Abstract
The current model of picornavirus protein formation implies that initiation of protein synthesis occurs at a single site on the viral RNA, and that the large polypeptide formed is later cleaved. A direct test of this model was made in vitro by studying the incorporation of [35S]methionine from rabbit liver Met-tRNAMMet and fMet-tRNAFMet into encephalomyocarditis virus RNA-coded proteins in extracts of Ehrlich ascites cells. The incorporation of N-formylmethionine was complete within 5 min, while utilization of Met-tRNAMMet continued for 20 min. Tryptic digests of [35S]methionine-labeled products from Met-tRNAMMet analyzed by anion-exchange chromatography yielded more than 30 peptides, as compared to about 15 [35S]methionine-labeled peptides from purified encephalomyocarditis virus. In contrast, products labeled with fMet-tRNAFMet yielded one major 26S-labeled tryptic peptide. The N-terminal location of methionine in this peptide was verified by Edman degradation. One predominant N-terminal tryptic peptide was also obtained with fMet-tRNAFMet when mouse Elberfeld and mengo-virus RNAs were used as messengers. On the basis of N-terminal compared with internal labeling of the products, no evidence for in vitro post-translational cleavage was found. The results are consistent with a single initiation site for synthesis of picornavirus proteins.
Keywords: translation in vitro, Met-tRNA, tryptic mapping, encephalomyocarditis RNA
Full text
PDF

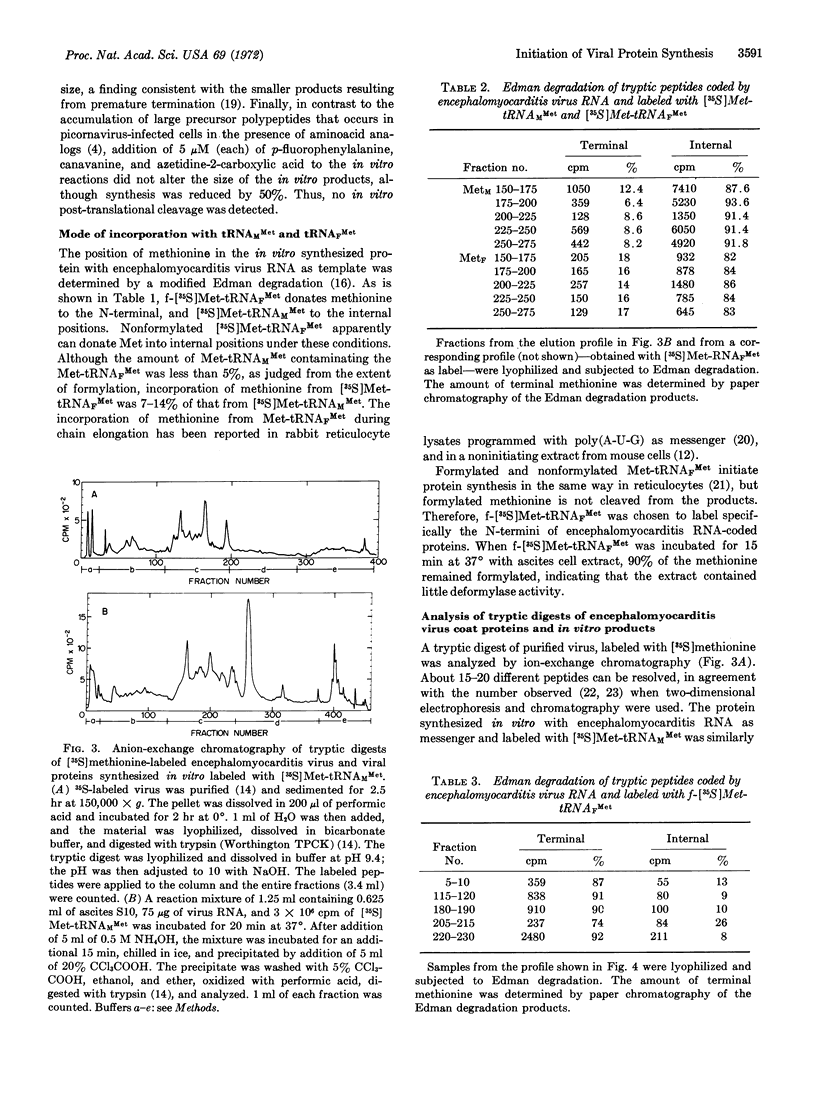
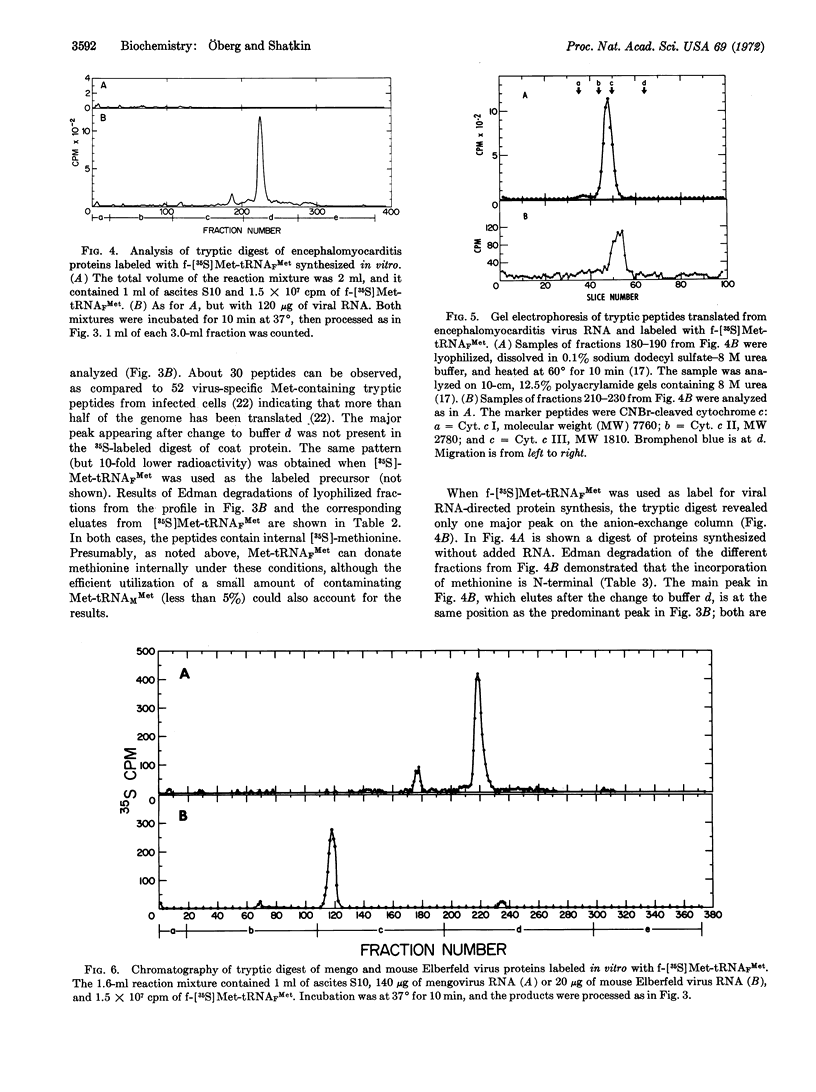

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boime I., Aviv H., Leder P. Protein synthesis directed by encephalomyocarditis virus RNA. II. The in vitro synthesis of high molecular weight proteins and elements of the viral capsid. Biochem Biophys Res Commun. 1971 Nov 5;45(3):788–795. doi: 10.1016/0006-291x(71)90486-4. [DOI] [PubMed] [Google Scholar]
- Butterworth B. E., Hall L., Stoltzfus C. M., Rueckert R. R. Virus-specific proteins synthesized in encephalomyocarditis virus-infected HeLa cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3083–3087. doi: 10.1073/pnas.68.12.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth B. E., Rueckert R. R. Gene order of encephalomyocarditis virus as determined by studies with pactamycin. J Virol. 1972 May;9(5):823–828. doi: 10.1128/jvi.9.5.823-828.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee N. K., Bose K. K., Woodley C. L., Gupta N. K. Protein synthesis in rabbit reticulocytes: factors controlling terminal and internal methionine codon (AUG) recognition by methionyl tRNA species. Biochem Biophys Res Commun. 1971 May 21;43(4):771–779. doi: 10.1016/0006-291x(71)90683-8. [DOI] [PubMed] [Google Scholar]
- Chatterjee N. K., Kerwar S. S., Weissbach H. Initiation of protein synthesis in HeLa cells. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1375–1379. doi: 10.1073/pnas.69.6.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerman H. W., Steers E., Jr, Redfield B. G., Weissbach H. Methionyl soluble ribonucleic acid transformylase. I. Purification and partial characterization. J Biol Chem. 1967 Apr 10;242(7):1522–1525. [PubMed] [Google Scholar]
- Dobos P., Kerr I. M., Martin E. M. Synthesis of capsid and noncapsid viral proteins in response to encephalomyocarditis virus ribonucleic acid in animal cell-free systems. J Virol. 1971 Oct;8(4):491–499. doi: 10.1128/jvi.8.4.491-499.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drews J., Grasmuk H., Weil R. Function of Met-tRNA fMet and Met-tRNA Met in peptide-chain elongation in cell-free systems from mouse-liver and ascites-tumor cells. Eur J Biochem. 1972 Apr 11;26(3):416–425. doi: 10.1111/j.1432-1033.1972.tb01782.x. [DOI] [PubMed] [Google Scholar]
- Eggen K. L., Shatkin A. J. In vitro translation of cardiovirus ribonucleic acid by mammalian cell-free extracts. J Virol. 1972 Apr;9(4):636–645. doi: 10.1128/jvi.9.4.636-645.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N. K., Chatterjee N. K., Bose K. K., Bhaduri S., Chung A. Roles of methionine transfer RNA's in protein synthesis in rabbit reticulocytes. J Mol Biol. 1970 Nov 28;54(1):145–154. doi: 10.1016/0022-2836(70)90452-3. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Kiehn E. D. Specific cleavage of viral proteins as steps in the synthesis and maturation of enteroviruses. Proc Natl Acad Sci U S A. 1968 Jul;60(3):1015–1022. doi: 10.1073/pnas.60.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housman D., Jacobs-Lorena M., Rajbhandary U. L., Lodish H. F. Initiation of haemoglobin synthesis by methionyl-tRNA. Nature. 1970 Aug 29;227(5261):913–918. doi: 10.1038/227913a0. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Polypeptide cleavages in the formation of poliovirus proteins. Proc Natl Acad Sci U S A. 1968 Sep;61(1):77–84. doi: 10.1073/pnas.61.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E., Tovell D. R. Characterization of the polypeptides formed in response to encephalomyocarditis virus ribonucleic acid in a cell-free system from mouse ascites tumor cells. J Virol. 1972 Jul;10(1):73–81. doi: 10.1128/jvi.10.1.73-81.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerwar S. S., Spears C., Weissbach H. Studies on the initiation of protein synthesis in animal tissues. Biochem Biophys Res Commun. 1970 Oct 9;41(1):78–84. doi: 10.1016/0006-291x(70)90471-7. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Bacteriophage f2 RNA: control of translation and gene order. Nature. 1968 Oct 26;220(5165):345–350. doi: 10.1038/220345a0. [DOI] [PubMed] [Google Scholar]
- Mathews M., Korner A. Mammalian cell-free protein synthesis directed by viral ribonucleic acid. Eur J Biochem. 1970 Dec;17(2):328–338. doi: 10.1111/j.1432-1033.1970.tb01170.x. [DOI] [PubMed] [Google Scholar]
- Rekosh D. Gene order of the poliovirus capsid proteins. J Virol. 1972 Mar;9(3):479–487. doi: 10.1128/jvi.9.3.479-487.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield P., Zamecnik P. C. Cupric ion catalysis in hydrolysis of aminoacyl-tRNA. Biochim Biophys Acta. 1968 Feb 26;155(2):410–416. doi: 10.1016/0005-2787(68)90185-8. [DOI] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr Determination of the gene sequence of poliovirus with pactamycin. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2852–2856. doi: 10.1073/pnas.68.11.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr Evidence for large precursor proteins in poliovirus synthesis. Proc Natl Acad Sci U S A. 1968 Mar;59(3):966–971. doi: 10.1073/pnas.59.3.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Taber R., Rekosh D., Baltimore D. Effect of pactamycin on synthesis of poliovirus proteins: a method for genetic mapping. J Virol. 1971 Oct;8(4):395–401. doi: 10.1128/jvi.8.4.395-401.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


