Abstract
Observational studies in primary hyperaldosteronism (PA) suggest a positive relationship between aldosterone and parathyroid hormone (PTH); however, interventions to better characterize the physiologic relationship between the renin-angiotensin-aldosterone system (RAAS) and PTH are needed. We evaluated the effect of individual RAAS components on PTH using 4 interventions in humans without PA. PTH was measured before and after: Study 1) low-dose angiotensin II [AngII] infusion (1 ng/kg/min) and captopril administration (25 mg × 1); Study 2) high-dose AngII infusion (3 ng/kg/min); Study 3) blinded crossover randomization to aldosterone infusion (0.7 µg/kg/hr) and vehicle; and Study 4) blinded randomization to spironolactone (50mg/daily) or placebo for 6 weeks. Infusion of AngII at 1 ng/kg/min acutely increased aldosterone (+148%) and PTH (+10.3%), while AngII at 3 ng/kg/min induced larger incremental changes in aldosterone (+241%) and PTH (+36%) (P<0.01). Captopril acutely decreased aldosterone (−12%) and PTH (−9.7%) (P<0.01). In contrast, aldosterone infusion robustly raised serum aldosterone (+892%) without modifying PTH. However, spironolactone therapy over 6 weeks modestly lowered PTH when compared to placebo (P<0.05). In vitro studies revealed the presence of AngII type I and mineralocorticoid receptor mRNA and protein expression in normal and adenomatous human parathyroid tissues. We observed novel pleiotropic relationships between RAAS components and the regulation of PTH in individuals without PA: the acute modulation of PTH by the RAAS appears to be mediated by AngII, whereas the long-term influence of the RAAS on PTH may involve aldosterone. Future studies to evaluate the impact of RAAS inhibitors in treating PTH-mediated disorders are warranted.
Keywords: Parathyroid hormone, Renin-Angiotensin-Aldosterone System, vitamin D, Spironolactone, calcium
INTRODUCTION
Characterization of the complex endocrine relationships between sodium- and calcium-regulatory hormones is evolving. Calcium1,2, vitamin D3–9, and parathyroid hormone (PTH)10–12 have been implicated in regulating the renin-angiotensin-aldosterone system (RAAS), and the RAAS, in turn, has been implicated in regulating these calcium-regulatory hormones13–16. In particular, recent observational studies in individuals with primary hyperaldosteronism (PA) suggest that excess aldosterone may result in hyperparathyroidism15–17. A better understanding of the normal physiologic relationship between the RAAS and PTH is of clinical relevance since inappropriate activity of both PTH and the RAAS may negatively impact cardiovascular18–26 and skeletal health27,28.
Human evidence characterizing the positive relationship between RAAS activity and PTH has largely originated from observational studies in disease states such as hyperaldosteronism15,16 and chronic kidney disease (CKD)29. Spironolactone therapy is associated with lower fracture risk in heart failure27; patients with PA have reduced bone mineral density and a higher rate of osteoporosis30; and PA is associated with elevated PTH levels that are lowered following clinically indicated surgical or medical therapy for PA14–16. Furthermore, the use of RAAS inhibitors is associated with lower PTH levels in CKD29.
The aforementioned studies provided noteworthy evidence to advance the understanding of RAAS-PTH interactions; however, they were observational in nature and were conducted in populations with severe disease (heart failure, PA, and CKD) where abnormalities in calcium, renal function, and volume homeostasis can be confounding. We hypothesized that physiologic interactions between the RAAS and PTH exist and that characterization of these physiologic relationships may improve the understanding of the pathophysiology and treatment of disease states such as PA and hyperparathyroidism (HPTH). To this end, we evaluated the impact of modulating individual RAAS components on PTH from four previously conducted human intervention studies.
METHODS
General Overview of Study Protocols
We conducted post-hoc analyses of controlled RAAS and calcium-regulatory hormone interventions from four human interventional study protocols in individuals without PA, HPTH, CKD, or heart disease. The subjects in all four studies were overweight or obese, as previously reported4, but had comparable 25-hydroxyvitamin D (25[OH]D) concentrations (Table 1). All study protocols were conducted in a Clinical Research Center (CRC) under conditions of controlled posture, diet, and time of day, and after withdrawal of medications known to modulate the RAAS. Studies 1 and 2 examined the relationship between acute, generalized RAAS modulation and PTH. In Study 1, we performed secondary analyses to evaluate the acute PTH-responses to an infusion of angiotensin II (AngII) and to an angiotensin converting enzyme (ACE) inhibitor (captopril) – interventions expected to acutely stimulate and inhibit both circulating AngII and aldosterone, respectively. Furthermore, these PTH-responses were evaluated in subjects while vitamin D deficient and again following treatment with high-dose vitamin D3 therapy, since modulation of vitamin D status modulates PTH and has been shown to modulate the tissue-responsiveness to AngII in humans4,6,31. In Study 2, we evaluated the dose-dependent relationship between PTH and the RAAS in a similar population to Study 1. Studies 3 and 4 focused specifically on the relationship between aldosterone and PTH. In Study 3, we evaluated the acute effect of aldosterone on PTH in subjects who were randomized to receive an infusion of aldosterone or vehicle in a blinded manner and then crossed over to receive the alternate infusion. Study 4 examined the impact of 6 weeks of double-blinded randomization to either spironolactone or placebo on PTH. Lastly, we performed in vitro studies to assess the expression of AngII type I receptor (AT1R) and mineralocorticoid receptor (MR) in normal and adenomatous human parathyroid tissue. All subjects provided informed consent, and all study procedures described below were approved by the Institutional Review Boards of Brigham and Women’s Hospital (Boston, USA) (Studies 1, 2, 4, and in vitro studies) and Vanderbilt University Medical Center (Nashville, USA) (Study 3).
Table 1. Demographic and Biochemical Characteristics of the Study Populations Prior to Commencing Study Protocols.
Data are presented as means ± SEM, where applicable. All non-African American subjects were Caucasian with the exception of one Asian-American subject in Study 2.
| Characteristic | Study 1 | Study 2 | Study 3 | Study 4 |
|---|---|---|---|---|
| n | 14 | 14 | 10 | 27 |
| Age (years) | 50 ± 2 | 49 ± 3 | 47 ± 3 | 43 ± 2 |
| % Female | 64 | 21 | 70 | 63 |
| % African-American | 70 | 36 | 30 | 41 |
| BMI (kg/m2) | 36.0 ± 1 | 29.5 ± 1 | 33.4 ± 1 | 37.1 ± 1 |
| 25(OH)D (ng/mL) | 16.6 ± 1.9 | 22.6 ± 2.7 | 19.2 ± 2.2 | 21.3 ± 1.5 |
Study 1: Population and Study Protocol
The Study 1 population and protocol has been previously described4 though the data and analyses presented here are novel. The complete study population and protocol details are available in the Online Supplement (see http://hyper.ahajournals.org).
Study 2: Population and Study Protocol
Subjects from Studies 2–4 have never been previously reported. Study 2 is an ongoing interventional physiology study recruiting participants to establish genotype/phenotype correlations in hypertension (NCT01426529). The inclusion criteria and study protocol for study 2 can be seen in the Online Supplement (see http://hyper.ahajournals.org).
Study 3: Population and Study Protocol
Study 3 recruited nondiabetic participants aged 18 to 70 years with the metabolic syndrome to assess the effects of aldosterone on glucose metabolism. In total, 10 subjects who completed the study protocol and had available frozen samples for secondary analysis of PTH were included (NCT00732160).
Study 3 participants were maintained on a liberal sodium diet that included >160mmol/day of sodium, 100mmol/d of potassium, 1,000mg calcium, and calories calculated for weight maintenance. Antihypertensive medications were withdrawn for a minimum of 3 weeks prior to study procedures. Subjects reported for admission to the Vanderbilt CRC in the evening and were randomized to an infusion of aldosterone (0.7mcg/kg/hour in 5% dextrose water; Professional Compounding Corporation of America) or vehicle for 12.5 hours starting at 10PM. The following day, subjects received infusion of the other study drug (aldosterone/vehicle) in the same fashion. Samples were drawn before infusion and at 10 hours during each infusion for PTH analysis. To mitigate the potassium-wasting effect of aldosterone infusion, potassium chloride was administered as needed to maintain serum potassium ≥3.7mmol/L during each infusion.
Study 4: Population and Study Protocol
Study 4 recruited obese, normotensive participants aged 18–70 years to evaluate the effect of mineralocorticoid receptor (MR) antagonist therapy versus placebo on vascular function in a randomized and double-blinded design (NCT01406015). In total, 32 subjects completed the study, of which 27 had available blood and urine to conduct additional analyses. These 27 subjects were selected for our analyses prior to un-blinding of their intervention status or knowledge of their intervention outcomes.
Subjects in Study 4 completed the same diets and washout procedures as described above for Studies 1 and 2 prior to CRC admission. On the morning of their first study visit, subjects had blood sampling that was used in this secondary analysis to measure PTH and other relevant calcium-regulatory components. Subjects also underwent other procedures during this study visit following blood sampling (including vascular hemodynamic protocols) that are not described herein as they are not relevant to the current study aims. All subjects were discharged home with a double-blinded randomized study drug that was 1 tablet daily of either spironolactone 50mg or placebo. After 6 weeks of therapy, subjects returned on the study diet for a second overnight visit to the CRC, and study procedures were repeated the following morning.
Laboratory Assays
Full details of the timing and methods of laboratory assays are provided in the Online Supplement (see http://hyper.ahajournals.org).
In vitro Studies to assess RAAS receptors in Human Parathyroid Tissue
To evaluate the roles of AngII and aldosterone in parathyroid regulation, we obtained normal and adenomatous parathyroid tissue from a patient with primary hyperparathyroidism who underwent a routine neck exploration for parathyroidectomy with hemithyroidectomy. Histopathology confirmed the procurement of a single parathyroid adenoma and a single normal parathyroid gland adherent to thyroid tissue. The full methodological details describing protein and mRNA analysis are in the Online Supplement (see http://hyper.ahajournals.org).
Statistical Analyses
We here describe post hoc analyses of the aforementioned four interventional study protocols with the objective of evaluating the influence of specific RAAS modulations on PTH in relatively healthy populations without PA, CKD, heart disease, or HPTH. All variables presented were normally distributed and were not transformed in any way. All values are presented as means ± standard error of the means (SEM). A two-tailed P-value < 0.05 was considered statistically significant.
In all studies, paired t-tests were used when comparing intra-individual changes in normally distributed variables. Since basal PTH values were altered by the vitamin D3 intervention in Study 1 (an expected physiologic change), we present PTH values not only as absolute values but also as the percent change from baseline to allow a proportional comparison of PTH-responses. In Study 3, where subjects were randomized and crossed over to both interventions (aldosterone and vehicle infusion), we also compare the intra-individual change in each variable due to intervention using paired t-tests. In Study 4, the impact of the intervention (spironolactone or placebo) on each independent population was compared using an ANOVA model, where the post-intervention PTH was the outcome and adjustment for the pre-intervention PTH value was included. Analyses were conducted using SAS 9.2 (SAS Institute, Cary, N.C., U.S.A.).
RESULTS
Study 1: PTH-responses to acute RAAS modulations and the influence of Vitamin D status
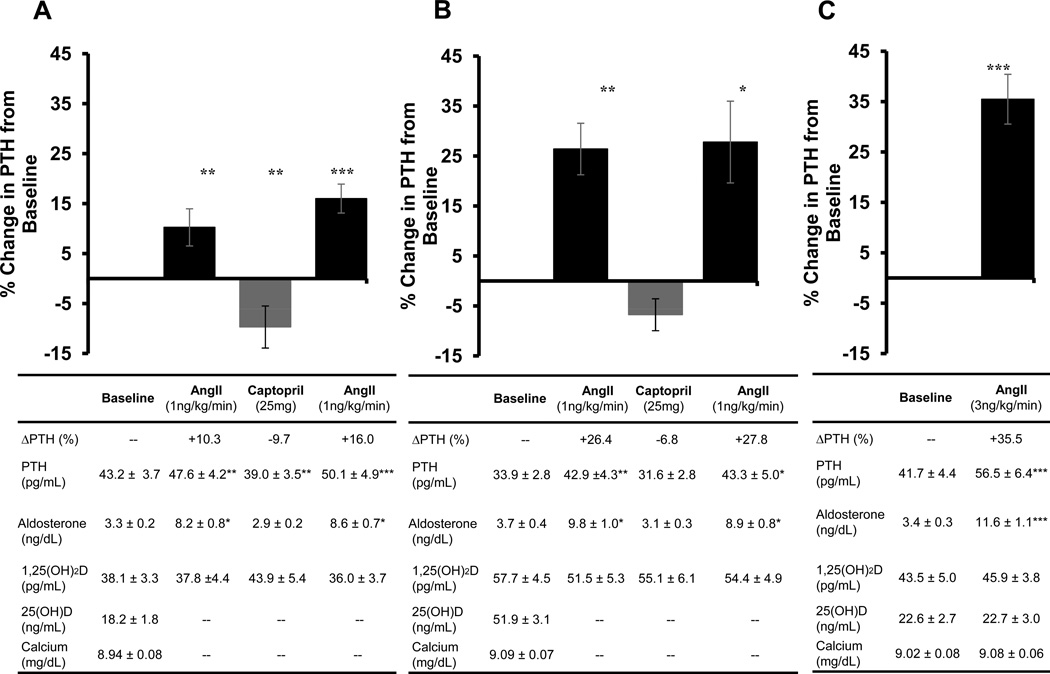
Infusion of AngII (1 ng/kg/min) in a vitamin D deficient state resulted in an expected rise in serum aldosterone (+148%) (as previously described)4, but also a +10.3% increase in PTH from baseline (Figure 1a). A single dose of captopril lowered serum aldosterone levels (−12%) and also acutely lowered PTH below basal levels (−9.7%), and thereafter, a second infusion of AngII following captopril administration resulted in an enhanced stimulation of PTH (+16.0% from baseline) (Figure 1a). When these study procedures were repeated following high-dose vitamin D3 therapy to induce a vitamin D sufficient state, significant changes in PTH-responses to RAAS provocations were observed: the PTH-response to an initial AngII infusion was more robust (+26.4% from baseline) (Figure 1b); captopril induced a smaller non-significant acute reduction in PTH (−6.8%) and had a negligible effect on the magnitude of subsequent AngII-mediated PTH stimulation (Figure 1b). These results parallel our prior observations in these same study subjects, where vitamin D3 therapy augmented the tissue (vascular and adrenal) response to AngII infusion and marginalized the influence of an ACE inhibitor4.
Figure 1. Acute modulation of PTH with RAAS manipulation.
Changes in PTH are displayed graphically as the percent change in PTH from baseline in response to either AngII infusion or ACE inhibition. Absolute values of PTH and related parameters are presented in the table below each graph. A) Study 1 (vitamin D deficient phase): The acute PTH-response to a 1 ng/kg/min infusion of AngII before and after a single dose of Captopril 25mg prior to vitamin D3 intervention. B) Study 1 (vitamin D sufficient phase): The acute PTH-response to a 1 ng/kg/min infusion of AngII before and after a single dose of Captopril 25mg after vitamin D3 intervention. C) Study 2: The acute PTH-response to a 3 ng/kg/min AngII infusion.
Data are presented as means ± SEM. All comparisons are made with the visit baseline values.
*p<0.05, **p<0.01, ***p<0.001.
Study 2: PTH-Responses to Higher Dose AngII Infusion
Study 2 investigated the impact of a higher dose AngII infusion on PTH. In comparison with Study 1, the infusion of 3 ng/kg/min of AngII resulted in greater increments in aldosterone (+241%) and a more robust PTH-response (+36% from baseline), which were independent of any acute changes in serum calcium or 1,25-dihydroxyvitamin D (1,25[OH]2D) concentrations (Figure 1c). When integrated with the findings from Study 1 (Figure 1a–c), an incremental relationship between the RAAS and PTH was observed, which was modulated by AngII dose, as well as by ACE inhibition and vitamin D status (both of which sensitize the tissue responsiveness to AngII infusion)4,6,31–33.
Study 3: PTH-responses to Aldosterone Infusion
To isolate whether the aforementioned acute modulations in PTH induced by AngII infusion were mediated by AngII itself or aldosterone, Study 3 investigated the impact of an aldosterone infusion on PTH. Baseline PTH levels before aldosterone and vehicle infusions were similar (Supplementary Table S1 - see http://hyper.ahajournals.org). The infusion of aldosterone resulted in a robust increase in serum aldosterone concentration (+892%) that was substantially higher than that induced by AngII infusions in Studies 1 and 2, as well as non-significant trends of increased blood pressure and decreased potassium. However, PTH was not modified by the infusion of aldosterone when compared to vehicle (Supplementary Table S1).
Study 4: PTH-responses to Chronic Mineralocorticoid Receptor Antagonist Therapy
Although an aldosterone infusion did not influence PTH in the acute setting, we evaluated the effect of chronic aldosterone blockade on PTH in Study 4 because the findings of Rossi et al. and Tomaschitz et al. have suggested that chronic aldosterone excess in PA may increase PTH15,16. The effect of chronic spironolactone therapy was evident within the treatment group via increments in aldosterone concentrations and decrements in blood pressure (Table 2). When comparing the pre- versus post-intervention effect of each treatment arm, neither placebo nor spironolactone therapy significantly altered the levels of PTH, serum calcium, 25(OH)D, or markers of bone formation and resorption over 6 weeks (Table 2). However, analyses comparing the change in these parameters induced by each intervention revealed a decrease in serum calcium and increase in PTH in the placebo group (possibly mediated by chronic and persistent vitamin D deficiency) that was significantly blunted by spironolactone treatment (Table 2, ANOVA P-value). A similar non-significant trend was seen with respect to C-telopeptide levels.
Table 2. Clinical and Biochemical Results for Study 4: Double-blinded randomization to spironolactone or placebo for 6 weeks.
Subjects were randomized to either spironolactone (50mg/day) or placebo for 6 weeks with blood measurements before and after the intervention period. The pre- and post-intervention values for each variable are presented with a P-value reflecting the paired comparison. In addition, the difference in the change in each variable induced by the interventions is presented in the far right column.
| Clinical and Biochemical Parameters |
Placebo | Spironolactone | P-value | ||||
|---|---|---|---|---|---|---|---|
| Age (y) | 40.8 ± 3 | 45.4 ± 3 | 0.32 | ||||
| % Female | 69 | 57 | 0.54 | ||||
| % African American | 54 | 29 | 0.82 | ||||
| BMI (kg/m2) | 36.1 ± 1 | 38.1 ± 2 | 0.42 | ||||
| Pre | Post |
Pre vs Post P-value |
Pre | Post |
Pre vs Post P-value |
ANOVA P-value |
|
| Systolic Blood Pressure (mmHg) | 119 ± 4 | 120 ± 3 | 0.70 | 123 ± 3 | 117 ± 3 | 0.0004 | <0.05 |
| Diastolic Blood Pressure (mmHg) | 71 ± 2 | 72 ± 2 | 0.54 | 72 ± 2 | 70 ± 2 | 0.19 | 0.21 |
| Serum Potassium (mmol/L) | 4.2 ± 0.1 | 4.1 ± 0.1 | 0.51 | 4.1 ± 0.1 | 4.2 ± 0.1 | 0.28 | 0.47 |
| Aldosterone (ng/dL) | 3.6 ± 0.4 | 3.1 ± 0.3 | 0.30 | 3.3 ± 0.4 | 6.6 ± 1.4 | 0.04 | <0.05 |
| Urine Aldosterone Excretion Rate (µg/24h) | 11.1 ± 1.7 | 9.2 ± 1.9 | 0.38 | 7.5 ± 1.2 | 12.2 ± 2.0 | 0.06 | 0.18 |
| Serum Calcium (mg/dL) | 9.0 ± 0.1 | 8.8 ± 0.1 | 0.10 | 8.9 ± 0.1 | 9.0 ± 0.1 | 0.49 | <0.05 |
| 25(OH)D (ng/mL) | 18.4 ± 2.0 | 19.6 ± 2.3 | 0.32 | 24.0 ± 1.9 | 24.0 ± 2.1 | 0.93 | 0.484 |
| PTH (pg/mL) | 41 ± 4 | 45 ± 4 | 0.07 | 38 ± 3 | 37 ± 3 | 0.31 | <0.05 |
| C-terminal telopeptide (ng/mL) | 0.46 ± 0.1 | 0.50 ± 0.1 | 0.09 | 0.51 ± 0.1 | 0.48 ± 0.1 | 0.28 | 0.07 |
| Procollagen type 1 N-terminal propeptide (µg/L) | 39 ± 4 | 41 ± 4 | 0.60 | 44 ± 5 | 43 ± 5 | 0.58 | 0.57 |
In vitro Studies to assess RAAS-related receptors in Human Parathyroid Tissue
AT1R and MR protein were detected in normal human parathyroid tissue (Supplementary Figure S1 - (see http://hyper.ahajournals.org); normal parathyroid tissue had relatively similar AT1R and MR protein levels when compared to mouse adrenal tissue. In addition, AT1R and MR were detected in parathyroid adenoma tissue of the same patient, with a 2–4-fold increase in expression when compared to normal parathyroid and mouse adrenal tissues. To further confirm AT1R and MR expression in parathyroid tissue, we quantified mRNA levels and observed expression in normal parathyroid tissue and a 3–4-fold increase in expression within adenomatous tissue (Supplementary Figure S1).
DISCUSSION
Our data demonstrate novel physiologic relationships between PTH and RAAS activity in humans without PA for the first time. Despite the notable limitations of our methods (the use of 4 small and separate study populations of obese individuals, post hoc analyses, and the absence of data on ionized and urinary calcium that could help uncover underlying mechanisms), we have nonetheless demonstrated new pleiotropic relationships between RAAS components and PTH that may have important implications for the treatment of PTH-mediated disorders.
Relationships between the RAAS and calcium-regulatory hormones that were initially identified in the past13,14,34,35 have recently been revisited and complemented by data that shed new insights into the complex interactions between these hormone systems: vitamin D3–6,11 and calcium1,2 appear to regulate renin, and a reciprocal relationship between the RAAS and PTH has been proposed15,16,36,37. The latter has largely stemmed from observational studies in PA that implicate aldosterone as a regulator of PTH. Inappropriately elevated serum aldosterone has been associated with high PTH levels that are lowered with treatment of PA14–16. Together these studies describe a pathophysiology in states of chronic aldosterone excess whereby hyperparathyroidism is induced, possibly secondary to aldosterone-mediated hypercalciuria14–16. Herein, we extend the findings of these prior studies with analyses from controlled human interventional protocols to evaluate the physiologic relationship between the RAAS and PTH in the absence of PA. Our findings, in the context of prior work by Tomaschitz et al and Rossi et al36,37, raise important questions that warrant discussion for future studies. Is PTH regulated by AngII, aldosterone, or both? Does the physiologic relationship between PTH and the RAAS differ with respect to the duration of RAAS modulations (acute versus chronic), or in physiologic versus pathophysiologic states? What significance do these findings have for cardiovascular and skeletal health?
Is PTH Regulated by AngII?
Identifying whether PTH is regulated by AngII, or aldosterone, or both, could have implications for pharmacologic therapies. Our results demonstrate that raising circulating AngII and aldosterone acutely stimulates PTH in a dose-dependent manner, while lowering endogenous AngII and aldosterone acutely with an ACE inhibitor lowers PTH (Supplementary Table S2: columns a+b - (see http://hyper.ahajournals.org). Since elevated aldosterone levels have been associated with elevated PTH levels in PA14,16,17, we hypothesized that an acute aldosterone infusion would also raise PTH. However, despite an acute 10-fold increase in serum aldosterone concentrations in Study 3, PTH remained unchanged (Table S2: column d), implicating AngII as the primary stimulus for PTH in the acute setting. The role of AngII as the mediator of these effects was further supported by the fact that the regulation of PTH was influenced by interventions known to sensitize tissue-responsiveness to AngII4,33: both ACE inhibition and vitamin D3 therapy independently enhanced the PTH-response to AngII infusion. We previously showed that vitamin D deficiency blunts tissue-responsiveness to exogenous AngII by inducing a state of high tissue-RAAS activity4,6,31; this blunted sensitivity to AngII could be corrected by lowering the tissue-RAAS with an ACE inhibitor or vitamin D3 therapy4,33. Consistent with this phenomenon, in Study 1 we observed an increase in PTH with AngII infusion that was augmented following captopril in the vitamin D deficient state. The PTH-response to AngII was further enhanced in the same subjects following vitamin D3 therapy, which also marginalized the influence of captopril on subsequent AngII infusions (presumptively due to maximal suppression of the endogenous tissue-RAAS with vitamin D3 therapy)4. Our identification of AT1R in normal and adenomatous human parathyroid tissue is a novel finding that further supports an acute and direct effect of AngII on PTH; we speculate that given the acuity of the findings, AngII may regulate the secretion of pre-formed PTH. Whether the effect of AngII on PTH persists beyond the acute period remains unknown (Table S2: column c); however, the finding that a parathyroid adenoma expresses higher levels of AT1R than normal parathyroid may impicate AngII in the pathogenesis and/or progression of hyperparathyroidism. An ongoing interventional study, The RAAS-PARC Study (NCT01691781), will provide insight into PTH-responses to acute and chronic ACE inhibition in individuals with primary hyperparathyroidism in comparison to normal healthy controls.
Is PTH Regualted by Aldosterone?
Alternatively, our findings suggest that in the chronic setting, it may be aldosterone rather than AngII which directly and/or indirectly stimulates PTH. This mechanism has been proposed13–17 in observational reports in patients with PA (a condition where aldosterone levels are chronically elevated with concomitant suppression of AngII and renin). Some of these observational reports included abnormalities in renal function and changes in serum and urinary calcium, which may have also contributed to PTH effects14–16. In addition, chronic MR antagonism has even been observationally associated with reduced fracture risk27, implicating PTH regulation by aldosterone as a potential mediator of this outcome. In our Study 4, chronic MR antagonism may have modestly lowered PTH and raised serum calcium. Although no change in PTH was observed in the group randomized to spironolactone treatment, PTH was significantly impacted by the spironolactone intervention when compared to the placebo-treated control (Table S2: column e). This phenomenon may have reflected a mild progressive secondary hyperparathyroidism in the placebo group due to chronic vitamin D deficiency that was blunted by spironolactone. Interestingly, this observation was seen despite the fact that MR antagonism should raise AngII concentrations and in a population with low aldosterone levels (subjects did not have PA and were on a liberal sodium diet); it is possible that the effect of spironolactone would have been more evident in a population with higher serum aldosterone16, hyperparathyroidism, longer duration of treatment, or larger sample size. Although we did not have urine calcium measurements in this study, prior research has shown that chronic aldosterone excess results in hypercalciuria and hypocalcemia16,38,39. Since we, along with Maniero et al10, have identified the presence of MR in normal and abnormal parathyroid tissue, we speculate that aldosterone action through the MR may impact PTH over the long-term by influencing PTH synthesis directly at the parathyroid gland and/or indirectly via renal regulation of calcium handling. The influence of MR antagonists on PTH may thus be most evident in populations with PA or HPTH, rather than relatively healthy individuals such as those in our studies. Studies of biochemical and clinical outcomes of MR antagonism in larger targeted populations will clarify this effect. The EPATH study (ISRCTN33941607)40 is designed to conclusively evaluate the influence of long-term MR antagonism on clinical outcomes in a large study population with primary hyperparathyroidism.
Is PTH Regulated by Both AngII and Aldosterone?
Overall, our findings suggest pleiotropic effects of RAAS components on PTH that may vary with time of exposure. Although we used PTH levels as our outcome, it is important to realize that in endocrine systems, where feedback mechanisms are complex, the absolute circulating level of any hormone may not be sufficient to evaluate biologic and clinical outcomes; the setting of a new homeostatic equilibrium may not be detected by circulating levels alone. Such a phenomenon, whereby levels of one hormone are maintained at the expense of changes to others, is common. For example, although ACE inhibitors acutely lower AngII and aldosterone concentrations, chronic ACE inhibition results in a re-equilibration and normalization of aldosterone levels41, yet the clinical benefits associated with chronic ACE inhibition remain highly favorable42. The reestablishment of the PTH-RAAS equilibrium may be most evident in physiologic states where both calcium- and sodium-regulatory hormones can modulate their activity appropriately. In contrast, in pathophysiologic states, such as PA or HPTH, normal mechanisms may be overwhelmed by the effects of excess aldosterone or PTH.
Strengths and Limitations
Our study has notable strengths and limitations. We describe post hoc analyses of previously conducted studies, which were not principally designed for evaluation of RAAS-PTH releationships. However, though these analyses were secondary in nature, we used data from 4 human intervention studies that employed careful methods to control modulators of the RAAS (including dietary electrolytes, posture, and antihypertensive medications) and calcium-regulatory hormones (including dietary calcium and vitamin D status). The sample sizes were small but were more than sufficient to detect robust changes in PTH (Studies 1 and 2) and expected changes in serum aldosterone levels and blood pressure due to aldosterone infusion (Study 3) and spironolactone (Study 4). It is possible that a larger population in Study 4 would have permitted greater power in detecting MR antagonist-induced changes in PTH and serum calcium; however, we suspect these would still have been modest in magnitude since the study population did not have PA or HPTH. Although the studies were performed in separate populations, as necessitated by the complex protocols performed in Clinical Research Centers, these populations were similar across demographic variables and though obese, were otherwise largely healthy. Obesity has been linked to a shift in the 25(OH)D-PTH relationship43; however, acute changes in PTH were observed using within-individual comparisons. We present possible explanations to account for a direct mechanism behind the RAAS-mediated effects we observed; however, calcium modulation has been implicated as potential indirect mediator of RAAS-PTH interactions12, and we did not have detailed electrolyte assessment, including magnesium and ionized and urinary calcium in all of our protocols. Of note, prior studies failed to show any modulation in ionized calcium with the AngII infusion doses we used35, thereby supporting a possible direct AngII-mediated effect on PTH, as we propose. Finally, further studies will be needed to evaluate the role of additional factors in the modulation of the RAAS and PTH, including Klotho44,45, changes in dietary electrolytes36,46–48, and the use of more physiologic doses of angiotensin II (since we used doses necessary to elicit observable effects)49.
PERSPECTIVES
In summary, we describe novel and pleiotropic relationships between PTH and the RAAS in individuals without PA. AngII appears be an acute modulator of PTH, potentially through direct stimulation of PTH release via the AT1R at the parathyroid gland. In contrast, aldosterone may be involved in the modulation of PTH in the chronic setting via indirect and/or direct mechanisms. Our findings build upon observations in pathophysiologic states10,13,16 by providing new insights into the complex relationships between calcium- and sodium-regulatory hormones that may exist in normal physiology. Ongoing40 and future interventional studies are likely to improve our understanding of these interactions and how they may influence the cardiovascular and skeletal outcomes associated with PTH-mediated disorders.
Supplementary Material
NOVELTY & SIGNIFICANCE.
What is new?
We used interventional human studies to characterize novel physiologic and pleiotropic relationships between the RAAS and PTH and conducted in vitro studies to support our clinical findings.
What is relevant?
Understanding how components of the RAAS modulate PTH may improve the understanding and treatment of PTH-mediated disorders.
Summary
Relationships between PTH and the RAAS are pleiotropic. Angiotensin II acutely stimulates PTH release, possibly via direct action at the parathyroid gland, while aldosterone may be involved in the chronic stimulation of PTH via direct and/or indirect interactions.
ACKNOWLEDGMENTS
SOURCES OF FUNDING: Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award numbers: K23 HL111771-01 (A.V), K23 HL08236-05 (J.S.W.), R01 HL104032 (L.H.P.), K24 HL103845 (G.K.A.), 5T32 HL007609-24 (A.E.G.), K23 DK081662 (J.M.L.). Research was also supported by a Brigham and Women’s Hospital Biomedical Research Institute Grant (A.V.), a Harvard Medical School Research Fellowship (J.M.B.), and the American Cancer Society under award MRSG-13-062-01 (D.T.R.). This project was supported by Clinical Translational Science Awards (UL1RR025758, UL1 RR024975) and grant M01-RR02635 to Harvard University, Brigham and Women’s Hospital, and Vanderbilt University from the National Center for Research Resources, and by the Specialized Center of Research in Molecular Genetics of Hypertension Grant P50HL055000. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST/DISCLOSURES: None.
REFERENCES
- 1.Atchison DK, Harding P, Beierwaltes WH. Hypercalcemia reduces plasma renin via parathyroid hormone, renal interstitial calcium, and the calcium-sensing receptor. Hypertension. 2011;58:604–610. doi: 10.1161/HYPERTENSIONAHA.111.172890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ortiz-Capisano MC, Reddy M, Mendez M, Garvin JL, Beierwaltes WH. Juxtaglomerular cell CaSR stimulation decreases renin release via activation of the PLC/IP3 pathway and the ryanodine receptor. Am J Physiol Renal Physiol. 2012;304:F248–F256. doi: 10.1152/ajprenal.00451.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaidya A, Sun B, Larson C, Forman JP, Williams JS. Vitamin D3 therapy corrects the tissue sensitivity to angiotensin ii akin to the action of a converting enzyme inhibitor in obese hypertensives: an interventional study. J Clin Endocrinol Metab. 2012;97:2456–2465. doi: 10.1210/jc.2012-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaidya A, Forman JP, Hopkins PN, Seely EW, Williams JS. 25-Hydroxyvitamin D is associated with plasma renin activity and the pressor response to dietary sodium intake in Caucasians. J Renin Angiotensin Aldosterone Syst. 2011;12:311–319. doi: 10.1177/1470320310391922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaidya A, Forman JP, Williams JS. Vitamin D and the vascular sensitivity to angiotensin II in obese Caucasians with hypertension. J Hum Hypertens. 2011;25:672–678. doi: 10.1038/jhh.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaidya A, Sun B, Forman JP, Hopkins PN, Brown NJ, Kolatkar NS, Williams GH, Williams JS. The Fok1 vitamin D receptor gene polymorphism is associated with plasma renin activity in Caucasians. Clin Endocrinol (Oxf) 2011;74:783–790. doi: 10.1111/j.1365-2265.2011.03991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaidya A, Williams JS. The relationship between vitamin D and the renin-angiotensin system in the pathophysiology of hypertension, kidney disease, and diabetes. Metabolism. 2012;61:450–458. doi: 10.1016/j.metabol.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 10.Maniero C, Fassina A, Guzzardo V, Lenzini L, Amadori G, Pelizzo MR, Gomez-Sanchez C, Rossi GP. Primary hyperparathyroidism with concurrent primary aldosteronism. Hypertension. 2011;58:341–346. doi: 10.1161/HYPERTENSIONAHA.111.173948. [DOI] [PubMed] [Google Scholar]
- 11.Tomaschitz A, Pilz S, Ritz E, Grammer T, Drechsler C, Boehm BO, März W. Independent association between 1,25-dihydroxyvitamin D, 25-hydroxyvitamin D and the renin-angiotensin system: The Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Clin Chim Acta. 2010;411:1354–1360. doi: 10.1016/j.cca.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 12.Pilz S, Tomaschitz A, März W, Cavalier E, Ritz E. Aldosterone and parathyroid hormone: a complex and clinically relevant relationship. Calcif Tissue Int. 2010;87:373–374. doi: 10.1007/s00223-010-9409-5. [DOI] [PubMed] [Google Scholar]
- 13.Resnick LM, Laragh JH. Calcium metabolism and parathyroid function in primary aldosteronism. Am J Med. 1985;78:385–390. doi: 10.1016/0002-9343(85)90328-6. [DOI] [PubMed] [Google Scholar]
- 14.Rossi E, Sani C, Perazzoli F, Casoli MC, Negro A, Dotti C. Alterations of calcium metabolism and of parathyroid function in primary aldosteronism, and their reversal by spironolactone or by surgical removal of aldosterone-producing adenomas. Am J Hypertens. 1995;8:884–893. doi: 10.1016/0895-7061(95)00182-O. [DOI] [PubMed] [Google Scholar]
- 15.Maniero C, Fassina A, Seccia TM, Toniato A, Iacobone M, Plebani M, De Caro R, Calò LA, Pessina AC, Rossi GP. Mild hyperparathyroidism: a novel surgically correctable feature of primary aldosteronism. J Hypertens. 2012;30:390–395. doi: 10.1097/HJH.0b013e32834f0451. [DOI] [PubMed] [Google Scholar]
- 16.Pilz S, Kienreich K, Drechsler C, Ritz E, Fahrleitner-Pammer A, Gaksch M, Meinitzer A, März W, Pieber TR, Tomaschitz A. Hyperparathyroidism in patients with primary aldosteronism: cross-sectional and interventional data from the GECOH study. J Clin Endocrinol Metab. 2012;97:E75–E79. doi: 10.1210/jc.2011-2183. [DOI] [PubMed] [Google Scholar]
- 17.Rossi GP, Ragazzo F, Seccia TM, Maniero C, Barisa M, Calò LA, Frigo AC, Fassina A, Pessina AC. Hyperparathyroidism can be useful in the identification of primary aldosteronism due to aldosterone-producing adenoma. Hypertension. 2012;60:431–436. doi: 10.1161/HYPERTENSIONAHA.112.195891. [DOI] [PubMed] [Google Scholar]
- 18.Hagström E, Hellman P, Larsson TE, Ingelsson E, Berglund L, Sundström J, Melhus H, Held C, Lind L, Michaëlsson K, Arnlöv J. Plasma parathyroid hormone and the risk of cardiovascular mortality in the community. Circulation. 2009;119:2765–2771. doi: 10.1161/CIRCULATIONAHA.108.808733. [DOI] [PubMed] [Google Scholar]
- 19.Rubin MR, Maurer MS, McMahon DJ, Bilezikian JP, Silverberg SJ. Arterial stiffness in mild primary hyperparathyroidism. J Clin Endocrinol Metab. 2005;90:3326–3330. doi: 10.1210/jc.2004-1400. [DOI] [PubMed] [Google Scholar]
- 20.van Ballegooijen AJ, Visser M, Kestenbaum B, Siscovick DS, de Boer IH, Gottdiener JS, Defilippi CR, Brouwer IA. Relation of Vitamin D and Parathyroid Hormone to Cardiac Biomarkers and to Left Ventricular Mass (from the Cardiovascular Health Study) Am J Cardiol. 2013;111:418–424. doi: 10.1016/j.amjcard.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deo R, Katz R, Shlipak MG, Sotoodehnia N, Psaty BM, Sarnak MJ, Fried LF, Chonchol M, de Boer IH, Enquobahrie D, Siscovick D, Kestenbaum B. Vitamin D, parathyroid hormone, and sudden cardiac death: results from the Cardiovascular Health Study. Hypertension. 2011;58:1021–1028. doi: 10.1161/HYPERTENSIONAHA.111.179135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Ballegooijen AJ, Reinders I, Visser M, Dekker JM, Nijpels G, Stehouwer CD, Pilz S, Brouwer IA. Serum parathyroid hormone in relation to all-cause and cardiovascular mortality: the hoorn study. J Clin Endocrinol Metab. 2013;98:E638–E645. doi: 10.1210/jc.2012-4007. [DOI] [PubMed] [Google Scholar]
- 23.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 24.Tomaschitz A, Pilz S, Ritz E, Morganti A, Grammer T, Amrein K, Boehm BO, März W. Associations of plasma renin with 10-year cardiovascular mortality, sudden cardiac death, and death due to heart failure. Eur Heart J. 2011;32:2642–2649. doi: 10.1093/eurheartj/ehr150. [DOI] [PubMed] [Google Scholar]
- 25.Tomaschitz A, Pilz S, Ritz E, Meinitzer A, Boehm BO, März W. Plasma aldosterone levels are associated with increased cardiovascular mortality: the Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Eur Heart J. 2010;31:1237–1247. doi: 10.1093/eurheartj/ehq019. [DOI] [PubMed] [Google Scholar]
- 26.Tomaschitz A, Pilz S, Pieske B, Ritz E, März W, Meinitzer A, Dobnig H, Amrein K, Kienreich K, Verheyen N, Kraigher-Krainer E, Drechsler C, Colantonio C, Wagner D, Fahrleitner-Pammer A. Circulating Aldosterone and Mortality in Female Nursing Home Residents. Exp Gerontol. 2013;48:313–318. doi: 10.1016/j.exger.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Carbone LD, Cross JD, Raza SH, Bush AJ, Sepanski RJ, Dhawan S, Khan BQ, Gupta M, Ahmad K, Khouzam RN, Dishmon DA, Nesheiwat JP, Hajjar MA, Chishti WA, Nasser W, Khan M, Womack CR, Cho T, Haskin AR, Weber KT. Fracture risk in men with congestive heart failure risk reduction with spironolactone. J Am Coll Cardiol. 2008;52:135–138. doi: 10.1016/j.jacc.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 28.Ceccoli L, Ronconi V, Giovannini L, Marcheggiani M, Turchi F, Boscaro M, Giacchetti G. Bone health and aldosterone excess. Osteoporos Int. 2013 May 22; doi: 10.1007/s00198-013-2399-1. in press. [DOI] [PubMed] [Google Scholar]
- 29.Koiwa F, Komukai D, Hirose M, Yoshimura A, Ando R, Sakaguchi T, Komatsu Y, Shinoda T, Inaguma D, Joki N, Nishida H, Ikeda M, Shigematsu T. Influence of renin-angiotensin system on serum parathyroid hormone levels in uremic patients. Clin Exp Nephrol. 2012;16:130–135. doi: 10.1007/s10157-011-0534-x. [DOI] [PubMed] [Google Scholar]
- 30.Salcuni AS, Palmieri S, Carnevale V, Morelli V, Battista C, Guarnieri V, Guglielmi G, Desina G, Eller-Vainicher C, Beck-Peccoz P, Scillitani A, Chiodini I. Bone involvement in aldosteronism. J Bone Miner Res. 2012;27:2217–2222. doi: 10.1002/jbmr.1660. [DOI] [PubMed] [Google Scholar]
- 31.Forman JP, Williams JS, Fisher ND. Plasma 25-hydroxyvitamin D and regulation of the renin-angiotensin system in humans. Hypertension. 2010;55:1283–1288. doi: 10.1161/HYPERTENSIONAHA.109.148619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fisher ND, Price DA, Litchfield WR, Williams GH, Hollenberg NK. Renal response to captopril reflects state of local renin system in healthy humans. Kidney Int. 1999;56:635–641. doi: 10.1046/j.1523-1755.1999.00579.x. [DOI] [PubMed] [Google Scholar]
- 33.Redgrave J, Rabinowe S, Hollenberg NK, Williams GH. Correction of abnormal renal blood flow response to angiotensin II by converting enzyme inhibition in essential hypertensives. J Clin Invest. 1985;75:1285–1290. doi: 10.1172/JCI111828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Resnick LM, Müller FB, Laragh JH. Calcium-regulating hormones in essential hypertension. Relation to plasma renin activity and sodium metabolism. Ann Intern Med. 1986;105:649–654. doi: 10.7326/0003-4819-105-5-649. [DOI] [PubMed] [Google Scholar]
- 35.Grant FD, Mandel SJ, Brown EM, Williams GH, Seely EW. Interrelationships between the renin-angiotensin-aldosterone and calcium homeostatic systems. J Clin Endocrinol Metab. 1992;75:988–992. doi: 10.1210/jcem.75.4.1400892. [DOI] [PubMed] [Google Scholar]
- 36.Tomaschitz A, Ritz E, Pieske B, Fahrleitner-Pammer A, Kienreich K, Horina JH, Drechsler C, März W, Ofner M, Pieber TR, Pilz S. Aldosterone and parathyroid hormone: a precarious couple for cardiovascular disease. Cardiovasc Res. 2012;94:10–19. doi: 10.1093/cvr/cvs092. [DOI] [PubMed] [Google Scholar]
- 37.Rossi GP. Hyperparathyroidism, arterial hypertension and aortic stiffness: a possible bidirectional link between the adrenal cortex and the parathyroid glands that causes vascular damage? Hypertens Res. 2011;34:286–288. doi: 10.1038/hr.2010.251. [DOI] [PubMed] [Google Scholar]
- 38.Rastegar A, Agus Z, Connor TB, Goldberg M. Renal handling of calcium and phosphate during mineralocorticoid "escape" in man. Kidney Int. 1972;2:279–286. doi: 10.1038/ki.1972.107. [DOI] [PubMed] [Google Scholar]
- 39.Rossi E, Perazzoli F, Negro A, Sani C, Davoli S, Dotti C, Casoli MC, Regolisti G. Acute effects of intravenous sodium chloride load on calcium metabolism and on parathyroid function in patients with primary aldosteronism compared with subjects with essential hypertension. Am J Hypertens. 1998;11:8–13. doi: 10.1016/s0895-7061(97)00366-x. [DOI] [PubMed] [Google Scholar]
- 40.Tomaschitz A, Fahrleitner-Pammer A, Amrein K, Ritz E, Pieske BM, Kienreich K, Horina JH, Schmidt A, Kraigher-Krainer E, Meinitzer A, Pilz S, Colantonio C, Verheyen N. Effect of eplerenone on parathyroid hormone levels in patients with primary hyperparathyroidism: a randomized, double-blind, placebo-controlled trial. BMC Endocr Disord. 2012;12:19. doi: 10.1186/1472-6823-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacFadyen RJ, Lee AF, Morton JJ, Pringle SD, Struthers AD. How often are angiotensin II and aldosterone concentrations raised during chronic ACE inhibitor treatment in cardiac failure? Heart. 1999;82:57–61. doi: 10.1136/hrt.82.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 43.Shapes SA, Lee EJ, D S, Durazo-Arvizu R, Schneider SH. The effect of obesity on the relationship between serum parathyroid hormone and 25-hydroxyvitamin D in women. J Clin Endocrinol Metab. 2013;98:E886–E890. doi: 10.1210/jc.2012-3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Voelkl J, Alesutan I, Leibrock CB, Quintanilla-Martinez L, Kuhn V, Feger M, Mia S, Ahmed MS, Rosenblatt KP, Kuro-O M, Lang F. Spironolactone ameliorates PIT1-dependent vascular osteoinduction in klotho-hypomorphic mice. J Clin Invest. 2013;123:812–822. doi: 10.1172/JCI64093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fischer SS, Kempe DS, Leibrock CB, Rexhepaj R, Siraskar B, Boini KM, Ackermann TF, Föller M, Hocher B, Rosenblatt KP, Kuro-O M, Lang F. Hyperaldosteronism in Klotho-deficient mice. Am J Physiol Renal Physiol. 2010;299:F1171–F1177. doi: 10.1152/ajprenal.00233.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tordoff MG. NaCl ingestion ameliorates plasma indexes of calcium deficiency. Am J Physiol. 1997;273:R423–R432. doi: 10.1152/ajpregu.1997.273.1.R423. [DOI] [PubMed] [Google Scholar]
- 47.McCarron DA, Rankin LI, Bennett WM, Krutzik S, McClung MR, Luft FC. Urinary calcium excretion at extremes of sodium intake in normal man. Am J Nephrol. 1981;1:84–90. doi: 10.1159/000166496. [DOI] [PubMed] [Google Scholar]
- 48.van der Kleij FG, de Jong PE, Henning RH, de Zeeuw D, Navis G. Enhanced responses of blood pressure, renal function, and aldosterone to angiotensin I in the DD genotype are blunted by low sodium intake. J Am Soc Nephrol. 2002;13:1025–1033. doi: 10.1681/ASN.V1341025. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt BM, Georgens AC, Martin N, Tillmann HC, Feuring M, Christ M, Wehling M. Interaction of rapid nongenomic cardiovascular aldosterone effects with the adrenergic system. J Clin Endocrinol Metab. 2001;86:761–767. doi: 10.1210/jcem.86.2.7259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.