Abstract
BACKGROUND/OBJECTIVES:
Previous studies in Gambian women with a low calcium intake have described decreases in whole-body and regional bone mineral content (BMC) and areal bone mineral density (aBMD) during the first year of lactation. The aim of this study was to examine whether these effects are reversed after lactation.
SUBJECTS/METHODS:
Thirty-three Gambian women who had a previous dual-energy X-ray absorptiometry (DXA) scan at 52 weeks lactation (L52) were invited to participate in a follow-up study when neither pregnant nor lactating (NPNL) for ⩾3 months and/or when 52 weeks postpartum in a subsequent lactation (F52). Whole body, lumbar spine and hip bone mineral were measured by DXA. Anthropometry and dietary assessments were also conducted. Repeated-measures analysis of covariance was used to determine differences from L52 at NPNL and F52.
RESULTS:
Twenty-eight women were scanned at NPNL and 20 at F52. The mean±s.d. calcium intake of the 33 women at NPNL and F52 was 360±168 mg/day. BMC, aBMD and size-adjusted BMC (SA-BMC) at all sites were higher at NPNL than L52. Percent increases in SA-BMC (mean±s.e.m.) were significant (P<0.0001): whole body=2.7±0.4% lumbar spine=4.9±1.0% total hip=3.7±1.0%. There were no significant differences in any measurements between the two lactation time points (L52 and F52).
CONCLUSIONS:
This study of Gambian women with low calcium intakes demonstrates that bone mineral mobilised during lactation is recovered after lactation. Successive periods of long lactation are not associated with progressive skeletal depletion.
Keywords: lactation, bone, calcium, DXA, The Gambia
Introduction
Maternal calcium metabolism is altered during pregnancy and lactation to support the increased demand for calcium required for fetal development and breast milk production.1, 2, 3, 4, 5, 6, 7 Deposition of calcium for bone mineral accretion of the fetus reaches 250–300 mg/day during the third trimester of pregnancy.2, 5 Postpartum, calcium secretion into breast milk averages about 200 mg/day.7 These increased calcium requirements are met by changes in maternal physiology that mobilise calcium from the mother's skeleton. In addition, calcium absorption increases during pregnancy and calcium excretion decreases during lactation.2, 5, 6, 7
During the first months of lactation, maternal bone mineral content (BMC) decreases at trabecular skeletal sites, such as the lumbar spine and hip (reviewed in detail in Olausson et al.5). The magnitude of the decrease depends on the intensity and duration of breast-feeding. However, the response to lactation differs widely between mothers.5 This mobilisation of calcium from the maternal skeleton could potentially lead to a decrease in bone mineral status and an increased risk of fracture in later life. Studies in well-nourished mothers from affluent countries have demonstrated that replenishment of mobilised bone mineral occurs in the later stages of lactation and after breast-feeding has stopped. However, there are few data on the long-term effects of lactation on the maternal skeleton in women with a low calcium intake from developing countries.
Dietary studies in the rural community of West Kiang, The Gambia, have consistently shown that the traditional adult diet provides on average 300–400 mg Ca/day.8, 9 This intake is well below international recommendations by about two- to threefold.10, 11, 12 The majority of Gambian women have many children and breastfeed each child for 18–24 months. Formula-milks and other breast-milk substitutes do not feature in the infant diet; complementary foods are usually introduced when the infant is 4–6 months old but mothers continue to breast-feed on demand. At 52 weeks lactation, the mean breast-milk output has been measured to be 760 ml/day.13 In a previous study of this Gambian population, changes in bone mineral status during the first year of lactation were measured using dual-energy X-ray absorptiometry (DXA). In contrast to studies in well-nourished women, at 1 year post-partum there was little evidence of recovery of bone mineral, especially at the hip.8 This suggests that the ability of Gambian women to replenish bone mineral mobilised earlier in lactation may be compromised, possibly because of their low calcium intake, and that long-term, this might result in maternal bone mineral depletion after several lactations. Alternatively, replenishment may occur later in lactation, when the intensity of breast-feeding diminishes, or when menstruation resumes and/or lactation has ceased.5
To investigate these possibilities, the bone mineral status of Gambian women who had previously had a DXA scan at 52 weeks of lactation was studied. The follow-up measurements were made at a time when they were neither pregnant nor lactating (NPNL) and at 52 weeks postpartum during a subsequent lactation (F52).
Subjects and methods
Participants
Thirty-three women from the villages of Keneba and Manduar in rural West Kiang, The Gambia, who had a DXA scan performed at 52 weeks of lactation (L52) in 1997–2000 as part of a previous study were invited to have repeat DXA scans when they were at least 3 months post lactation and not pregnant for at least 3 months (NPNL), and/or during a subsequent lactation at 52 weeks postpartum (F52). The aim of the study was to investigate long-term effects of lactation on maternal bone. Measurements at F52 were included to allow for the fact that women in this region commonly become pregnant with a subsequent child before or only shortly after stopping breast-feeding, potentially making it difficult to obtain NPNL measurements.
The women had a mean age of 28.6±8.4 years (range 17.6—45.4 years) and a median parity of 4 (interquartile range (IQR): 2–6.25, range: 1–10) at L52. Female staff visited the women every 4–5 weeks throughout the study to administer a questionnaire that asked about their current pregnancy, menstrual and lactation status, and to record the date of delivery of the infant from the infant welfare card. In this way, it was possible to ascertain when the woman would become eligible for NPNL and F52 measurements. The order of the measurements was not critical for the study and depended on when each woman met the relevant criteria and agreed to participate. The joint Medical Research Council (MRC)/Gambia Government Ethics Committee approved this study. All women gave informed written consent after an explanation of the study in their own language.
Data collection and procedures
Bone mineral status was measured using a dual-energy X-ray absorptiometer (Lunar DPX+, software versions 4.7b and 4.7e; GE Lunar, Madison, WI, USA) located at MRC, Keneba, The Gambia. The primary variables of interest were BMC (g), bone area (BA, cm2), areal bone mineral density (aBMD, g/cm2) and size-adjusted BMC (g; SA-BMC=BMC adjusted for BA, weight and height). The sites measured were the whole-body, lumbar spine (L1–L4), total hip and hip sub-regions (femoral neck, trochanter and femoral shaft). Calibration and performance of the DXA were monitored regularly and showed satisfactory stability and precision; the coefficient of variation of spine phantom measurements over the period of the study was 0.6% with no evidence of drift.
Anthropometry
Women wearing light clothing and no shoes were weighed to the nearest 0.1 kg using scales that were checked regularly (Seca scales, Chasmors Ltd, London, UK). Height was measured to the nearest 0.1 cm on a stadiometer (supplied by Chasmors Ltd) by trained members of the study team using standardised protocols. Stadiometers were checked before each session for accuracy using a calibrated pole. The women were measured after removing any head-dress, standing with flat feet and positioned to ensure a horizontal Frankfort plane. Because there was no evidence of a change in height over time, the average height was calculated for each individual.
Dietary records
A 2-day weighed dietary record was obtained at each time point using a similar method to those described in previous Gambian studies.14 The coded records were analysed using an electronic nutrient database for Gambian foods. This contains food composition data obtained by direct measurement combined with information on recipes and ingredients.15 Initially, the computation of calcium intake from the food records was carried out using a version of the in-house computer programme Diet In Data Out that was adapted for Gambian foods. Subsequently an updated version of this programme, Diet In Nutrients Out, was used. Calcium from drinking water was not quantified because the calcium concentration of water in this region is low (<10 mg/l).9 None of the women had consumed calcium supplements in the pregnancy and lactation before the L52 time point or in the intervening years to the NPNL and/or F52 time points.
Age and parity information
A register of all births and antenatal records for all women in the community has been kept for many years at MRC Keneba.16 Accurate information on age and parity for the women at each time point was obtained from these records.
Statistics
Statistical analyses were conducted with DataDesk (software version 6.2.1; Data Description Inc, Ithaca, NY, USA). Descriptive statistics are reported as mean±s.d. and differences as mean±s.e.m. for all continuous variables. These data for categorical variables are presented as median (IQR). The data were transformed to natural logarithms to investigate proportional effects.17
Changes within individuals in weight and the various bone measures between the three time points were investigated using repeated-measures analysis of variance (ANOVA) or analysis of covariance (ANCOVA), as appropriate. This was performed using hierarchical linear models that included subject ID and time point (L52/NPNL/F52). Scheffé post hoc tests were used to consider the significance of differences within individuals between the time points. When the dependent variable is in natural logarithms, the coefficient for a discrete variable, once multiplied by 100, corresponds closely to the percentage effect as defined by [(difference/mean) × 100].18 All percentage changes reported in this paper were derived in this way. The F52 measurements preceded NPNL in 73% of the 15 women who participated at both time points. The order of the measurements did not affect the results materially and was omitted in models described in this paper.
Adjustment of BMC for bone and body size17 was achieved at each skeletal site in two ways. First, for the calculation of mean±s.d. values, logarithmic regression models were constructed at each time point with BA, weight and height as independent variables. The residuals for each subject were then evaluated and added to the loge (mean BMC) value for the dependent variable at that time point and calculating the antilogarithm. Second, for the calculation of changes within individuals between time points, BA and weight were added to the hierarchical linear models of BMC as described above. Nonsignificant variables (P>0.05) were removed by backwards elimination to produce parsimonious models.
Regression analysis was used to consider whether time since resumption of menses or time since cessation of the previous lactation predicted change in SA-BMC at NPNL. These elapsed times were estimated from the prospective monthly questionnaire; a conservative assumption of an additional 3 months was made for those women whose menses had already returned and/or were not lactating at enrolment into the follow-up study. Such an assumption is likely to have been a variable underestimation and therefore limited any detailed summary description or inclusion as explanatory variables in statistical models. However, no significant associations between these elapsed times and change in bone measures were identified and were not considered further. Regression analysis was also used to test for differences in SA-BMC at F52 between women who had resumed menstruation and those who had not by adjusting BMC for BA, weight and height and including menses (Yes/No=1/0) in the models.
Possible relationships with the age or parity of the woman with either absolute values of the bone and anthropometric measures at NPNL or F52 or in change from L52 were considered by including age as a continuous variable, or parity as a discrete variable dichotomised at the median (parity 1–4/parity 5–11=1/0). Age and parity are highly correlated in this population and could not be included together in the models.
Similar time trends in the bone measures were observed if the data were restricted to those women who were measured at both NPNL and F52, but significances were attenuated because of the smaller sample size (data not presented).
Results
All 33 women agreed to participate in this study. These women had been scanned at 368±8 days postpartum in the index lactation. Twenty-eight women were measured at NPNL and twenty at F52 (366±33 days postpartum). Fifteen women participated on both occasions. The mean±s.d. time intervals from L52 to NPNL and L52 to F52 were 4.7±1.3 years and 4.6±1.1 years, respectively. The mean height of the women was 1.61±0.07 m. The calcium intake was: 350±164 mg/day at NPNL and 367±186 mg/day at F52, an overall average in the 33 women of 360±168 mg/day. The median (IQR) increase in parity (additional periods of pregnancy and lactation) by NPNL was 1 (0–1); an increase of 0 in 39%, 1 in 43% and 2 in 18% of the women. At NPNL, all women had resumed menstruation. At F52, the median (IQR) increase in parity was 1 (1–2); an increase of 1 in 60% and 2 in 40% of the women. At F52, all 20 women were still breast-feeding and 8 had resumed menstruation (40%).
Tables 1 and 2 show the weight and DXA results for women at the three time points. Figure 1 illustrates the changes in SA-BMC from L52 at NPNL and F52 by skeletal site. Women at NPNL were slightly heavier than at L52 (1.6 kg, 3.4%) but this was not significant (P=0.2) and overall, there was no significant change in weight.
Table 1. Changes in maternal anthropometry and bone mineral measures at whole body and lumbar spine.
| Value at L52(n=33)Mean±s.d. | % Change NPNL—L52 (n=28) Mean±s.e.m. | % Change F52—L52 (n=20)Mean±s.e.m. | Time-point effect P-valuea | |
|---|---|---|---|---|
| Weight (kg) | 54.5±8.9 | 3.4±1.9 | 1.0±2.1 | 0.2 |
| Whole body | ||||
| BMC (g) | 2212±312 | 5.0±0.9* | 2.1±1.0 | 0.0001 |
| BA (cm2) | 2036±208 | 2.2±0.7** | 1.1±0.9 | 0.02 |
| aBMD (g/cm2) | 1.083±0.067 | 2.9±0.4* | 0.9±0.5 | 0.0001 |
| SA-BMCb (g) | 2167±118 | 2.7±0.4* | 0.8±0.5 | 0.0001 |
| Lumbar spinec | ||||
| BMC (g) | 47.7±9.6 | 8.8±1.0* | 2.4±1.1 | 0.0001 |
| BA (cm2) | 47.8±5.6 | 2.3±0.4* | 1.2±0.4** | 0.0001 |
| aBMD (g/cm2) | 0.991±0.111 | 6.6±0.8* | 1.2±0.9 | 0.0001 |
| SA-BMCb (g) | 47.1±4.6 | 4.9±1.0* | 0.3±0.9 | 0.0001 |
Abbreviations: aBMD, areal bone mineral density; ANOVA, analysis of variance; ANCOVA, analysis of covariance; BMC, bone mineral content; BA, bone area; F52, 52 weeks in a subsequent lactation; L52, 52 weeks postpartum in index lactation; SA-BMC, size-adjusted BMC.
From hierarchical repeated-measures ANOVA or ANCOVA with subject (ID) and time point (L52/NPNL/F52) as independent variables.
Values of SA-BMC at each time point calculated by including BA, body weight and height in a logarithmic model; evaluating the residual for each subject; adding the residual to loge (mean BMC) value and calculating the antilogarithm. Changes in SA-BMC between time points obtained from ANCOVA models of BMC with adjustments for BA and weight; weight was not a significant predictor of change in whole body or lumbar spine and was removed from the models presented here. Significance of change from L52 as given by Scheffé post-hoc tests in the ANOVA or ANCOVA models: *P <0.0001; **P=0.02; all others P >0.1.
One lumbar spine scan omitted at NPNL because of poor quality ie n=27.
Table 2. Changes in maternal bone mineral measures at the hip.
| Value at L52 (n=31) Mean±s.d. | % Change NPNL—L52 (n=28) Mean±s.e.m | % Change F52—L52 (n=20) Mean±s.e.m | Time-point effect P-valuea | |
|---|---|---|---|---|
| Total hip | ||||
| BMC (g) | 29.1±3.9 | 3.7±1.0*** | 0.6±1.2 | 0.003 |
| BA (cm2) | 28.6±2.2 | −0.9±0.5 | −0.5±0.6 | 0.2 |
| aBMD (g/cm2) | 1.015±0.096 | 4.7±1.0* | 1.1±1.2** | 0.0001 |
| SA-BMC (g)b | 27.9±2.6 | 3.7±1.0* | 0.7±1.1 | 0.002 |
| Femoral neck | ||||
| BMC (g) | 4.36±0.72 | 4.8±1.3*** | 0.9±1.6 | 0.003 |
| BA (cm2) | 4.38±0.49 | 2.5±0.8** | 1.1±1.0 | 0.02 |
| aBMD (g/cm2) | 0.993±0.096 | 2.4±1.3 | −0.2±1.4 | 0.1 |
| SA-BMC (g)b | 4.28±0.41 | 2.6±1.3 | 0.0±1.4 | 0.1 |
| Trochanter | ||||
| BMC (g) | 8.30±1.86 | 1.1±1.9 | 0.4±2.2 | 0.8 |
| BA (cm2) | 10.5±1.56 | −4.8±1.5**** | −2.2±1.8 | 0.01 |
| aBMD (g/cm2) | 0.784±0.088 | 5.9±1.3* | 2.6±1.5 | 0.0002 |
| SA-BMC (g)b | 7.46±0.68 | 4.9±1.4* | 2.2±1.5 | 0.006 |
| Femoral shaft | ||||
| BMC (g) | 16.4±1.8 | 4.6±1.0* | 0.5±1.2 | 0.0001 |
| BA (cm2) | 13.7±0.9 | 0.6±0.6 | 0.0±0.7 | 0.5 |
| aBMD (g/cm2) | 1.198±0.128 | 4.0±1.0***** | 0.5±1.2 | 0.0007 |
| SA-BMC (g)b | 16.2±1.6 | 3.3±0.9* | 0.2±1.0 | 0.002 |
Abbreviations: aBMD, areal bone mineral density; ANOVA, analysis of variance; ANCOVA, analysis of covariance; BMC, bone mineral content; BA, bone area; F52, 52 weeks in a subsequent lactation; L52, 52 weeks postpartum in index lactation; NPNL, neither pregnant nor lactating; SA-BMC, size-adjusted BMC.
From hierarchical repeated-measures ANOVA or ANCOVA with subject (ID) and time point (L52/NPNL/F52) as independent variables.
Values of SA-BMC at each time point calculated by including BA, body weight and height in a logarithmic model; evaluating the residual for each subject; adding the residual to the loge (mean BMC) value and calculating the antilogarithm. Changes in SA-BMC between time points obtained from ANCOVA models of BMC with adjustments for BA and weight. Significance of change from L52 as given by Scheffé post-hoc tests in the ANOVA or ANCOVA models: *P<0.0001; **P=0.02; ***P=0.004; ****P=0.01; *****P=0.001; all others P>0.1.
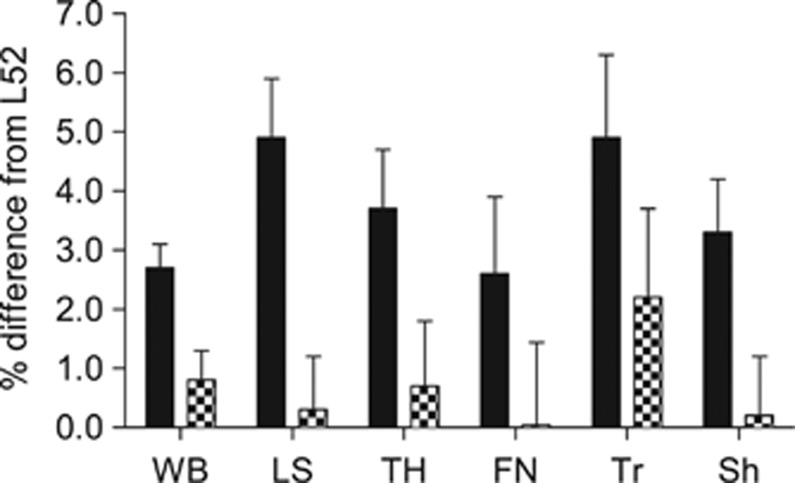
Figure 1.
Changes in size-adjusted BMC (SA-BMC) from 52 weeks postpartum in index lactation (L52). Black columns, neither pregnant nor lactating (NPNL); hatched columns, 52 weeks in a subsequent lactation (F52). Significance of increases: at NPNL=P<0.0001 at all sites except femoral neck (FN; not significant); at F52 none were significant. LS, lumbar spine; Sh, femoral shaft; TH, total hip; Tr, trochanter; WB, whole body.
At the whole body and lumbar spine, BMC, aBMD and SA-BMC were consistently significantly higher at NPNL compared with L52, but not at F52 (Table 1). Total body and spine BA changed in line with BMC but the magnitude of the change was smaller (Table 1). Weight was not a significant predictor in the SA-BMC models of the whole body and lumbar spine. The changes within individuals by NPNL in whole body and lumbar spine SA-BMC were +2.7% and +4.9%, respectively (P<0.0001).
At the total hip, and sub-regions, BMC, aBMD and SA-BMC were also significantly higher at NPNL than L52 (Table 2), except for aBMD and SA-BMC at the femoral neck and BMC at the trochanter. At F52, there was a tendency for a small increase in aBMD and SA-BMC compared with L52 at the total hip and trochanter, but this was only significant for aBMD at the total hip (P=0.02). At NPNL, BA was significantly greater at the femoral neck (P=0.02) but smaller at the trochanter (P=0.01). There were no significant changes in BA at the total hip or femoral shaft. Weight was a significant positive predictor in SA-BMC models at the total hip and femoral shaft (P=0.006 and 0.001, respectively), and was close to significance at the femoral neck (P=0.06) and trochanter (P=0.07). However, removing the weight adjustment made little difference to the observed changes between time points.
Age was a significant negative predictor of lumbar spine bone mineral measures at NPNL and F52 (BMC, aBMD, SA-BMC, all P<0.01) and of BA and statural height (significant at NPNL only, P<0.01), with nonsignificant trends in a similar direction for parity. However, neither age nor parity were significant predictors of change in the lumbar spine bone measures at either time point. Bone measures of the whole body and hip were not predicted by age or parity, nor was body weight.
The women who had resumed menstruation by F52 had 7–10% greater SA-BMC than those who had not. This was significant at the whole body and lumbar spine (Table 3). Their weight also tended to be greater, but weight was a significant contributor to the size-adjustment models for BMC only at the total hip.
Table 3. Differences at F52 in weight and size-adjusted bone mineral content between mothers who had resumed menstruation and those who had not.
| Resumed (n=8) Mean±s.d. | Not resumed (n=12) Mean±s.d. | % Difference at F52aResumed vs. not resumed | ||
|---|---|---|---|---|
| |
|
|
Mean±s.e.m. |
P-value |
| Weight (kg) | 55.9±6.3 | 53.1±8.0 | 5.7±6.1 | 0.4 |
| SA-BMC (g)b | ||||
| Whole body | 2256±46 | 2104±134 | 7.3±2.4 | 0.008 |
| Lumbar spine | 52.4±2.8 | 47.3±4.9 | 10.9±4.2 | 0.02 |
| Total hip | 28.7±3.5 | 26.5±2.9 | 8.1±5.4 | 0.1 |
Abbreviations: ANCOVA, analysis of covariance; BA, bone area; BMC, bone mineral content; F52, follow-up lactation at 52 weeks postpartum; SA-BMC, size-adjusted bone mineral content.
From ANCOVA models of BMC with adjustment for BA, weight and height at F52 and with menses (yes/no).
Values of SA-BMC at each time point calculated by including BA, body weight, and height in a logarithmic model; evaluating the residual for each subject; adding the residual to the loge (mean BMC) value and calculating the antilogarithm.
Discussion
Breast-feeding is associated with physiological decreases in the BMC of the mother during the early months of lactation.5 This has raised concerns that breast-feeding may be a risk factor for osteoporosis, and hence, fracture in later life. Studies of well-nourished women in affluent countries have discounted this possibility because the period of bone mineral mobilisation is followed by bone mineral replenishment during the later stages of lactation and following the cessation of breast-feeding.3, 5, 19, 20, 21, 22 Also post-menopausal women who breast-fed do not appear to be at greater risk of osteoporotic fracture.5 However, the situation is not clear for women with low calcium intakes who have many children and breast-feed for long periods, such as women in The Gambia and other traditional African societies.
The study reported here demonstrates that whole body, lumbar spine and hip BMC and aBMD of Gambian women, with calcium intakes of 360 mg/day, were significantly higher post-lactation (NPNL) than at 52 weeks of lactation. This observation remained after size-adjustment to take account of changes in BA and body weight. This suggests that a low dietary calcium intake two- to threefold below current international recommendations can support the restoration of bone mobilised during pregnancy and lactation. The extent to which this restoration is complete cannot be determined from this study because no pre-pregnancy values were available for the L52 measurement and because the number of women participating was relatively small. However, the average increases in bone mineral observed by NPNL at the whole body, lumbar spine and hip were of a similar magnitude to the mean decreases reported in lactation.5 In addition, the lack of any significant differences in SA-BMC between measurements made at 52 weeks postpartum in two separate lactations suggests that repeated periods of breast-feeding do not place a woman with a low calcium intake at risk of maternal skeletal depletion.
The changes in BMC and aBMD of the women across time may relate to alterations in body weight. However, weight changes were small and insignificant and were only a significant predictor of bone changes at the hip. They may also relate to changes in scanned BA, which can be due to increases or alterations in skeletal size. The data from this study provide further evidence for the redistribution of bone within the hip during and after lactation observed in previous studies, with an increase in BA at the femoral neck and a decrease in BA at the trochanter.3 However, changes in scanned BA can also be caused by technical artefacts associated with changes in BMC and in the orientation of the bone in the DXA beam.5, 23 Although the effects of such technical artefacts are reduced by the derivation of aBMD values, full adjustment of BMC for bone and body size (SA-BMC) minimises these contributions.6, 17 In addition, advancing age would be expected to change SA-BMC, and hence, alter the size of the observed increase at NPNL.5 In line with this possibility, age (or parity) was a negative predictor of SA-BMC, but only at the lumbar spine. Stature and lumbar spine BA were also negatively related to age suggesting that the greater lumbar spine SA-BMC in younger women may have been a reflection of secular changes in skeletal growth before adulthood. However, the contribution from ageing to change in SA-BMC over a 5-year period in women of reproductive age was likely to be small; indeed neither age nor parity predicted the changes in SA-BMC recorded in this study. Thus, the changes in SA-BMC observed in the Gambian women can be interpreted as being due primarily to lactation.
The mechanisms underpinning the mobilisation of bone mineral during lactation and its later restoration are not fully understood.2, 5 One possibility is that it is a function of the suppression and re-emergence of ovulation and menstruation,5, 20, 21, 24, 25 although the experimental evidence is not consistent.19, 22 At F52, SA-BMC was greater in those Gambian women who had resumed menstruating, providing evidence that the restoration of bone mineral may be driven by the hormonal changes accompanying ovulation. The longer period of lactational amenorrhoea, typical of Gambian women, therefore, partly explains the differences in the temporal pattern between Gambian and Western women observed in previous studies.8 It is likely that the women who had resumed menstruating by 52 weeks postpartum were those whose infants demanded less breast-milk and, consequently, were producing less breast-milk, thereby promoting both the earlier return of ovulation and the reversal of bone mineral mobilisation. In the absence of data on breast-milk output or feed frequency in this Gambian study, it is not possible to distinguish these inter-relationships further.
This study has shown that maternal bone mineral mobilised in lactation is replenished after lactation in Gambian women with a low calcium intake and that successive periods of long lactation are not associated with skeletal depletion.
Acknowledgments
This work was funded by the UK Medical Research Council (Unit Programme numbers U105960371 and U123261351). We thank the women who participated in this study and the staff of MRC Keneba, The Gambia, particularly Michael Mendy, Mustapha Ceesay, Mariama Jammeh, Fatou Manneh, Buba Ceesay, Morikebba Sanyang and Lamin Jammeh. We also are grateful for the contributions of staff at MRC Human Nutrition Research, Cambridge, especially Sheila Levitt, Celia Prynne and Jenny Thompson.
The authors declare no conflict of interest.
Footnotes
YS conducted the research for his MSc dissertation, supervised by GRG and AP; LMAJ and AP were the principal investigators and were responsible for the study design and supervised data collection; YS and AP analysed the data and drafted the manuscript; GG and MAL provided senior scientific oversight of the data collection and bone densitometry, respectively, and critically reviewed the manuscript; all authors approved the final manuscript and AP had overall responsibility for the decision to publish.
References
- Abrams SA. Calcium turnover and nutrition through the life cycle. Proc Nutr Soc. 2001;60:283–289. doi: 10.1079/pns200082. [DOI] [PubMed] [Google Scholar]
- Kovacs CS. Calcium and bone metabolism disorders during pregnancy and lactation. Endocrinol Metab Clin North Am. 2011;40:795–826. doi: 10.1016/j.ecl.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Laskey M, Prentice A. Bone mineral changes during and after lactation. Obstet Gynecol. 1999;94:608–615. doi: 10.1016/s0029-7844(99)00369-5. [DOI] [PubMed] [Google Scholar]
- Miller SC, Bowman BM. Rapid inactivation and apoptosis of osteoclasts in the maternal skeleton during the bone remodeling reversal at the end of lactation. Anat Rec (Hoboken) 2007;290:65–73. doi: 10.1002/ar.20403. [DOI] [PubMed] [Google Scholar]
- Olausson H, Goldberg GR, Laskey MA, Schoenmakers I, Jarjou LM, Prentice A. Calcium economy in human pregnancy and lactation. Nut Res Rev. 2012;25:40–67. doi: 10.1017/S0954422411000187. [DOI] [PubMed] [Google Scholar]
- Olausson H, Laskey MA, Goldberg GR, Prentice A. Changes in bone mineral status and bone size during pregnancy and the influences of body weight and calcium intake. Am J Clin Nutr. 2008;88:1032–1039. doi: 10.1093/ajcn/88.4.1032. [DOI] [PubMed] [Google Scholar]
- Prentice A. Micronutrients and the bone mineral content of the mother, fetus and newborn. J Nutr. 2003;133:1693S–1699S. doi: 10.1093/jn/133.5.1693S. [DOI] [PubMed] [Google Scholar]
- Jarjou LM, Laskey MA, Sawo Y, Goldberg GR, Cole TJ, Prentice A. Effect of calcium supplementation in pregnancy on maternal bone outcomes in women with a low calcium intake. Am J Clin Nutr. 2010;92:450–457. doi: 10.3945/ajcn.2010.29217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice A, Laskey MA, Shaw J, Hudson GJ, Day KC, Jarjou LM, et al. The calcium and phosphorus intakes of rural Gambian women during pregnancy and lactation. Br J Nutr. 1993;69:885–896. doi: 10.1079/bjn19930088. [DOI] [PubMed] [Google Scholar]
- Department of Health . Nutrition and Bone Health: with Particular Reference to Calcium and Vitamin D. The Stationery Office: London; 1998. [Google Scholar]
- Institute of Medicine Food and Nutrition Board . Dietary Reference Intakes for Calcium and Vitamin D. The National Academies Press: Washington DC; 2011. [PubMed] [Google Scholar]
- World Health Organization and Food and Agriculture Organization . Vitamin and Mineral Requirements in Human Nutrition, Second edition. WHO: Geneva; 2004. [Google Scholar]
- Jarjou LMA, Goldberg GR, Coward WA, Prentice A. Calcium intake of rural Gambian infants: a quantitative study of the relative contributions of breast milk and complementary foods at 3 and 12 months of age. Eur J Clin Nutr. 2012;66:673–677. doi: 10.1038/ejcn.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarjou LM, Prentice A, Sawo Y, Laskey MA, Bennett J, Goldberg GR, et al. Randomized, placebo-controlled, calcium supplementation study in pregnant Gambian women: effects on breast-milk calcium concentrations and infant birth weight, growth, and bone mineral accretion in the first year of life. Am J Clin Nutr. 2006;83:657–666. doi: 10.1093/ajcn.83.3.657. [DOI] [PubMed] [Google Scholar]
- Prynne CJ, Paul AA. Food Composition Table for use in The Gambia. Medical Research Council, Human Nutrition Research: Cambridge; 2011. [Google Scholar]
- Rayco-Solon P, Moore SE, Fulford AJ, Prentice AM. Fifty-year mortality trends in three rural African villages. Trop Med Int Health. 2004;9:1151–1160. doi: 10.1111/j.1365-3156.2004.01325.x. [DOI] [PubMed] [Google Scholar]
- Prentice A, Parsons TJ, Cole TJ. Uncritical use of bone mineral density in absorptiometry may lead to size-related artifacts in the identification of bone mineral determinants. Am J Clin Nutr. 1994;60:837–842. doi: 10.1093/ajcn/60.6.837. [DOI] [PubMed] [Google Scholar]
- Cole TJ. Sympercents: symmetric percentage differences on the 100 log(e) scale simplify the presentation of log transformed data. Stat Med. 2000;19:3109–3125. doi: 10.1002/1097-0258(20001130)19:22<3109::aid-sim558>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Holmberg-Marttila D, Sievanen H, Laippala P, Tuimala R. Factors underlying changes in bone mineral during postpartum amenorrhea and lactation. Osteoporos Int. 2000;11:570–576. doi: 10.1007/s001980070077. [DOI] [PubMed] [Google Scholar]
- Kalkwarf HJ, Specker BL. Bone mineral loss during lactation and recovery after weaning. Obstet Gynecol. 1995;86:26–32. doi: 10.1016/0029-7844(95)00083-4. [DOI] [PubMed] [Google Scholar]
- Kolthoff N, Eiken P, Kristensen B, Nielsen SP. Bone mineral changes during pregnancy and lactation: a longitudinal cohort study. Clin Sci (Lond) 1998;94:405–412. doi: 10.1042/cs0940405. [DOI] [PubMed] [Google Scholar]
- Ritchie LD, Fung EB, Halloran BP, Turnlund JR, van Loan MD, Cann CE. A longitudinal study of calcium homeostasis during human pregnancy and lactation and after resumption of menses. Am J Clin Nutr. 1998;67:693–701. doi: 10.1093/ajcn/67.4.693. [DOI] [PubMed] [Google Scholar]
- Laskey MA, Murgatroyd PR, Prentice A. Comparison of narrow-angle fan-beam and pencil-beam densitometers: in vivo and phantom study of the effect of bone density, scan mode, and tissue depth on spine measurements. J Clin Densitom. 2004;7:341–348. doi: 10.1385/jcd:7:3:341. [DOI] [PubMed] [Google Scholar]
- Cross NA, Hillman LS, Allen SH, Krause GF. Changes in bone mineral density and markers of bone remodeling during lactation and postweaning in women consuming high amounts of calcium. J Bone Miner Res. 1995;10:1312–1320. doi: 10.1002/jbmr.5650100907. [DOI] [PubMed] [Google Scholar]
- Kalkwarf HJ, Specker BL, Bianchi DC, Ranz J, Ho M. The effect of calcium supplementation on bone density during lactation and after weaning. N Eng J Med. 1997;337:523–528. doi: 10.1056/NEJM199708213370803. [DOI] [PubMed] [Google Scholar]