins Leukot Essent Fatty Acids. 2001;65:223–227. doi: 10.1054/plef.2001.0315. [DOI] [PubMed] [Google Scholar]


