Highlights
-
•
The use of decision aids for immunisation decisions is under researched and controversial.
-
•
Parents receiving a decision aid or a leaflet had reduced decisional conflict for the MMR decision.
-
•
MMR uptake in the decision aid and control arms achieved levels required for population immunity.
-
•
Leaflet arm parents were less likely to vaccinate their child.
-
•
Childhood immunisation decision aids can achieve both informed decision-making and uptake.
Keywords: MMR, Measles, Decision aid, Decisional conflict, Leaflet
Abstract
Objective
To evaluate the effectiveness of a web based decision aid versus a leaflet versus, usual practice in reducing parents’ decisional conflict for the first dose MMR vaccination decision. The, impact on MMR vaccine uptake was also explored.
Design
Three-arm cluster randomised controlled trial. Setting: Fifty GP practices in the north of, England. Participants: 220 first time parents making a first dose MMR decision. Interventions: Web, based MMR decision aid plus usual practice, MMR leaflet plus usual practice versus usual practice only, (control). Main outcome measures: Decisional conflict was the primary outcome and used as the, measure of parents’ levels of informed decision-making. MMR uptake was a secondary outcome.
Results
Decisional conflict decreased post-intervention for both intervention arms to a level where, parents could make an informed MMR decision (decision aid: effect estimate = 1.09, 95% CI −1.36 to −0.82; information leaflet: effect estimate = −0.67, 95% CI −0.88 to −0.46). Trial arm was significantly, associated (p < 0.001) with decisional conflict at post-intervention. Vaccination uptake was 100%, 91%, and 99% in the decision aid, leaflet and control arms, respectively (χ2 (1, N = 203) = 8.69; p = 0.017). Post-hoc tests revealed a statistically significant difference in uptake between the information leaflet, and the usual practice arms (p = 0.04), and a near statistically significant difference between the, decision aid and leaflet arms (p = 0.05).
Conclusions
Parents’ decisional conflict was reduced in both, the decision aid and leaflet arms. The decision aid also prompted parents to act upon that decision and, vaccinate their child. Achieving both outcomes is fundamental to the integration of immunisation, decision aids within routine practice. Trial registration: ISRCTN72521372.
1. Introduction
In England and Wales, the decline in MMR uptake following Wakefield's now discredited study [1] continues to reverse [2]. However uptake remains below the 95% target required for population immunity at 92% (1st dose by 24 months) and 88% (1st and 2nd dose by 5 years) [3]. The incidence of measles is at its highest for 18 years [4]. More widely, over 20 000 cases of measles were reported in 51 countries within the WHO European Region from January to October 2012 [5]. In the USA, where childhood immunisation is mandatory, 211 cases of measles were confirmed in 2011 the highest levels since 1996 [6]. These outbreaks are partially attributable to vaccine refusal [6,7] and strategies targeting different groups of parents who do not vaccinate their children continue to be needed [8,9].
Typically Government information about MMR vaccination, for example ‘MMR the facts’ [10], emphasises the risks of not having the vaccine with the aim of increasing uptake. Patient decision aids are a different type of information resource that provide detailed information on the probable risks and benefits of having and not having the MMR vaccination. Decision aids also encourage people to actively evaluate this information in accordance with their values, to make a decision [11]. Encouraging people to deliberate about their MMR beliefs may affect their motivation to vaccinate [12–14] hence their underuse in this context.
Few studies have evaluated decision aids for childhood immunisation decisions. In New Zealand, a childhood immunisation paper based decision aid reduced parent's anxiety about making the decision and encouraged promptness in vaccination [15]. An Australian MMR vaccination web based decision aid resulted in parents having more positive views towards MMR, feeling more informed and leaning towards vaccination [16]. This decision aid was subsequently adapted for UK parents and its feasibility evaluated [17]. The findings suggested that the decision aid may support both informed decision-making and vaccination uptake. Finally an MMR leaflet [18] was compared with the leaflet plus a community-based decision support intervention [19]. Parents in both groups felt more able to make an informed decision with those receiving the community intervention significantly more likely to take their child to be vaccinated. These studies suggest that interventions focusing on the decision-making process for MMR vaccination are associated with parents making informed decisions, and may also impact positively on vaccine uptake. However, with the exception of Jackson et al. [19], evaluations have used quasi-experimental designs and so cannot provide conclusive evidence of effectiveness.
This paper presents the findings from the first cluster randomised controlled trial to evaluate the effectiveness of a decision aid versus a leaflet versus usual practice for a childhood immunisation decision. Our primary interest was whether the decision aid compared with a leaflet could support parents’ informed decision-making about the MMR vaccine. We were also interested in their impact on MMR uptake.
2. Materials and methods
This was a three-arm cluster randomised controlled trial: MMR decision aid plus usual practice, MMR leaflet plus usual practice and usual practice only (control). The study was approved by the York Ethics Committee (08/H1311/23), and registered on or about 31 October 2007 with the UK Clinical Research Network (UKCRN ID 4811) [20].
2.1. Participants and procedure
The UK childhood vaccination programme is administered primarily through primary care via General Practices (GPs). All 312 GP Practices within five Primary Care Providers (called Primary Care Trusts, PCTs) in the north of England were eligible to take part and offered £250 to participate. Uptake of first dose MMR ranged from 87% to 92% across the five PCTs at the time of the study [21]. First-time parents with a child aged 3–12 months being offered the first dose of the MMR vaccine were eligible. Parents were required to have an email address and sufficient English language skills to participate. On study completion, parents were offered a £10 gift voucher.
Eligible parents identified through GP practice registers were sent a postal invitation via their GP practice. Interested parents replied directly to the research team. Parents were then contacted by telephone (by SS, CJ) to confirm eligibility, enrol in the study and provide demographic data. The baseline questionnaire and consent form were subsequently sent to the parent. After all the baseline questionnaires had been sent out within a GP practice, that practice was randomised. On receipt of the completed baseline questionnaire and consent form, the appropriate intervention was delivered. At this point the researchers (SS, CJ) and participants were no longer blind to allocation. Only the statistician (WH) remained blind. The follow-up questionnaire was sent two weeks later. First dose MMR uptake data were collected from GP practices when children reached 15 months of age. Recruitment and follow-up occurred May 2009 to end September 2010.
2.2. Randomisation
Simple randomisation using a computer-generated random list allocated GP practices on a 1:1:1 basis. An independent researcher who had no contact with participants generated the allocation sequence and assigned the GP practices to their allocated arm.
2.3. Interventions
The interventions were delivered at the parent level.
2.3.1. MMR decision aid plus usual practice
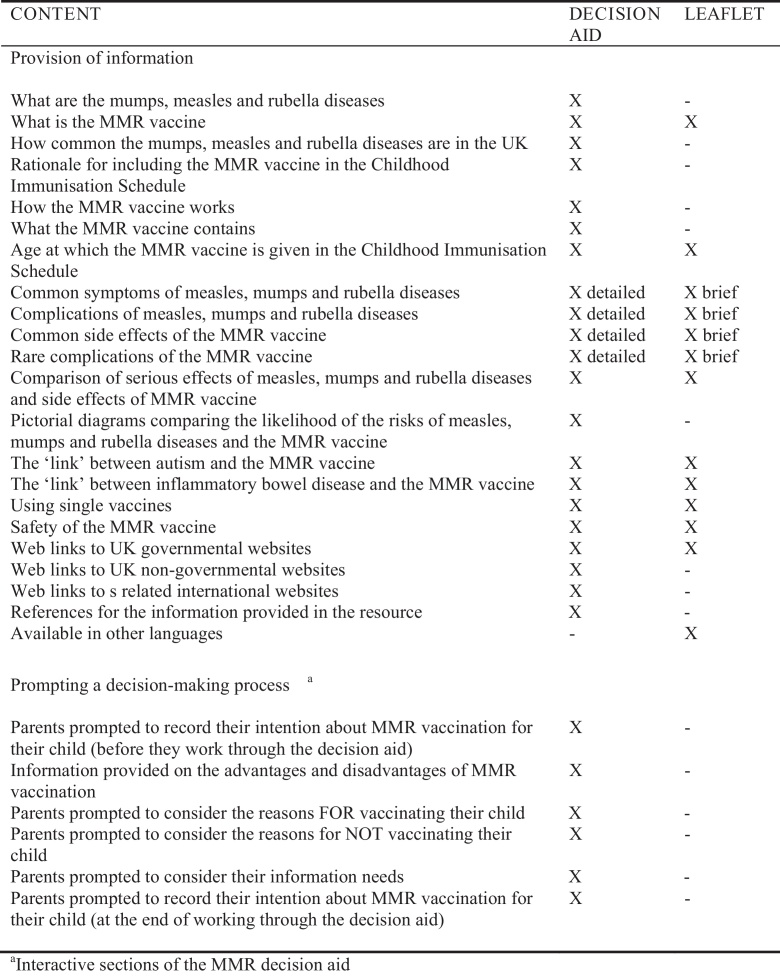
Parents were posted the web link for the MMR decision aid and to reduce contamination risk were provided with a personal login to access it. They continued to receive usual practice (described below) from their GP practice. The decision aid was a modified version of the Australian MMR decision aid [16]. It can be accessed at www.leedsmmr.co.uk. Prior to this trial, it was assessed against the International Patient Decision Aids Standards [IPDAS, 22]. A description of the modified version, its adaptation and piloting is published elsewhere [17]. An overview is presented in Fig. 1.
Fig. 1.
Comparison of the content provided in the MMR decision aid and MMR leaflet.
2.3.2. MMR leaflet plus usual practice
Parents were sent the Health Scotland leaflet ‘MMR your questions answered’ [18] and received usual practice. Our previous research [19] found this leaflet to significantly reduce parents’ decisional conflict. An overview is presented in Fig. 1. The leaflet does not meet IPDAS criteria [22] to be a decision aid.
2.3.3. Usual practice only (control)
Parents received the usual service provided by their GP practice. Parents of children registered with a GP are invited to have their child vaccinated for the first dose MMR at 12–13 months. Telephone interviews with participating GP practices indicated that usual practice typically included an appointment for the first dose MMR vaccination, a leaflet (usually ‘MMR the Facts’ [10]), and the offer of a consultation if the parent had concerns.
2.4. Measures
Demographic data were collected during the telephone contact as described above: date of birth, ethnicity, highest educational qualification, employment status, household income, sex and date of birth of the child.
Outcome data were collected at the individual parent level in the baseline and 2-weeks post-intervention questionnaires. Previously, we found two weeks was sufficient time for parents to utilise the resource they had been sent [19]. The baseline questionnaire is provided as supplementary material.
2.4.1. Primary outcome
Decisional conflict [23] assesses a parent's perception that their decision was informed, in accordance with their values, and can be acted upon. This 16-item validated scale has five sub-sections: informed, values clarity, support, uncertainty and effective decision. Scores range from 1 (no decisional conflict) to 5 (extremely high decisional conflict). Scores lower than two are associated with implementing informed decisions, higher scores are interpreted as delaying decisions or feeling unsure about their implementation [23].
2.4.2. Secondary outcomes
First dose MMR vaccination uptake data for children of participating parents were obtained from GP practice records (by practice administrators) when the child was aged 15 months.
Subscales of the decisional conflict scale: as presented above.
Knowledge about MMR and the measles disease was measured using 11 multiple choice items used in our previous research [17,19].
MMR immunisation cognitions: The Theory of Planned Behaviour [24] informed items to assess parents’ cognitions (attitude, subjective norm, perceived behavioural control) towards having their child immunised with MMR at the recommended ages.
MMR immunisation trade-off beliefs: We used a previously modified version of the Beliefs about Flu Vaccination Questionnaire to assess parents’ trade-off beliefs on vaccine necessity versus vaccine concerns for MMR [25]. This measure has not been validated for MMR yet is associated with parents’ decisional conflict for MMR [19]. We calculated a single MMR immunisation ‘trade-off’ beliefs score. A positive score indicates that the parent perceives that necessity outweighs the concerns.
Anxiety was assessed by the short form State-Trait Anxiety Index [26].
2.4.3. Parents’ engagement with the decision aid or leaflet
Data were collected in the post-intervention questionnaire. Parents in the decision aid arm were asked ‘Did you use the MMR website?’ (Yes the whole website/Yes but only some of it/No). The time parents spent working through the decision aid was captured by Google analytics. Parents in the leaflet arm were asked ‘Did you read the MMR leaflet?’ (Yes the whole leaflet/Yes but only some of it/No). All parents were asked how long (minutes) they had spent looking at other information about MMR before or since their child was born.
2.5. Sample size
Based on the primary outcome of decisional conflict [23], to detect an effect size of 0.5 [17] with a minimum power of 0.80 for a 2-sided significance level of 0.05 and independent sampling, the target sample size required was 402 parents (134 parents per arm) from 42 clusters to achieve 360 parents (120 per arm) completing the study. This estimate took into account 10% attrition and clustering effects within GP practices of a sample size inflation factor of 1.9 using an ICC of 0.1 and an estimated 10 parents per cluster [19].
2.6. Statistical analysis
2.6.1. Baseline balance
Differences in participants’ demographic characteristics and baseline decisional conflict score across the three trial arms were assessed using one-way ANOVA (age of parent, age of child, decisional conflict) and chi squared analysis (relationship to child, ethnicity, marital status, education, employment).
2.6.2. Primary outcome
Intervention effect was assessed at the parental level but within GP practice clusters. Analyses were performed on an intention to treat basis. All reported p-values are two sided, with p < 0.05 considered as significant. Analyses were carried out using STATA 11.0.
A multilevel model with GP practice at the upper level was constructed but found to not significantly improve the model (ICC = 1.74 × 10−19, p > 0.999). Decisional conflict was analysed using Analysis of Covariance, where decisional conflict post-intervention was regressed onto decisional conflict at baseline, parental demographics, trial arm and secondary outcomes at baseline (see Section 2.4). The model was simplified by removing non-significant items until only statistically significant items remained. Individual items were re-entered one at a time to check if they influenced the simplified model. If an influence was detected, the item was included.
Due to missing values resulting from non-completion of questionnaire items, complete case analyses corresponded to only 55% of the data (121/220 parents). We assumed that data were missing at random (MAR) [27]. For each missing value variable we imputed data using multiple imputation from all variables that we considered could reasonably influence the missing values. Although we cannot be conclusive that MAR is justified sufficient predictors of the missing values were included to make this assumption plausible [28]. Five imputed datasets were generated. The complete case model agreed on the importance of all significant variables in the model fitted to the imputed datasets and results were found to be similar indicating that minimal bias would have been introduced due to missing values. The best fit model was determined for the imputed datasets and aggregated results from the five imputed datasets are presented.
2.6.3. Secondary outcomes
Fisher's Exact test assessed the impact of the intervention on MMR uptake. Analysis was performed on an intention to treat basis.
Descriptive statistics (means, 95% confidence intervals) were calculated for the subscales of the decisional conflict scale at both time points and compared across allocated arm using repeated measures ANOVAs. Where statistically significant effects were identified, post hoc tests were performed. As the focus of this paper is the impact of the interventions on informed decision-making and vaccine uptake, changes in other secondary outcome measures (knowledge, MMR immunisation cognitions, MMR immunisation trade-off beliefs and anxiety) are not reported.
Frequencies were calculated for parents’ self-reported use of the decision aid and leaflet. The mean amount of time parents spent working through the MMR decision aid was reported by Google analytics. One way ANOVA compared how long parents had spent looking at other information about MMR across the three arms.
3. Results
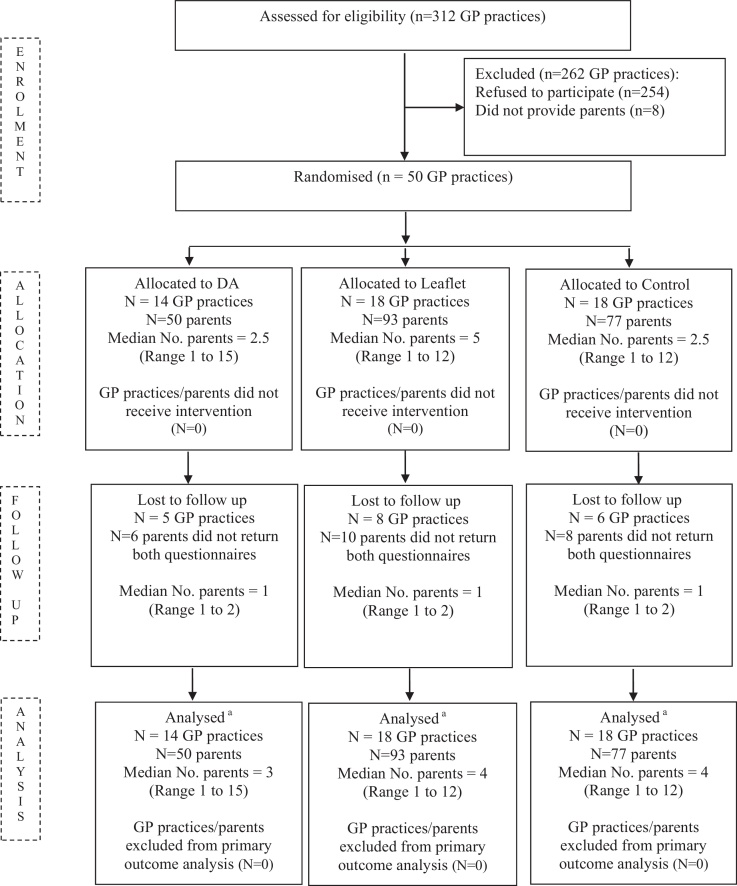
Fifty eight (19%) of 312 GP practices agreed to participate, of which 50 (16%) provided parents and were randomised. A total of 1179 parents were sent an invitation letter. Of these 250 (21%) replied. Thirty did not meet the inclusion criteria. Of the 220 eligible parents recruited 196 (89%) completed the study. This was equivalent to 54% of the original target sample completing the study. However, the ICC (1.74 × 10−19) and the average cluster size (4 parents) were both lower than the estimates used in the sample size calculation (0.1, 10 parents, respectively) thus providing an adequate sample to draw meaningful conclusions [29]. Participant flow through the study is presented in Fig. 2.
Fig. 2.
Participant flow through the trial.
3.1. Baseline characteristics
Baseline characteristics are presented in Table 1. There were no statistically significant differences in parental or child characteristics across the three trial arms (all p > 0.1). Most parents were white British mothers, in their early 30s, married or co-habiting. Approximately half were educated beyond 18 years and in full-time employment. These characteristics are consistent with parents who find it difficult to make an MMR decision [30,31]. Children were 8–9 months old at recruitment.
Table 1.
Baseline characteristics by arm.
| No. (%) |
|||
|---|---|---|---|
| Decision aid | Leaflet | Control | |
| GP practices | 14 (28) | 18 (36) | 18 (36) |
| Parents | 50 (23) | 93 (42) | 77 (35) |
| Relationship to child | |||
| Mother | 46 (92) | 87 (94) | 73 (95) |
| Father | 4 (8) | 6 (6) | 4 (5) |
| Ethnicity | |||
| White British | 49 (98) | 82 (88) | 70 (91) |
| Other | 1 (2) | 9 (10) | 7 (9) |
| Missing | 0 (0) | 2 (2) | 0 (0) |
| Marital status | |||
| Single/never married | 2 (4) | 4 (4) | 4 (5) |
| Married/live with partner | 47 (94) | 86 (93) | 73 (95) |
| Separated/divorced/widowed | 0 (0) | 2 (2) | 0 (0) |
| Missing | 1 (2) | 1 (1) | 0 (0) |
| Education | |||
| Up to 18 years | 22 (44) | 32 (34) | 31 (40) |
| Beyond 18 years | 28 (56) | 60 (65) | 46 (60) |
| Missing | 0 (0) | 1 (1) | 0 (0) |
| Employment | |||
| Full time | 27 (54) | 44 (47) | 36 (47) |
| Part time | 17 (34) | 29 (31) | 29 (38) |
| Other | 6 (12) | 19 (20) | 11 (14) |
| Missing | 0 (0) | 1 (2) | 1 (1) |
| Mean age (SD) of parent, years | 32.20 (5.51) | 33.29 (5.58) | 31.43 (5.25) |
| Mean age (SD) of child, months | 9.00 (2.35) | 8.04 (2.63) | 8.33 (2.40) |
3.2. Impact of the decision aid and leaflet interventions on informed decision-making (decisional conflict)
At baseline there was a statistically significant difference in decisional conflict across the three arms F(2,192) = 3.42, p = 0.04). Parents in the decision aid arm had statistically significantly higher decisional conflict than parents in the control arm (p = 0.01). However, parents in all three arms reported levels of decisional conflict associated with difficulties in making an informed decision (Table 2). Post-intervention, mean decisional conflict had decreased for parents in both intervention arms to below 2, a level associated with informed decision-making. Trial arm was significantly associated (p < 0.001) with decisional conflict post-intervention. The greatest reduction in decisional conflict occurred for parents in the decision aid arm, and this was evident for all five subscales (all p < 0.001; Table 2). There was no reduction in decisional conflict (or any of the subscales) over time in the control arm (all p > 0.1; Table 2).
Table 2.
Descriptive data for decisional conflict scale and subscales.
| Decision aid |
Leaflet |
Control |
p-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | n | Mean | 95% CI | n | Mean | 95% CI | n | |||
| Decisional conflicta | Baseline | 2.82 | 2.57–3.07 | 44 | 2.59 | 2.40–2.77 | 83 | 2.41 | 2.21–2.60 | 68 | <0.001b |
| Post-intervention | 1.57c | 1.37–1.78 | 43 | 1.89c | 1.75–2.02 | 80 | 2.49 | 2.29–2.70 | 67 | ||
| Uncertaintya | Baseline | 2.97 | 2.67–3.27 | 47 | 2.63 | 2.38–2.88 | 86 | 2.47 | 2.19–2.75 | 70 | <0.001b |
| Post-intervention | 1.65c | 1.44–1.87 | 44 | 2.07c | 1.87–2.27 | 81 | 2.53 | 2.25–2.81 | 68 | ||
| Informeda | Baseline | 3.06 | 2.78–3.34 | 48 | 2.98 | 2.76–3.21 | 86 | 2.83 | 2.59–3.06 | 72 | <0.001b |
| Post-intervention | 1.45c | 1.27–1.63 | 44 | 1.91c | 1.73–2.09 | 82 | 2.85 | 2.60–3.09 | 69 | ||
| Values claritya | Baseline | 2.86 | 2.58–3.13 | 46 | 2.71 | 2.48–2.94 | 86 | 2.51 | 2.25–2.78 | 72 | <0.001b |
| Post-intervention | 1.45c | 1.29–1.60 | 44 | 1.79c | 1.64–1.94 | 82 | 2.50 | 2.27–2.73 | 69 | ||
| Supporta | Baseline | 2.55 | 2.33–2.77 | 47 | 2.38 | 2.20–2.55 | 86 | 2.34 | 2.14–2.53 | 70 | <0.001b |
| Post-intervention | 1.53c | 1.32–1.73 | 43 | 1.92c | 1.76–2.07 | 82 | 2.52 | 2.31–2.72 | 69 | ||
| Effective decisiona | Baseline | 2.44 | 2.23–2.65 | 47 | 2.23 | 2.06–2.39 | 87 | 2.10 | 1.91–2.29 | 71 | <0.001b |
| Post-intervention | 1.44c | 1.30–1.59 | 44 | 1.81c | 1.67–1.95 | 81 | 2.22 | 2.03–2.40 | 68 | ||
Decisional conflict and subscales: values range from 1 (no decisional conflict) to 5 (high decisional conflict). A decisional conflict score lower than two is associated with implementing an informed decision [23]. A score lower than two on the subscales represents levels of uncertainty, being informed, values clarity, support and effective decision-making associated with informed decision-making [23].
Statistically significant value for time by arm interaction identified in repeated measures ANOVA.
Statistically significant within-arm difference (p < 0.001) from baseline to post-intervention identified in post hoc tests.
Three outcome measures at baseline were associated with decisional conflict post-intervention (Table 3). Specifically, higher decisional conflict, higher anxiety and less positive immunisation trade-off beliefs (i.e. less sure that the necessity of the vaccine outweighs the concerns) at baseline were associated with higher decisional conflict post-intervention.
Table 3.
Coefficients for the ANCOVA modelling of post-intervention decisional conflict on potential covariates.
| Variable | Effect estimatea (95% confidence interval) | p-Value |
|---|---|---|
| Control (n = 93) | – | <0.001 |
| Leaflet (n = 77) | –0.67 (–0.88 to –0.46) | |
| Decision aid (n = 50) | –1.09 (–1.36 to –0.82) | |
| Decisional conflict at baseline | 0.37 (0.24–0.49) | <0.001 |
| Anxiety at baseline | 0.010 (0.001–0.019) | 0.041 |
| MMR immunisation trade-off beliefs | –0.030 (–0.054 to –0.005) | 0.019 |
Results are presented per one point increase of decisional conflict.
3.3. Impact of the decision aid and leaflet interventions on MMR uptake
MMR vaccination uptake data for 203 children (93%) were collected from GP practices. Of these parents 48 were in the decision aid arm, 85 in the leaflet arm and 70 in the control arm. Vaccination uptake was 100%, 91% and 99%, respectively (χ2 (1, N = 203) = 8.69; p (using Fishers exact test) = 0.017). Post-hoc tests revealed a statistically significant difference in uptake between the leaflet and control arms (8%, 95% CI 1–15%, p = 0.04), and between the decision aid and leaflet arms (9%, 95% CI 3–16%, p = 0.05), but not between the decision aid and control arms (1%, 95% CI −1 to 4%, p = 0.99).
3.4. Parents’ engagement with the decision aid and leaflet interventions
Thirty nine of the 50 parents in the decision aid arm responded to the question about their use of the decision aid, 87% reported working through the entire website. The mean length of time taken was 11.04 min. Parents spent most time on ‘Frequently Asked Questions’ (M = 4.56 min). Eighty of the 93 parents in the leaflet arm responded to the question about their use of the leaflet, 98% read the whole leaflet.
The mean time parents spent looking at ‘other MMR information’ was 91.66 min (95% CI = 71.51–111.80, n = 128). One-way ANOVA revealed no statistically significant difference between the three arms (F (2,125) = 0.15, p = 0.86).
4. Discussion
This is the first cluster randomised controlled trial evaluating the effectiveness of a decision aid versus a leaflet versus usual practice for a childhood immunisation decision. Impact was explored on two outcomes relevant to childhood immunisation, informed decision-making and MMR uptake [32–34]. Only the decision aid achieved both outcomes. Several study limitations are acknowledged. Nineteen per cent of GP practices agreed to take part however only 16% provided parents (21% of all parents invited). Possible deterrents to practice enrolment were concerns about associated workload, ambivalence towards decision aids and fears of a negative impact on vaccination uptake. As a consequence we did not meet our initial recruitment target. However, we consider the study was sufficiently powered to draw meaningful conclusions because the ICC and the number of parents per cluster were lower than estimates used in the sample size calculation, meaning that the sample size inflation factor, and therefore the required sample size, had been over-estimated [29]. We do not know if parents who took part were different to non-responders, although their characteristics were consistent with parents who find making the MMR decision difficult [30,31]. We are confident that the sample is broadly representative of the target audience for this decision aid, that is ‘hesitant’ and ‘late or selective’ vaccinators [8,30] representing 20–30% and 2–27% of parents, respectively [33,35–38]. Due to the study timeframe we recorded MMR uptake at 15 months of age instead of 24 months which is used for national COVER (Cover of Vaccination Evaluated Rapidly; [39]) data. Uptake may have been higher (in the leaflet and control arms) by 24 months. Finally, complete case analysis for the primary outcome was undertaken on just 55% of the data. Although we found no evidence of selection bias this remains a possibility.
Our finding that parents receiving the decision aid had lower decisional conflict than those receiving usual practice is consistent with evaluations of decision aids in other contexts [40]. The positive effect of the leaflet mirrors our previous research [19]. The positive impact of the decision aid on both vaccination uptake (which was higher than uptake across the five participating PCTs at the time of the study [21]) and decisional conflict is in keeping with previous quasi-experimental evaluations of childhood immunisation decision aids [15–17]. What are the possible explanations for first, the differences between vaccination uptake in the leaflet and decision aid arms (in which parents had levels of decisional conflict associated with informed decision-making); and second for the high uptake in the control arm when parents experienced high levels of decisional conflict?
First, the decision aid framed MMR vaccination as a choice between two options, to have or to not have the vaccination, rather than as an opportunity to have a vaccination as presented in the leaflet. Importantly, it included an interactive values clarification exercise [41,42] which prompted parents to deliberate about their child having the vaccine or catching the diseases. It is plausible that this deliberation process may have enabled parents to use the information effectively [13,14] to make a decision consistent with their values [43], thus prompting action. Reading the information leaflet appears to have been sufficient for parents to feel that they had made an informed decision but they may have based their decision on the issues they focused on at that time, which are often emotions such as anticipated regret [13,14], rather than the evidence. This is consistent with the findings of our previous study [19] and other childhood immunisation research [13,14,43]. It seems likely that the lower rate of uptake in the leaflet arm reflected parental inertia rather than vaccination rejection.
Second, uptake in the control group (99%) was very similar to uptake in the decision aid arm (100%). One could then question whether interventions to support decision-making are worthwhile as an uptake rate of ≥95% is a successful population health outcome [9]. However, the two arms differed significantly in the extent to which decision-making was informed. Post-intervention, decisional conflict was reduced to a level associated with informed decision-making in parents receiving the decision aid while in the control arm was unchanged from baseline. So while parents exposed to the decision aid were making a deliberative informed decision to have their child vaccinated, parents in the control arm may have been adopting a position of ‘unquestioning acceptor’ [33]. Our earlier research [44] found that whilst most parents took their children to be vaccinated, two thirds reported that they had not made an informed decision, with some experiencing decision regret.
Finally, it may be argued that our findings can be attributed to the mode of information delivery (internet versus paper). However, increasing evidence indicates that it is the components of the decision aid (rather than the mode of delivery) that enable more informed decisions to be acted upon [45].
5. Conclusions
The concern about using decision aids in the context of immunisation is that their focus on individual choice and autonomy potentially conflicts with public health goals by deterring uptake of programmes with evidence of effectiveness [46–48]. The consequences of which are particularly important when a parent's decision to not vaccinate their child has implications for the wider community [46]. Our findings, building on those of others [15,16], suggest this concern is unfounded. Moreover, simply providing information without supporting parents to use it effectively may deter timely uptake of the first MMR dose, although this warrants further investigation. The challenge then is two-fold; to encourage health professionals and public health organisations to value decision support for immunisation decisions [49]; and to identify ways of implementing tools like decision aids into routine practice that are not burdensome to parents or health professionals [34,49,50]. The first should be achievable as health professionals and public health organisations are central in addressing parental concerns about vaccine safety [33,44]; and these tools appear to help meet uptake targets attached to financial incentives [34,51]. Regarding implementation, the web address for a decision aid could be incorporated into invitation letters sent to parents to use in advance of the appointment so they can come to the appointment ready to discuss MMR if they so choose. For parents who may be less likely to access this type of support, perhaps due to health literacy barriers [52] or cultural norms that don’t value patient autonomy [53], other approaches to decision support need to be explored.
Acknowledgements
The study was funded by the National Institute for Health Research, Research for Patient Benefit Programme (ref. PB-PG-0107-12048). This paper presents independent research commissioned by the National Institute for Health Research. The views expressed are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health. We would like to thank the GP practices, our parent participants, Judy Taylor (project secretary) and the Primary Care Research Network for their contributions to this trial. Thank you also to David Torgerson for commenting on earlier drafts of this paper.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplementary material related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2013.10.025.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Wakefield A.J., Murch S.H., Anthony A., Linnell J., Casson D.M., Malik M. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 1998;351:637–641. doi: 10.1016/s0140-6736(97)11096-0. [DOI] [PubMed] [Google Scholar]
- 2.Smith A., Yarwood J., Salisbury D.M. Tracking mothers’ attitudes to MMR immunisation 1996-2006. Vaccine. 2007;25:3996–4002. doi: 10.1016/j.vaccine.2007.02.071. [DOI] [PubMed] [Google Scholar]
- 3.Health Protection Agency [Internet]. Quarterly Vaccine Coverage Data Tables. COVER Q12-4 Jan to March 2013. London: Health Protection Agency; [cited 10 July 2013]. Available from: http://www.hpa.org.uk/web/HPAweb&HPAwebStandard/HPAweb_C/1211441442288.
- 4.Health Protection Agency [Internet]. Measles at highest level for 18 years. London: Health Protection Agency; [updated 8 February 2013; cited 13 February 2013]. Available from http://www.hpa.org.uk/NewsCentre/NationalPressReleases/2013PressReleases/130208Measlesathighestlevelfor18years/?printable=true.
- 5.World Health Organization European Region [Internet]. Experts meet to discuss verification process for measles and rubella elimination. Strasbourg: WHO; [updated 1 February 2013; cited 5 February 2013]. Available from http://www.euro.who.int/en/what-we-do/health-topics/communicable-diseases/measles-and-rubella/news/news/2013/02/experts-meet-to-discuss-verification-process-for-measles-and-rubella-elimination.
- 6.Poland G.A., Jacobson R.M. The re-emergence of measles in developed countries: time to develop the next-generation measles vaccines? Vaccine. 2012;30:103–104. doi: 10.1016/j.vaccine.2011.11.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raspose A. Re-emerging infectious diseases: need for improving uptake of existing vaccines. J Vaccines Vaccin. 2012;3:6. [Google Scholar]
- 8.McIntyre P., Leask J. Improving uptake of MMR vaccine. Brit Med J. 2008;336:729–730. doi: 10.1136/bmj.39503.508484.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization [Internet]. Measles deaths decline, but elimination progress stalls in some regions. Improved vaccination rates critical for success. Geneva: WHO; [updated 13 January 2010; cited 9 February 2013]. Available from http://www.who.int/mediacentre/news/notes/2013/measles_20130117/en/index.html.
- 10.Department of Health . HMSO; London: 2004. MMR the facts. [Google Scholar]
- 11.Bekker H.L. The loss of reason in patient decision aid research: do checklists affect the validity of informed choice interventions? Patient Educ Couns. 2010;78:357–364. doi: 10.1016/j.pec.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Bekker H.L. Decision aids and uptake of screening. Brit Med J. 2010;341:c5407. doi: 10.1136/bmj.c5407. [DOI] [PubMed] [Google Scholar]
- 13.Wroe A.L., Bhan A., Salkovskis P., Bedford H. Feeling bad about immunising our children. Vaccine. 2005;23:1428–1433. doi: 10.1016/j.vaccine.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Wroe A.L., Turner H., Salkovskis P. Understanding and predicting parental decisions about early childhood immunizations. Health Psych. 2004;23:33–41. doi: 10.1037/0278-6133.23.1.33. [DOI] [PubMed] [Google Scholar]
- 15.Wroe A.L., Turner N., Owens R.G. Evaluation of a decision-making aid for parents regarding childhood immunizations. Health Psychol. 2005;24:539–547. doi: 10.1037/0278-6133.24.6.539. [DOI] [PubMed] [Google Scholar]
- 16.Wallace C., Leask J., Trevena L.J. Effects of a web based decision aid on parental attitudes to MMR vaccination: a before and after study. Brit Med J. 2006;332:146–149. doi: 10.1136/bmj.38678.681840.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson C., Cheater F.M., Peacock R., Leask J., Trevena L. Evaluating a web based decision aid to support informed decision making by UK parents: a before and after feasibility study. Health Ed J. 2010;69:74–83. [Google Scholar]
- 18.NHS Health Scotland; Edinburgh: 2005. NHS Health Scotland. MMR your questions answered. [Google Scholar]
- 19.Jackson C., Cheater F.M., Harrison W., Peacock R., Bekker H., West R. Randomised cluster trial to support informed parental decision-making for the MMR vaccine. BMC Pub Health. 2011;11:475. doi: 10.1186/1471-2458-11-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson C. (principal investigator). MMR decision-making. Identifier: 4811. http://public.ukcrn.org.uk/Search/StudyDetail.aspx?StudyID=4811.
- 21.Health Protection Agency [Internet]. Quarterly Vaccine Coverage Data Tables. COVER Q10-2 July to Sept 2010. London: Health Protection Agency; [cited 10 July 2013]. Available from http://www.hpa.org.uk/web/HPAweb&HPAwebStandard/HPAweb_C/1211441442288.
- 22.Elwyn G., O’Connor A., Stacey D., Volk R., Edwards A., Coulter A. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. Brit Med J. 2006;333:417. doi: 10.1136/bmj.38926.629329.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Connor A.M. Validation of a decisional conflict scale. Med Decis Making. 1995;15:25–33. doi: 10.1177/0272989X9501500105. [DOI] [PubMed] [Google Scholar]
- 24.Conner M., Norman P., editors. Predicting health behaviours. Open University Press; Philadelphia: 1995. [Google Scholar]
- 25.Bekker H.L., Gough D., Williams M. Attendances choices about the influenza immunisation programme: evidence for targeting patients’ beliefs. Psychol Health Med. 2003;8:279–287. [Google Scholar]
- 26.Marteau T.M., Bekker H.L. The development of a six-item short form of the state scale of the Spielberger State-Trait Inventory (STAI) Brit J Clin Psychol. 1992;31:301–306. doi: 10.1111/j.2044-8260.1992.tb00997.x. [DOI] [PubMed] [Google Scholar]
- 27.Rubin D.B. Inference and Missing Data. Biometrika. 1976;63:581–592. [Google Scholar]
- 28.Cattle B.A., Baxter P.D., Greenwood D.C., Gale C.P., West R.M. Multiple imputation for completion of a national clinical audit dataset. Stat Med. 2011;30:2736–2753. doi: 10.1002/sim.4314. [DOI] [PubMed] [Google Scholar]
- 29.Hayes R.J., Alexander N.D.E., Bennett S., Cousens S.N. Design and analysis issues in cluster-randomized trials of interventions against infectious diseases. Stat Methods Med Res. 2000;9:95–116. doi: 10.1177/096228020000900203. [DOI] [PubMed] [Google Scholar]
- 30.Brown K.F., Kroll J.S., Hudson M.J. Factors underlying parental decisions about combination childhood vaccinations including MMR: a systematic review. Vaccine. 2010;28:4235–4248. doi: 10.1016/j.vaccine.2010.04.052. [DOI] [PubMed] [Google Scholar]
- 31.Pearce A., Law C., Elliman D., Cole T.J., Bedford H. Factors associated with uptake of measures, mumps and rubella vaccine (MMR) and use of single antigen vaccines in a contemporary UK cohort: prospective cohort study. Brit Med J. 2008;336:729. doi: 10.1136/bmj.39489.590671.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Department of Health [Internet]. The NHS White Paper, Equity and excellence. London: The Stationary Office [updated July 2010; cited 9 July 2013]. Available from https://www.gov.uk/government/publications/liberating-the-nhs-white-paper.
- 33.Leask J., Kinnersley P., Jackson C., Cheater F.M., Bedford H., Rowles G. Communicating with parents about vaccination: guidelines for health professionals. BMC Pediatr. 2012;12:154. doi: 10.1186/1471-2431-12-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coulter A., Collins A. The Kings Fund; London: 2011. Making shared decision-making a reality. No decision about me without me. [Google Scholar]
- 35.Gust D.A., Brown C., Sheedy K., Hibbs B., Weaver D., Nowak G. Immunization attitudes and beliefs among parents: beyond a dichotomous perspective. Am J Health Behav. 2005;29:81–92. doi: 10.5993/ajhb.29.1.7. [DOI] [PubMed] [Google Scholar]
- 36.Benin A.L., Wisler-Scher D.J., Colson E., Shapiro E.D., Holmboe E.S. Qualitative analysis of mothers’ decision-making about vaccines for infants: the importance of trust. Pediatrics. 2006;117:1532–1541. doi: 10.1542/peds.2005-1728. [DOI] [PubMed] [Google Scholar]
- 37.Downs J.S., de Bruin W.B., Fischhoff B. Parents’ vaccination comprehension and decisions. Vaccine. 2008;26:1595–1607. doi: 10.1016/j.vaccine.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 38.Opel D.J., Taylor J.A., Mangione-Smith R., Solomon C., Zhao C., Catz S. Validity and reliability of a survey to identify vaccine-hesitant parents. Vaccine. 2011;29:6598–6605. doi: 10.1016/j.vaccine.2011.06.115. [DOI] [PubMed] [Google Scholar]
- 39.Health Protection Agency [Internet]. Quarterly Vaccine Coverage Data Tables. London: Health Protection Agency; [cited 10 July 2013]. Available from http://www.hpa.org.uk/web/HPAweb&HPAwebStandard/HPAweb_C/1211441442288.
- 40.Stacey D, Bennett CL, Barry MJ, Col NF, Eden KB, Holmes-Rovner M, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Systemat Rev 2011;10 [Art. No.:CD001431]. [DOI] [PubMed]
- 41.Pieterse A.H., de Vries M., Kunneman M., Stiggelbout A.M., Feldman-Stewart D. Theory-informed design of values clarification methods: a cognitive psychological perspective on patient health-related decision making. Soc Sci Med. 2013;77:156–163. doi: 10.1016/j.socscimed.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 42.Feldman-Stewart D., Tong C., Siemens R., Alibhai S., Pickles T., Robinson J. The impact of explicit values clarification exercises in a patient decision aid emerges after the decision is actually made: evidence from a randomized controlled trial. Med Decis Making. 2012;32:616–626. doi: 10.1177/0272989X11434601. [DOI] [PubMed] [Google Scholar]
- 43.Connolly T., Reb J. Toward interactive, Internet-based decision aid for vaccination decisions: better information alone is not enough. Vaccine. 2012;30:3813–3818. doi: 10.1016/j.vaccine.2011.12.094. [DOI] [PubMed] [Google Scholar]
- 44.McMurray R., Cheater F., Weighall A., Nelson C., Schweiger M., Mukherjee S. Managing controversy through consultation: a qualitative study of communication and trust around MMR vaccination decisions. Brit J Gen Pract. 2004;54:520–525. [PMC free article] [PubMed] [Google Scholar]
- 45.Bekker H.L., Hewison J., Thornton J.G. Understanding why decision aids work: linking process and outcome. Patient Educ Couns. 2003;50:323–329. doi: 10.1016/s0738-3991(03)00056-9. [DOI] [PubMed] [Google Scholar]
- 46.Wood-Harper J. Informing education policy on MMR: balancing individual freedoms and collective responsibilities for the promotion of public health. Nurs Ethics. 2005;12:43–58. doi: 10.1191/0969733005ne757oa. [DOI] [PubMed] [Google Scholar]
- 47.Raffle A.E. Information about screening: is it to achieve high uptake or to ensure informed choice? Health Expect. 2001;4:92–98. doi: 10.1046/j.1369-6513.2001.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Entwistle V., Carter S.M., Trevena L., Flitcroft K., Irwig L., McCaffery K. Communicating about screening. Brit Med J. 2008;337:789–791. doi: 10.1136/bmj.a1591. [DOI] [PubMed] [Google Scholar]
- 49.Légaré F, Ratté S, Stacey D, Kryworuchko J, Gravel K, Graham ID, et al. Interventions for improving the adoption of shared decision making by healthcare professionals (Review). Cochrane Database Systemat Rev 2010;5 [Art. No.: CD006732]. [DOI] [PubMed]
- 50.Légaré F., Ratté S., Gravel K., Graham I.D. Barriers and facilitators to implementing shared decision-making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Educ Couns. 2008;73:526–535. doi: 10.1016/j.pec.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 51.NHS Employers [Internet]. Quality and Outcomes Framework. London: NHS Employers; [updated 10 May 2013; cited 11 July 2013]. Available from: http://www.nhsemployers.org/payandcontracts/generalmedicalservicescontract/qof/pages/qualityoutcomesframework.aspx.
- 52.Smith S.K., Dixon A., Trevena L., Nutbeam D., McCaffery K. Exploring patient involvement in healthcare decision making across different education and functional health literacy groups. Soc Sci Med. 2009;69:1805–1812. doi: 10.1016/j.socscimed.2009.09.056. [DOI] [PubMed] [Google Scholar]
- 53.Charles C., Gafni A., Whelan T., O’Brien M.A. Cultural influences on the physician-patient encounter: the case of shared treatment decision-making. Patient Educ Couns. 2006;63:262–267. doi: 10.1016/j.pec.2006.06.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.