Abstract
Background/Objectives:
Snacks contribute toward a significant proportion of human total daily energy intake. This study investigated the effects of almonds, a satiating and nutrient-rich, common snack, on postprandial glycemia, appetite, short-term body weight and fasting blood parameters when consumed with meals or alone as a snack.
Methods:
This was a 4-week randomized, parallel-arm study that entailed consuming almonds (43 g/day) with breakfast (BF) or lunch (LN), alone as a morning (MS) or afternoon (AS) snack or no almonds (CL). Participants (N=137) with increased risk for type 2 diabetes completed an oral glucose tolerance test (OGTT) and acute-feeding session at baseline, followed by almond consumption for 4 weeks before repeating the OGTT and acute-feeding trials. Anthropometric, biochemical and appetite responses were assessed.
Results:
Almonds lowered serum glucose responses postprandially. Effects were most prominent in the snack groups. Almonds, consumed as snacks, also reduced hunger and desire to eat during the acute-feeding session. After 4 weeks, anthropometric measurements and fasting blood biochemistries did not differ from the control group or across intervention groups. Without specific guidance, daily energy intake was reduced to compensate for energy from the provided almonds. Dietary monounsaturated fat and α-tocopherol intakes were significantly increased in all almond groups.
Conclusion:
Almonds provide post-ingestive metabolic and appetitive benefits and did not increase the risk for weight gain. This suggests that almonds may be a healthful snack option.
Keywords: randomized-controlled trial, almonds, body weight, blood glucose, appetite
Introduction
The National Health and Nutrition Examination Surveys (NHANES) document changes in US consumer eating patterns over the past 3 decades.1 In 2006, almost all adults (97%) reported consuming at least one snack per day2 compared with 71% in 1977. Snacking was self-defined by the survey respondents and most likely referred to eating events that occurred between self-described main meals. Not only has snacking become more prevalent, the number of snacks consumed per day has also increased. One daily snacking event was included by the population in 1977 and that increased to about 2.2 snacks per day in 2006. In conjunction with increased snacking frequency, snack size has increased from 165 to 264 kcal/day over the same period of time.1
Higher eating frequency is associated with greater energy intake, especially after correcting for under-reporting.3 Population studies report that body weight is positively associated with eating frequency in both children4, 5 and adults.6, 7, 8, 9 Because the number of meals has remained relatively stable over the last few decades,1 an increase in eating frequency is likely due to more frequent snacking. Findings indicate that compensation for snacking or eating events occurring several hours before meals lead to poor dietary compensation.10 Increased portion size has also been implicated in weight gain,11 but no significant increase in portion sizes of main meals are reported in the NHANES 1977–2006 data,2 and supplemental energy provided with meals can lead to precise compensation.10 This suggests that eating frequency may be particularly problematic for weight gain, especially when there is no reduction in portion sizes as eating frequency increases.6, 7, 9, 12 However, there are several fundamental issues that require clarification to confirm this hypothesis. One major issue is the lack of consensus for the definition of a snack. Snacks are variously defined by the timing of their ingestion, their nutrient profile or energy content with possible variable implications for their association with health outcomes.13 However, there are foods and drinks that are commonly consumed with a meal or alone as snacks (for example, potato chips and soda), making the classification of snacks based on energy or nutrient profile questionable. With respect to overall health concerns with snacking, nutrient profiles of snack foods must be considered. Some foods/beverages may be nutrient poor, whereas others are good sources of essential nutrients and may be healthful dietary components.14, 15, 16, 17 Thus, balancing the effects of snacking on energy versus nutrient intake is vital for assessing an items role in a healthful diet.
Almonds provide varied healthful effects. They are rich in nutrients such as monounsaturated fats, magnesium, protein and vitamin E as well as fiber and phytochemicals.18 Although high in energy, humans compensate for energy from almonds due to their high satiety value.19 Moreover, the absorption of energy from almonds is inefficient18 and they may raise resting energy expenditure with chronic consumption.19 Indeed, acute and longer-term almond ingestion helps regulate body weight,20, 21 modulates fluctuations of blood glucose22, 23 and reduces plasma lipids postprandially.24, 25 As snacking is almost universal in the United States, a priority should be to identify snacks that pose little risk for weight gain while providing health benefits. In this light, this study compared: (1) the acute post-ingestive effects of almond consumption with meals or alone as snacks and (2) the short-term effects of almond consumption on body weight, body composition and indicators of metabolism. We hypothesized that almonds, consumed as snacks would improve fasting and postprandial blood glucose, insulin and lipids profiles while not increasing the risk for weight and body fat mass gain.
Materials and methods
Study design
This was a 4-week randomized, controlled, parallel-arm study with five groups:
Control (CL): participants were asked to avoid all nuts and seeds during the study period.
Breakfast (BF) (meal group): participants were instructed to consume 43 g of almonds (independent analyses indicating they contain 245 kcal, 21.0 g fat, 9.2 g carbohydrates, 9.0 g protein, 5.3 g fiber and 9.9 mg α-tocopherol) with their daily BF for 4 weeks. The quantity of almonds selected was based on the recommendations from the Dietary Guidelines for Americans 2010.26
Lunch (LN) (meal group): participants received instructions similar to the BF group, except that 43 g of almonds were consumed with their customary LN.
Morning snack (MS) (snack group): participants were instructed to consume 43 g of almonds as a MS for 4 weeks. MS was defined as an eating event that occurred between the participants' customary BF and LN, with ∼2 h after BF and before LN.
Afternoon snack (AS) (snack group): instructions were similar to the MS group, except that almonds were consumed between lunch and dinner, with at least 2 h before and after these meals.
Participants and recruitment
Participants were at increased risk for type 2 diabetes based on their being either overweight or obese (body mass index, BMI>27 kg/m2) or had normal weight (BMI 18.5–24.9 kg/m2) with a strong family history for this disease. Other inclusion criteria were as follows: age 18–60 years; both males and females; not allergic to nuts and test meals; non-diabetic (2-h postprandial glucose <11.1 mmol/l as determined by oral glucose tolerance test, OGTT); not taking any medication known to affect glycemia; metabolism or appetite; regular BF eaters; and weight stable (<5 kg weight change in the past 3 months). Participants were recruited through advertisements in the local media. After being screened, eligible participants were randomized into one of the five study arms using a computerized random number generator by the primary investigator. To detect a difference of one s.d. between control and intervention groups at 90% statistical power, 25 participants were required in each group. To allow for dropouts, 30 participants were recruited in each study group.
Experiment protocol and measurements
OGTT sessions
At baseline, a standard OGTT (75 g dextrose load) was performed following an overnight fast. Serum glucose and insulin concentrations were measured at 0 and 120 min. During the same session, height, weight (Tanita, model TBF-410), body fat (air displacement plethysmography, BOD POD), waist circumference and blood pressure were measured. Participants also completed a 24-h dietary recall using a multi-pass interview method with a dietitian and standard questionnaires that assessed personality and eating behaviors and habitual physical activity levels.27, 28, 29, 30, 31, 32, 33, 34 Participants were asked to record their appetitive sensations hourly, measured as ‘hunger', ‘fullness' and ‘desire to eat' on personal digital assistants, using visual analog scales, during waking hours for a day before attending each visit. Participants repeated these activities after 4 weeks of almond consumption.
Acute-feeding sessions
Within a week following the OGTT, participants returned, after an overnight fast of at least 10 h, for an acute-feeding session that began between 0700 and 0800 hours and lasted for 515 min (8 h 35 min). Upon arrival, an indwelling catheter was placed in an arm vein. Participants rested for 15 min before a baseline blood sample was drawn. Immediately following, a standard BF(Table 1) was served and participants were instructed to consume the entire portion within 10 min. Subsequent blood samplings were performed at 15, 45, 60, 90, 120, 180 and 240 min after the BF. Next, a standard LN (Table 1) was provided to the participants who were allotted 10 min to consume the meal before blood samples were taken at 265, 295, 310, 340, 370, 430 and 490 min of the session. Participants randomized into the BF and LN groups received 43 g of almonds together with their meals, the MS and AS groups consumed 43 g of almonds 120 min after BF and LN, whereas the CL group did not receive any snack. Participants also recorded their ‘hunger', ‘fullness' and ‘desire to eat' sensations when blood samples were taken. During the acute-feeding session, activities that may alter appetite sensations (for example, sleeping and watching movies containing food cues) were prohibited. Postprandial glucose, insulin and triacylglycerol concentrations were measured for all 15 samples collected from each participant. Participants in all almond groups were also asked to rate: (1) their liking for the ingested almonds using a general labeled magnitude scale and (2) how often they would consume almonds using the ‘Food Action Rating Scale'.35 This session was repeated again after 4 weeks of almond supplementation.
Table 1. Standard test meals used at acute-feeding session.
| Nutrient | Breakfast | Lunch |
|---|---|---|
| Energy, kcal | 310 | 225 |
| Fat, g | 0.0 | 0.5 |
| Carbohydrate, g | 64.0 | 51.5 |
| Protein, g | 8.0 | 6.0 |
| Menu | Cream of wheat Orange juice | White bagel, grape jelly and water |
Weekly follow-ups (∼30 minutes each)
Following the baseline acute-feeding session, participants were asked to consume 43 g of almonds every day at times based on their assigned group. The CL group was instructed to avoid all nuts and seeds in the diet during the study period. All almonds were dry-roasted and lightly salted (200 mg Na per 43 g almonds) and were preweighed and packaged for the participants' convenience. Participants were not given specific dietary advice other than to follow their habitual dietary practices and physical activities. A ruse was introduced to enhance the compliance with almond consumption at the designated times of day. Breath samples were collected during follow-up sessions and participants were led to believe that that the analysis of their breath samples for an almond volatile would determine whether almonds had been ingested at the specified times. Actual compliance to almond consumption was assessed through reported dietary intake and fasting plasma α-tocopherol concentration. Participants attended weekly visits (week 1, 2 and 3) after an overnight fast, during which body weight was recorded and compliance to the study protocol was reinforced. During the week-2 visit, participants were also asked to complete a 24-h dietary intake assessment using the multi-pass interview method. Appetite sensation ratings were recorded on visual analog scales every waking hour for a day. On week-4, participants returned for OGTT and acute-feeding sessions as described above. This study was conducted according to the guidelines established in the Declaration of Helsinki and all procedures involving human participants were approved by the Purdue University Institutional Review Board and registered with ClinicalTrials.gov (identifier no. NCT01690936). Written informed consent was obtained from all participants.
Data and statistical analysis
Group characteristics at baseline were compared using one-way analysis of variance (ANOVA). Data are presented as mean±s.d. unless otherwise specified. Within and between-group comparisons of means from acute-feeding sessions (0, 15, 45, 60, 90, 120, 180, 240, 265, 195, 310, 340, 370, 430 and 490 min) and weekly follow-up sessions (baseline, week 1, 2, 3 and 4) were performed using the general linear model for repeated measures of ANOVA with the Bonferroni corrections. During the acute-feeding session, incremental area under the curve (AUC) for serum glucose concentrations 60-min post meal or snack times were calculated using the trapezoidal rule. Between-group comparisons were conducted using one-way ANOVA. All statistical analyses were performed using the Statistical Package for Social Sciences software (IBM SPSS version 20, IBM Corp., Armonk, NY, USA) and statistical significance was set at α<0.05, two-tailed.
Results
Participants
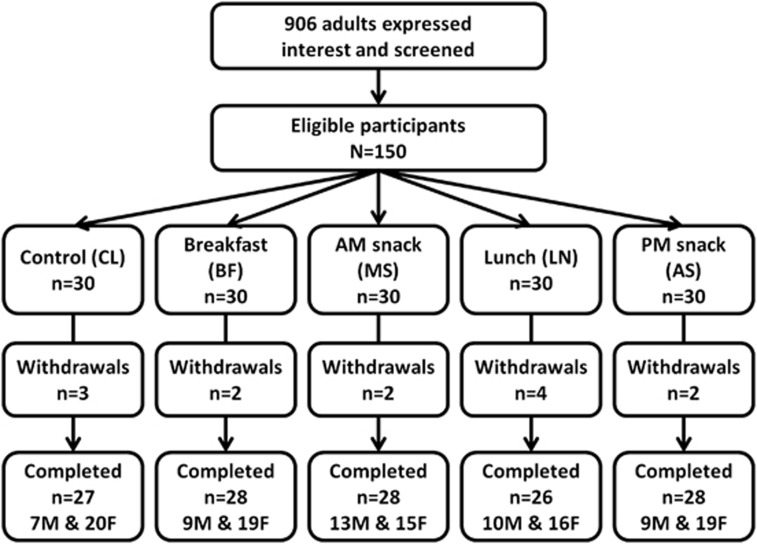
The study was conducted between 2010 and 2012, and 906 volunteers expressed interest in the study. One hundred and fifty participants met the eligibility criteria and were recruited. Participants were randomly assigned to the study groups (n=30 each group). A total of 137 (males, n=48; females, n=89) participants completed all study visits (Figure 1). Thirteen participants withdrew from the study due to time commitment (n=11), high fasting blood glucose (n=1) and high baseline blood pressure (n=1). The mean age of participants in each study group was CL=28.7±9.6 years, BF=32.9±11.5 years, MS=27.8±10.7 years, LN=29.3±13.5 years and AS=29.0±11.9 years (not significantly different between groups). Baseline mean anthropometry (Table 2A), fasting blood biochemistry (Table 2B) and dietary intake data (Table 2C) were not significantly different between groups (one-way ANOVA, P>0.05).
Figure 1.
Participant recruitment and randomization and study design.
Table 2. Anthropometric measurements (A), blood biochemistries (B) and dietary intakes (C) at baseline and week-4 of study.
| (A) |
Baseline |
Week-4 |
Repeated measures ANOVA (Greenhouse–Geisser)a |
|||
|---|---|---|---|---|---|---|
| Mean±s.d. | Mean± s.d. | Time, P | Group, P | Timeb Group, P | ||
| Body weight, kg | ||||||
| CL | n=27 | 77.2±16.8 | 77.2±17.2 | |||
| BF | n=28 | 80.5±15.0 | 80.7±14.8 | |||
| MS | n=28 | 83.2±21.1 | 83.2±21.3 | 0.023b | 0.510 | 0.357 |
| LN | n=26 | 84.8±13.7 | 85.1±13.4 | |||
| AS | n=28 | 81.8±14.6 | 81.9±15.8 | |||
| BMI, kg/m2 | ||||||
| CL | n=27 | 27.0±4.4 | 26.9±4.4 | |||
| BF | n=28 | 28.2±4.8 | 28.3±4.7 | |||
| MS | n=28 | 28.7±5.0 | 28.7±5.1 | 0.011b | 0.547 | 0.627 |
| LN | n=26 | 29.0±3.9 | 29.1±3.9 | |||
| AS | n=28 | 28.2±5.2 | 28.3±5.3 | |||
| Waist, cm | ||||||
| CL | n=27 | 81.9±12.4 | 82.3±12.4 | |||
| BF | n=28 | 85.3±12.2 | 85.0±12.4 | |||
| MS | n=28 | 85.6±11.4 | 85.8±11.6 | <0.001b | 0.585 | 0.010b |
| LN | n=26 | 87.0±9.9 | 87.2±9.7 | |||
| AS | n=28 | 86.2±11.2 | 86.0±11.0 | |||
| Body fat mass, kg | ||||||
| CL | n=27 | 26.3±10.5 | 26.2±10.4 | |||
| BF | n=28 | 28.1±11.2 | 28.0±11.2 | |||
| MS | n=28 | 28.1±10.2 | 28.1±10.9 | 0.151 | 0.988 | 0.922 |
| LN | n=26 | 28.5±9.2 | 28.2±9.3 | |||
| AS | n=28 | 27.3±10.7 | 27.0±11.2 | |||
| Systolic blood pressure, mmHg | ||||||
| CL | n=27 | 121.2±11.5 | 117.8±12.5 | |||
| BF | n=28 | 123.9±12.5 | 121.5±14.9 | |||
| MS | n=28 | 122.7±11.9 | 122.6±11.0 | 0.023b | 0.047b | 0.481 |
| LN | n=26 | 128.4±14.8 | 128.4±10.9 | |||
| AS | n=28 | 127.7±11.6 | 124.1±10.2 | |||
| Diastolic blood pressure, mmHg | ||||||
| CL | n=27 | 74.6±8.0 | 72.2±9.3 | |||
| BF | n=28 | 77.1±10.3 | 76.3±11.0 | |||
| MS | n=28 | 76.1±9.1 | 74.3±8.5 | 0.020b | 0.438 | 0.713 |
| LN | n=26 | 78.7±11.9 | 76.6±11.7 | |||
| AS | n=28 | 76.8±7.9 | 76.8±6.7 | |||
| (B) |
Baseline |
Week-4 |
Repeated measures ANOVA (Greenhouse–Geisser)a |
|||
|---|---|---|---|---|---|---|
| Mean± s.d. | Mean± s.d. | Time, P | Group, P | Timeb Group, P | ||
| Fasting serum glucose, mg/dl | ||||||
| CL | n=27 | 92.3±9.7 | 92.2±10.0 | |||
| BF | n=28 | 89.0±7.8 | 91.3±0.9 | |||
| MS | n=28 | 93.8±18.4 | 90.1±9.1 | 0.003b | 0.877 | 0.733 (adj. BMI 0.084) |
| LN | n=26 | 92.6±9.4 | 92.5±9.0 | |||
| AS | n=28 | 91.4±8.9 | 91.9±11.5 | |||
| Fasting serum triacylglycerol, mg/dl | ||||||
| CL | n=27 | 93.3±44.4 | 98.3±50.2 | |||
| BF | n=28 | 89.7±50.3 | 103.3±58.7 | |||
| MS | n=28 | 92.6±51.6 | 90.4±41.2 | 0.001b | 0.849 | 0.260 (adj. BMI 0.855) |
| LN | n=26 | 99.5±68.5 | 92.7±52.6 | |||
| AS | n=28 | 112.2±43.7 | 95.6±37.0 | |||
| Fasting serum total cholesterol, mg/dl | ||||||
| CL | n=27 | 151.9±25.3 | 147.2±24.0 | |||
| BF | n=28 | 152.3±23.2 | 153.4±26.9 | |||
| MS | n=28 | 151.1±34.7 | 153.0±36.2 | <0.001b | 0.864 | 0.530 (adj. BMI 0.822) |
| LN | n=26 | 154.1±32.4 | 149.0±26.4 | |||
| AS | n=28 | 157.5±31.4 | 151.8±33.0 | |||
| Fasting serum LDL cholesterol, mg/dl | ||||||
| CL | n=27 | 89.1±22.6 | 83.9±21.7 | |||
| BF | n=28 | 95.7±26.5 | 92.6±23.6 | |||
| MS | n=28 | 93.5±29.3 | 95.9±29.7 | <0.001b | 0.726 | 0.636 (adj. BMI 0.981) |
| LN | n=26 | 93.0±26.0 | 89.1±24.9 | |||
| AS | n=28 | 97.0±28.5 | 92.0±28.3 | |||
| Fasting serum HDL cholesterol, mg/dl | ||||||
| CL | n=27 | 52.7±11.6 | 53.0±12.3 | |||
| BF | n=28 | 48.3±14.8 | 49.6±18.3 | |||
| MS | n=28 | 47.7±9.4 | 47.3±9.2 | <0.001b | 0.502 | 0.178 (adj. BMI 0.376) |
| LN | n=26 | 51.0±15.7 | 52.0±14.7 | |||
| AS | n=28 | 47.9±10.7 | 46.9±10.4 | |||
| Fasting serum insulin, μU/ml | ||||||
| CL | n=27 | 10.5±5.0 | 9.4±5.7 | |||
| BF | n=28 | 9.0±5.7 | 11.1±11.3 | |||
| MS | n=28 | 13.4±12.9 | 10.7±6.4 | 0.024b | 0.176 | 0.309 (adj. BMI 0.962) |
| LN | n=26 | 13.2±5.7 | 13.5±8.1 | |||
| AS | n=28 | 11.2±6.5 | 13.3±8.7 | |||
| Fasting plasma α-tocopherol, mg/dl | ||||||
| CL | n=27 | 6.39±1.62 | 6.26±1.45 | |||
| BF | n=28 | 6.65±1.76 | 7.05±1.82 | |||
| MS | n=28 | 5.90±1.33 | 6.15±1.36 | 0.181 | 0.541 | 0.333 |
| LN | n=26 | 6.66±2.99 | 6.59±2.74 | |||
| AS | n=28 | 6.43±2.02 | 6.59±1.55 |
| (C) |
Baseline |
Week-4 |
Repeated measures ANOVA (Greenhouse-Geisser)a |
|||
|---|---|---|---|---|---|---|
| Mean±s.d. | Mean±s.d. | Time, P | Group, P | Timeb Group, P | ||
| Energy intake, kcal/day | ||||||
| CL | n=27 | 1890±539 | 1719±661 | |||
| BF | n=28 | 2026±754 | 1969±667 | |||
| MS | n=28 | 2223±795 | 2216±749 | 0.573 | 0.035b | 0.806 |
| LN | n=26 | 2036±718 | 2082±719 | |||
| AS | n=28 | 1948±748 | 1878±685 | |||
| Fat intake, g/day | ||||||
| CL | n=27 | 72.8±27.3 | 69.0±42.4 | |||
| BF | n=28 | 82.8±45.4 | 90.0±42.0 | |||
| MS | n=28 | 84.1±36.1 | 96.4±34.9 | 0.057 | 0.033b | 0.583 |
| LN | n=26 | 82.3±38.9 | 88.2±31.5 | |||
| AS | n=28 | 73.3±37.1 | 85.0±30.2 | |||
| Carbohydrate intake, g/day | ||||||
| CL | n=27 | 228.5±90.6 | 216.0±84.9 | |||
| BF | n=28 | 251.9±90.6 | 220.6±69.5 | |||
| MS | n=28 | 285.0±114.1 | 259.0±108.8 | 0.062 | 0.057 | 0.710 |
| LN | n=26 | 244.7±88.2 | 242.3±113.9 | |||
| AS | n=28 | 239.8±105.6 | 204.4±101.6 | |||
| Protein intake, g/day | ||||||
| CL | n=27 | 76.6±34.9 | 63.9±26.4 | |||
| BF | n=28 | 76.9±33.7 | 76.1±30.6 | |||
| MS | n=28 | 79.3±33.6 | 88.9±42.7 | 0.755 | 0.123 | 0.373 |
| LN | n=26 | 84.8±31.4 | 84.1±26.7 | |||
| AS | n=28 | 75.7±42.8 | 73.9±32.6 | |||
| Alcohol intake, g/day | ||||||
| CL | n=27 | 6.3±23.9 | 1.4±5.3 | |||
| BF | n=28 | 1.2±4.1 | 1.9±10.3 | |||
| MS | n=28 | 6.7±22.6 | 1.3±5.6 | 0.240 | 0.762 | 0.334 |
| LN | n=26 | 0.6±2.7 | 2.4±7.1 | |||
| AS | n=28 | 1.8±4.9 | 1.0±5.2 | |||
| Dietary cholesterol (mg/day) | ||||||
| CL | n=27 | 259±208 | 227±299 | |||
| BF | n=28 | 266±213 | 228±230 | |||
| MS | n=28 | 259±209 | 286±250 | 0.437 | 0.705 | 0.890 |
| LN | n=26 | 283±217 | 256±160 | |||
| AS | n=28 | 276±198 | 231±174 | |||
| Dietary saturated fat, g/day | ||||||
| CL | n=27 | 26.4±14.8 | 23.0±16.3 | |||
| BF | n=28 | 31.1±21.3 | 25.7±15.3 | |||
| MS | n=28 | 29.5±15.2 | 27.3±14.5 | 0.066 | 0.561 | 0.990 |
| LN | n=26 | 27.2±12.7 | 23.5±10.1 | |||
| AS | n=28 | 27.2±18.2 | 24.7±12.3 | |||
| Dietary monounsaturated fat, g/day | ||||||
| CL | n=27 | 24.9±9.9 | 22.5±14.5 | |||
| BF | n=28 | 28.4±16.0 | 36.3±13.2 | |||
| MS | n=28 | 28.7±14.0 | 39.0±11.6 | <0.001b | <0.001b | 0.036b |
| LN | n=26 | 29.0±15.6 | 37.3±12.1 | |||
| AS | n=28 | 27.3±14.5 | 35.8±12.7 | |||
| Dietary polyunsaturated fat, g/day | ||||||
| CL | n=27 | 15.3±7.2 | 18.0±12.5 | |||
| BF | n=28 | 16.2±8.5 | 21.3±13.6 | |||
| MS | n=28 | 19.1±11.3 | 22.8±11.2 | 0.008b | 0.064 | 0.323 |
| LN | n=26 | 19.9±13.0 | 20.7±10.9 | |||
| AS | n=28 | 17.6±9.1 | 17.8±7.9 | |||
| Dietary fiber, g/d | ||||||
| CL | n=27 | 14.7±8.3 | 18.4±11.5 | |||
| BF | n=28 | 21.2±12.6 | 19.7±8.3 | |||
| MS | n=28 | 21.2±11.9 | 23.4±12.2 | 0.128 | 0.010b | 0.706 |
| LN | n=26 | 17.0±6.0 | 19.0±9.0 | |||
| AS | n=28 | 16.7±7.9 | 15.9±6.2 | |||
| Dietary α-tocopherol, mg/day | ||||||
| CL | n=27 | 13.9±24.1 | 9.1±5.5 | <0.001b | <0.001b | <0.001b |
| BF | n=28 | 10.2±6.7 | 17.6±3.4 | |||
| MS | n=28 | 12.3±8.1 | 24.4±13.2 | |||
| LN | n=26 | 11.2±8.5 | 18.8±6.0 | |||
| AS | n=28 | 11.4±8.5 | 17.6±6.6 |
Abbreviations: AS, afternoon snack; BF, breakfast; BMI, body mass index; CL, control; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LN, lunch; MS, morning snack.
General linear model for repeated measures (RMANOVA) with Greenhouse–Geisser correction for data with the Mauchly's test of Sphericity P<0.05.
Statistical significance, P<0.05.
Acute post-ingestive effects of almonds eaten with meals or as snacks
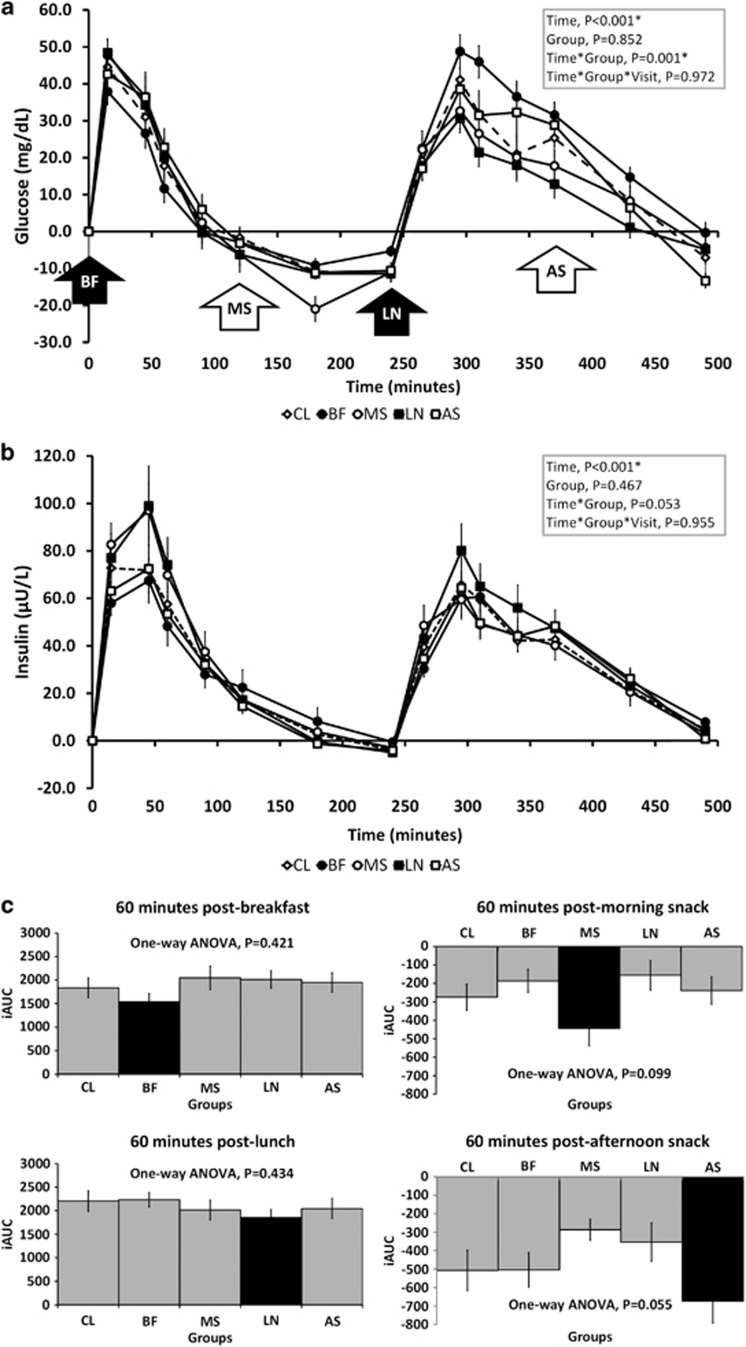
Figure 2 presents postprandial changes in glucose (A) and insulin (B) concentrations during the acute-feeding session at baseline. Overall, there were significant time and interaction effects on postprandial serum glucose (time, P<0.001; time × group, P=0.001) and triacylglycerol (time, P<0.001; time × group, P<0.001) concentrations. Incremental AUC revealed that groups receiving almonds had lower glycemic responses 60 min after almond ingestion (Figure 2c), and the effect approached statistical significance in both snack groups, MS and AS (one-way ANOVA, P=0.099 and P=0.055, respectively). Insulin concentrations changed significantly over time (P<0.001) and the interaction effects approached statistical significance (time × group, P=0.053). However, there was no clear pattern of change following almond ingestion. Insulin concentrations were lowest in the BF after BF but were highest in the LN group after lunch. Post hoc analyses comparing the combined meal (BF and LN), snack (MS and AS) and control groups approached statistical significance (P=0.078), with a trend of blunted glycemia in the snack groups compared with meal groups. The postprandial glucose and insulin responses did not significantly differ between baseline (initial almond exposure) and week-4 (after short-term daily almond exposure) (time × group × visits, P=0.972 and 0.848, respectively), suggesting minimal adaptation over 4 weeks of almond ingestion.
Figure 2.
Changes in serum glucose (a), insulin (b) and 60-min postprandial incremental AUC for glucose (c) during acute-feeding session at baseline. Legend: solid bars in (c) represent study groups that received 43 g almonds, whereas the lighter-shade bars were groups that received none.
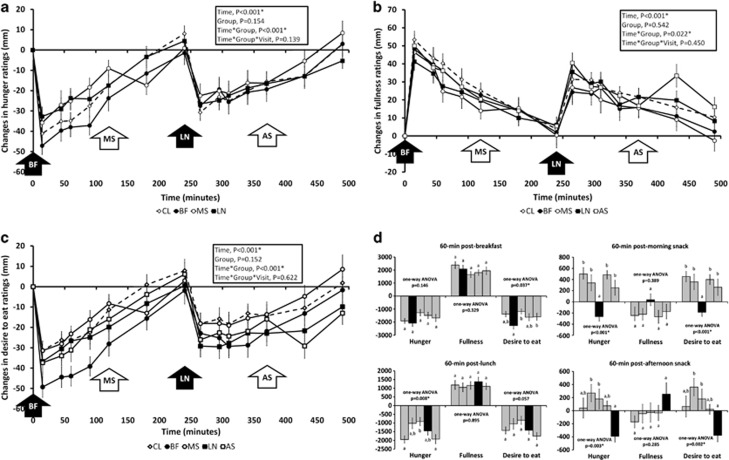
Significant time × treatment group interaction effects for ‘hunger', ‘fullness' and ‘desire to eat' ratings (time × group, P<0.001 for ‘hunger' and ‘desire to eat', and P=0.022 for ‘fullness') were observed during the acute-feeding session (Figures 3a–c). When examined as 60-min postprandial incremental AUC (Figure 3d), almonds ingested with BF led to a greater decrease of ‘desire to eat' (one-way ANOVA, P=0.037), whereas almond consumption with LN resulted in a greater suppression of hunger ratings (one-way ANOVA, P=0.008). In contrast, reported ‘hunger' and ‘desire to eat' ratings were reversed (Figure 3d) when almonds were consumed as morning and ASs (one-way ANOVA, P<0.05). When calculated as AUC, postprandial ‘hunger' and ‘desire to eat' ratings followed the order of combined snack groups (MS and AS) <combined meal groups (BF and LN) <control (CL) (one-way ANOVA, P=0.026 and P=0.023, respectively). Similar to postprandial blood biochemistries, these appetite ratings did not differ significantly between acute-feeding sessions conducted at baseline and at week-4 in any study group, suggesting minimal adaptation to short-term almond consumption.
Figure 3.
Changes in hunger (a), fullness (b), desire to eat (c) ratings and 60-min postprandial incremental AUC for these appetite ratings (d) during acute-feeding sessions. Legend: solid bars in (d) represent study groups that received 43 g almonds. Bars in groups represent study groups CL, BF, MS, LN and AS from left to right.
Short-term effects of almond consumption
Dietary intake and compliance
Despite the additional 250 kcal/day from almonds, daily energy intake in all almond groups was not significantly higher than baseline or the CL group (Table 2C). Almond supplementation also had no significant time or interaction effect on the intake of other nutrients at baseline, week-2 or week-4, except for dietary monounsaturated fat (interaction effects, P=0.036) and α-tocopherol intake (interaction effects, P<0.001). Post hoc analyses revealed that these effects derived mainly from the CL vs all almond groups. Almonds, when ingested with meals (BF and LN) or alone as snacks (MS and AS), were associated with comparable habitual energy intake (CL). Liking of almonds, measured at baseline and at week-4, decreased slightly but significantly in all groups receiving almonds (from 48.5±25.7 to 40.8±30.8 mm, time effects, P=0.009) but there was no difference between groups (interaction effects, P=0.303). Ratings on the Food Action Scale did not differ within or between groups after 4 weeks of almond consumption.
Although all almond groups significantly increased dietary α-tocopherol intake as calculated from their reported 24-h recall, changes in fasting plasma α-tocopherol concentrations only showed trends in that direction: CL=−0.8%±12.8% BF=+7.4%±16.3% MS=+5.4%±14.4% LN=−0.7%±12.8% and AS=+5.7%±16.5% (Table 2C).
Anthropometric measurements and blood pressure
Body weight and BMI fluctuated over the 4-week almond consumption period (time effects, P=0.023 and 0.011, respectively) but were not significantly different between study groups (Table 2A). Body fat mass, systolic and diastolic blood pressure changes were also not significantly different between study groups after 4 weeks. Although changes in waist circumference were significant within and between groups, the effects were mixed and small (interaction effects, P=0.010). Further analyses revealed that there was no differential effect of almonds, eaten together with meals (BF and LN combined, n=53) or alone as snacks (MS and AS combined, n=54) or between lean and overweight/obese status on all anthropometric measurements and blood pressure.
Fasting blood biochemistries
(Table 2) Fasting blood biochemistries changed significantly over time, but not significantly between groups or from the CL group (Table 2B). When BMI was included as a covariate in the general linear model analysis, there was a trend for almond consumption on fasting glucose concentrations (time × group × BMI effects, P=0.084), where mean fasting glucose concentrations were slightly elevated in the CL but decreased in all almond groups among normal-weight participants (n=30). This trend was not observed in overweight and obese individuals. Similar to anthropometric measurements, combining meal and snack groups did not yield significant effects on fasting blood biochemistry changes. Mean fasting plasma α-tocopherol concentrations were higher in all almond groups after 4 weeks but failed to reach statistical significance (Table 2B).
Free-living appetitive responses
Most participants recorded their hourly free-living appetite sensations between 1000 and 2200 hours, so data within this period of time were included in analyses. The ‘hunger', ‘fullness' and ‘desire to eat' ratings changed significantly during the 12-h period (time effects, P<0.001) but they were not significantly different between study groups nor between baseline and week-4 (time × group × visits, P>0.05).
Discussion
Snacking reportedly increases the risk for weight gain, but such a broad generalization may mask differential responses to selected foods. This study compared the acute post-ingestive and the short-term effects of incorporating almonds in a meal or consuming them alone as snacks. It also allowed the assessment of possible physiological adaptations to almond consumption that could accentuate or diminish behavioral responses after 4 weeks of daily ingestion.
Acute post-ingestive effects
This study supports the moderating effects of almond consumption on postprandial glycemia observed in a previous study.22, 36 Serum glucose concentrations 60 min after BF and LN meals were lower when 43 g of almonds were ingested with the meals. This may be attributable to the fiber and fat content of almonds. Fiber reduces glycemia by: (a) increasing the viscosity of intestinal contents hindering glucose diffusion; (b) lowering the glucose concentration by reducing carbohydrate availability in the gastrointestinal tract; and (c) capsulation of starch and hence impairing α-amylase activity.37 Fat derived from the almonds may have also caused lower postprandial glycemia by slowing gastric emptying times and dilution.38 The glycemia-lowering-effect of fats is primarily due to a decreased absorption rate rather than an increased clearance from the circulation.39 This is in agreement with our finding, where muted glucose responses were not due to higher postprandial insulin concentrations. However, serum glucose concentrations decreased when almonds were consumed alone as snacks (Figure 2) suggesting an enhancement of clearance. Future studies are required to better understand the underlying mechanisms that elevate glucose clearance from the circulation after almond snacks. Because of a reduction in glucose concentrations in the MS group, glycemic responses remained low following standard LN. A second-meal effect has been reported in a number of studies,40, 41, 42 but our data revealed that this effect was carried over from snack-to-meal instead of from meal-to-meal.
Appetitive sensations followed a predicted pattern after the meals including almonds. Hunger ratings were the lowest in the BF group immediately after BF but this hunger suppressing effect was less pronounced in the LN group after the LN meal (Figure 3d). This difference may be attributable to the higher energy BF that was about 85 kcal more than the LN provided to the participants (Table 1). Eating almonds both with meals and alone as snacks resulted in lower hunger levels before the subsequent meal, although the hunger levels were suppressed more and remained below baseline when consumed as ASs (Figure 3). Changes in the ‘desire to eat' ratings also followed similar patterns. The effects of almonds on appetitive sensations were most pronounced when they were ingested alone as snacks. The AUC of hunger, fullness and desire to eat ratings at 60-min were reversed relative to groups that did not receive a snack. Together, these findings indicate that there is a greater decrease in hunger levels and this translated into a longer inter-meal interval.43 Although hunger is a poor predictor of food intake,44 it has been shown to influence food-purchasing decisions among impulsive individuals.45 Similarly, lower desire-to-eat ratings reduce the likelihood of individuals responding to food cues and eating in the absence of hunger.46 In contrast to other work,42 fullness ratings were not significantly higher in the almond groups (Figures 3b, d). This leaves open questions about the influence of almonds on meal size.
Short-term effects of almond consumption
Changes of body weight in the groups consuming almonds were not significantly different from the control group despite the contribution of 250 kcal of energy from the almonds to the daily diet for 4 weeks. This outcome is consistent with previous observations that humans compensate for energy from almonds behaviorally (dietary compensation) and physiologically (inefficient energy absorption18 and increased thermogenesis19) and remain weight stable.20, 21, 47
In longitudinal studies, almonds have been shown to improve fasting glucose48, insulin49 and total and low-density lipoprotein cholesterol.24, 50 However, these effects were not seen in this study. The lack of effects on other fasting blood biochemical indices and blood pressure from almonds in our study relative to many previously published reports may be explained by differences in study design. Our study was shorter in duration and used a relatively low dose of almonds, which may have limited the power to detect effects. A dose-response effect of almonds has been observed in other studies, where higher almond intake was associated with greater lipid and glycemia-lowering effects.24, 50, 51 Furthermore, participants included in this study were relatively healthy (despite having elevated risks for type 2 diabetes). Their fasting lipid profiles and blood pressure were within the healthy range at baseline. Previous studies that reported significant improvements often included participants with hyperlipidemia50, 52, 53, 54, 55, 56 or type 2 diabetes.36, 48, 57, 58
Hourly appetite ratings recorded at baseline, week-2 and week-4 of this study did not differ between study groups, showing that participants maintained their hunger, fullness and desire to eat levels throughout the 4 weeks, even though some groups received an additional 43 g of almonds, an energy dense food, in their daily diet. This observation is consistent with previous evidence that entailed supplementing participants with fruits and vegetables;59 foods of low energy density. Thus, energy density does not seem to be the salient property. To a large degree, participants in the almond groups adjusted their energy intake to achieve a profile of appetitive sensations that resembled their customary patterns, so energy intake in response to almond supplementation did not affect body weight. Carbohydrate, protein and fat intake were also unaffected. However, the profile of fat intake was significantly altered, where monounsaturated fat intake increased significantly in the almond groups but decreased in the control group. Similar results were noted for dietary α-tocopherol intake. Almonds are good sources of these nutrients so such findings were not surprising. It should also be highlighted that the baseline intake of α-tocopherol was below the recommended intake of 15 mg/day in all groups but met the recommendations after 4 weeks in study groups that received almonds. This has been reported previously.19, 60 Increased reported intakes of monounsaturated fats and α-tocopherol indicate compliance with almonds supplementation. In this study, α-tocopherol intake from almonds (9.9 mg/43 g) was not associated with significant increases of fasting plasma α-tocopherol concentration but they changed in the predicted direction. A number of studies have previously shown that almond supplementation increased plasma α-tocopherol concentrations but larger doses of almonds were used in these studies.19, 58, 60, 61
Conclusion
In summary, consumption of 43 g of almonds modulated postprandial glycemia and suppressed hunger and desire to eat sensations especially after being consumed as snacks. Over a 4-week period, almond consumption helped meet recommended dietary intake of α-tocopherol and did not affect body weight (due largely to strong dietary compensation) or postprandial lipid profiles in healthy adults at risk for type 2 diabetes. These findings suggest that almonds may be a healthy snack option.
Acknowledgments
We thank the Almond Board of California for funding and supplying almonds for this study. We would also like to acknowledge Judy George and Robin Rhine for their assistance in phlebotomy procedures.
The authors declare no conflict of interest.
References
- US Department of Agriculture What we eat in America,2011
- Piernas C, Popkin BM. Snacking increased among U.S. adults between 1977 and 2006. J Nutr. 2010;140:325–332. doi: 10.3945/jn.109.112763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TT-K, Roberts SB, Howarth NC, McCrory MA. Effects of screening out implausible energy intake reports on relationships between diet and BMI. Obes Res. 2005;13:1205–1217. doi: 10.1038/oby.2005.143. [DOI] [PubMed] [Google Scholar]
- Locard E, Mamelle N, Bilette A, Miginiac M, Munoz F, Rey S. Risk factors of obesity in a five year old population. Parental versus environmental factors. Int J Obes Relat Metab Disord. 1992;16:721–729. [PubMed] [Google Scholar]
- Lioret S, Touvier M, Lafay L, Volatier JL, Maire B. Dietary and physical activity patterns in French children are related to overweight and socioeconomic status. J Nutr. 2008;138:101–107. doi: 10.1093/jn/138.1.101. [DOI] [PubMed] [Google Scholar]
- Bertéus Forslund H, Torgerson JS, Sjostrom L, Lindroos AK. Snacking frequency in relation to energy intake and food choicesin obese men and women compared to a reference population. Int J Obes. 2005;29:711–719. doi: 10.1038/sj.ijo.0802950. [DOI] [PubMed] [Google Scholar]
- Howarth NC, TT-K Huang, Roberts SB, Lin BH, McCrory MA. Eating patterns and dietary composition in relation to BMI in younger and older adults. Int J Obes. 2007;31:675–684. doi: 10.1038/sj.ijo.0803456. [DOI] [PubMed] [Google Scholar]
- Keski-Rahkonen A, Bulik CM, Pietilainen KH, Rose RJ, Kaprio J, Rissanen A. Eating styles, overweight and obesity in young adult twins. Eur J Clin Nutr. 2007;61:822–829. doi: 10.1038/sj.ejcn.1602601. [DOI] [PubMed] [Google Scholar]
- Bes-Rastrollo M, Sanchez-Villegas A, Basterra-Gortari FJ, Nunez-Cordoba JM, Toledo E, Serrano-Martinez M. Prospective study of self-reported usual snacking and weight gain in a Mediterranean cohort: the SUN project. Clin Nutr. 2010;29:323–330. doi: 10.1016/j.clnu.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Porrini M, Santangelo A, Crovetti R, Riso P, Testolin G, Blundell JE. Weight, protein, fat and timing of preloads affect food intake. Physiol Behav. 1997;62:563–570. doi: 10.1016/s0031-9384(97)00162-5. [DOI] [PubMed] [Google Scholar]
- Rolls BJ, Morris EL, Roe LS. Portion size of food affects energy intake in normal-weight and over-weight men and women. Am J Clin Nutr. 2002;76:1207–1213. doi: 10.1093/ajcn/76.6.1207. [DOI] [PubMed] [Google Scholar]
- Scherwitz L, Kesten D. Seven eating styles linked to overeating, overweight and obesity. Explore. 2005;1:342–359. doi: 10.1016/j.explore.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Gregori D, Maffeis C. Snacking and obesity: urgency of a definition to explore such a relationship. J Am Diet Assoc. 2007;107:562. doi: 10.1016/j.jada.2007.02.025. [DOI] [PubMed] [Google Scholar]
- Talegawkar SA, Johnson EJ, Carithers T, Taylor HA, Jr, Bogle ML, Tucker KL. Total alpha-tocopherol intakes are associated with serum alpha-tocopherol concentrations in African American adults. J Nutr. 2007;137:2297–2303. doi: 10.1093/jn/137.10.2297. [DOI] [PubMed] [Google Scholar]
- Stroehla BC, Malcoe LH, Velie EM. Dietary sources of nutrients among rural Native American and white children. J Am Diet Assoc. 2005;105:1908–1916. doi: 10.1016/j.jada.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Nicklas TA, Hampl JS, Taylor CA, Thompson VJ, Heird WC. Monounsaturated fatty acid intake by children and adults: temporal trends and demographic differences. Nutr Rev. 2004;62:132–141. doi: 10.1111/j.1753-4887.2004.tb00035.x. [DOI] [PubMed] [Google Scholar]
- Zizza C, Arsiwalla DD, Ellison KJ. Contribution of snacking to older adults' vitamin, carotenoid, and mineral intakes. J Am Diet Assoc. 2010;10:768–772. doi: 10.1016/j.jada.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Cassady BA, Hollis JH, Fulford AD, Considine RV, Mattes RD. Mastication of almonds: effects of lipid bioaccessibility, appetite, and hormone response. Am J Clin Nutr. 2009;89:794–800. doi: 10.3945/ajcn.2008.26669. [DOI] [PubMed] [Google Scholar]
- Hollis J, Mattes R. Effect of chronic consumption of almonds on body weight in healthy humans. Br J Nutr. 2007;98:651–656. doi: 10.1017/S0007114507734608. [DOI] [PubMed] [Google Scholar]
- Fraser GE, Bennett HW, Jaceldo KB, Sabate J. Effect on body weight of a free 76 Kilojoule (320 calorie) daily supplement of almonds for six months. J Am Coll Nutr. 2002;21:275–283. doi: 10.1080/07315724.2002.10719221. [DOI] [PubMed] [Google Scholar]
- Sabate J. Nut consumption and body weight. Am J Clin Nutr. 2003;78:647S–650S. doi: 10.1093/ajcn/78.3.647S. [DOI] [PubMed] [Google Scholar]
- Jenkins DJA, Kendall CWC, Josse AR, Salvatore S, Brighenti F, Augustin LSA, et al. Almonds decrease postprandial glycemia, insulinemia, and oxidative damage in healthy individuals. J Nutr. 2006;136:2987–2992. doi: 10.1093/jn/136.12.2987. [DOI] [PubMed] [Google Scholar]
- Jenkins DJA, Kendall CWC, Marchie A, Parker TL, Connelly PW, Qian W, et al. Dose response of almonds on coronary heart disease risk factors: blood lipids, oxidized low-density lipoproteins, lipoproteina), homocysteine, and pulmonary nitric oxide: a randomized, controlled, crossover trial. Circulation. 2002;106:1327–1332. doi: 10.1161/01.cir.0000028421.91733.20. [DOI] [PubMed] [Google Scholar]
- Sabate J, Haddad E, Tanzman J, Jambazian PR, Rajaram S. Serum lipid response to the graduated enrichment of a Step I diet with almonds: a randomized feeding trial. Am J Clin Nutr. 2003;77:1379–1384. doi: 10.1093/ajcn/77.6.1379. [DOI] [PubMed] [Google Scholar]
- Foster GD, Shantz KL, Vander Veur SS, Oliver TL, Lent MR, Virus A, et al. A randomized trial of the effects of an almond-enriched, hypocaloric diet in the treatment of obesity. Am J Clin Nutr. 2012;96:249–254. doi: 10.3945/ajcn.112.037895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Agriculture, US Department of Health and Human Services Dietary Guidelines for Americans7th edn.US Government Printing Office: Washington, DC, USA; 2010 [Google Scholar]
- Cappelleri JC, Bushmakin AG, Gerber RA, Leidy NK, Sexton CC, Kalsson J, et al. Evaluating the Power of Food Scale in obese subjects and a general sample of individuals: development and measurement properties. Int J Obes. 2009;33:913–922. doi: 10.1038/ijo.2009.107. [DOI] [PubMed] [Google Scholar]
- Cepeda-Benito A, Gleaves DH, Williams DL, Erath SA. The development and validation of the state and trait food-craving questionnaires. Behavior Ther. 2000;31:151–173. doi: 10.1016/s0005-7967(99)00141-2. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- Zung WWK. A self-rating depression scale. Arch Gen Psychiat. 1965;12:63–70. doi: 10.1001/archpsyc.1965.01720310065008. [DOI] [PubMed] [Google Scholar]
- Eysenck SBG, Eysenck HJ, Barrett P. A revised version of the psychoticism scale. Pers Individ Dif. 1985;6:21–29. [Google Scholar]
- Raudenbush B, Van Der Klaauw NJ, Frank RA. The contribution of psychological and sensory factors to food preference patterns as measured by the Food Attitudes Survey (FAS) Appetite. 1995;25:1–15. doi: 10.1006/appe.1995.0037. [DOI] [PubMed] [Google Scholar]
- Stephenson MT, Hyoyle RH, Palmgreen P, Slater MD. Brief measures of sensation seeking for screening and large-scale surveys. Drug Alcohol Depend. 2003;72:279–286. doi: 10.1016/j.drugalcdep.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Johansson G, Westerterp KR. Assessment of the physical activity level with two questions: validation with doubly labeled water. Int J Obes. 2008;32:1031–1033. doi: 10.1038/ijo.2008.42. [DOI] [PubMed] [Google Scholar]
- Schutz HG. A food action rating scale for measuring food acceptance. J Food Sci. 1965;30:365–374. [Google Scholar]
- Cohen AE, Johnston CS. Almond ingestion at mealtime reduces postprandial glycemia and chronic ingestion reduces hemoglobin A1c in individuals with well-controlled type 2 diabetes mellitus. Metabolism. 2011;30:1312–1317. doi: 10.1016/j.metabol.2011.01.017. [DOI] [PubMed] [Google Scholar]
- Ou S, Kwok K-c LiY, Fu L. In vitro study of possible role of dietary fiber in lowering postprandial serum glucose. J Agric Food Chem. 2001;49:1026–1029. doi: 10.1021/jf000574n. [DOI] [PubMed] [Google Scholar]
- Collier G, O'Dea K. The effect of coingestion of fat on the glucose, insulin, and gastric inhibitory polypeptide responses to carbohydrate and protein. Am J Clin Nutr. 1983;37:941–944. doi: 10.1093/ajcn/37.6.941. [DOI] [PubMed] [Google Scholar]
- Normand S, Khalfallah Y, Louche-Pelissier C, Pachiaudi C, Antoine J-M, Blanc S, et al. Influence of dietary fat on postprandial glucose metabolism (exogenous and endogenous) using intrinsically 13C-enriched durum wheat. Br J Nutr. 2001;86:3–11. doi: 10.1079/bjn2001359. [DOI] [PubMed] [Google Scholar]
- Wolever TMS, Jenkins DJA, Ocana AM, Rao VA, Collier GR. Second-meal effect: low-glycemic-index food eaten at dinner improve subsequent breakfast glycemic response. Am J Clin Nutr. 1988;48:1041–1047. doi: 10.1093/ajcn/48.4.1041. [DOI] [PubMed] [Google Scholar]
- Jenkins DJA, Wolever TMS, Taylor RH, Griffiths C, Krzeminska K, Lawrie JA, et al. Slower release dietary carbohydrate improves second meal tolerance. Am J Clin Nutr. 1982;35:1339–1346. doi: 10.1093/ajcn/35.6.1339. [DOI] [PubMed] [Google Scholar]
- Mori AM, Considine RV, Mattes RD. Acute and second-meal effects of almond form in impaired glucose tolerant adults: a randomized crossover trial. Nutr Metab. 2011;8:1–8. doi: 10.1186/1743-7075-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmonier C, Chapelot D, Louis-Sylvestre J. Effects of macronutrient content and energy density of snacks consumed in a satiety state on the onset of the next meal. Appetite. 2000;34:161–168. doi: 10.1006/appe.1999.0302. [DOI] [PubMed] [Google Scholar]
- Mattes R. Hunger ratings are not a valid proxy measure of reported food intake in humans. Appetite. 1990;15:103–113. doi: 10.1016/0195-6663(90)90043-8. [DOI] [PubMed] [Google Scholar]
- Nederkoorn C, Guerrieri R, Havermans RC, Roefs A, Jansen A. The interactive effect of hunger and impulsitivity on food intake and purchase in a virtual supermarket. Int J Obes. 2009;33:905–912. doi: 10.1038/ijo.2009.98. [DOI] [PubMed] [Google Scholar]
- Lambert LG, Neal T, Noyes J, Parker C, Worrel P. Food-related stimuli increase desire to eat in hungry and satiated human subjects. Curr Psychol. 1991;10:297–303. [Google Scholar]
- Garcia-Lorda P, Rangil IM, Salas-Salvado J. Nut consumption, body weight and insulin resistance. Eur J Clin Nutr. 2003;57:S8–S11. doi: 10.1038/sj.ejcn.1601802. [DOI] [PubMed] [Google Scholar]
- Lovejoy JC, Most MM, Lefevre M, Greenway FL, Rood JC. Effect of diets enriched in almonds on insulin action and serum lipids in adults with normal glucose tolerance or type 2 diabetes. Am J Clin Nutr. 2002;76:1000–1006. doi: 10.1093/ajcn/76.5.1000. [DOI] [PubMed] [Google Scholar]
- Jenkins DJA, Kendall CWC, Marchie A, Josse AR, Nguyen TH, Faulkner DA, et al. Effect of almonds on insulin secretion and insulin resistance in nondiabetic hyperlipidemic subjecys: a randomized controlled crossover trial. Metabolism. 2008;57:882–887. doi: 10.1016/j.metabol.2008.01.032. [DOI] [PubMed] [Google Scholar]
- Jenkins DJA, Kendall CWC, Marchie A, Josse AR, Nguyen TH, Faulkner DA, et al. Almonds reduce biomarkers of lipid peroxidation in older hyperlipidemic subjects. J Nutr. 2008;138:908–913. doi: 10.1093/jn/138.5.908. [DOI] [PubMed] [Google Scholar]
- Josse AR, Kendall CWC, Augustin LSA, Ellis PR, Jenkins DJA. Almonds and postprandial glycemia—a dose-response study. Metab Clin Exp. 2007;56:400–404. doi: 10.1016/j.metabol.2006.10.024. [DOI] [PubMed] [Google Scholar]
- Tamizifar B, Rismankarzadeh M, Vosoughi A, Rafieeyan M, Tamizafar B, Aminzade A. A low-dose almond-based diet decreases LDL-C while preserving HDL-C. ArchIraian Med. 2005;8:45–51. [Google Scholar]
- Jalali-Khanabadi BA, Mozaffari-Khosravi H, Parsaeyan N. Effects of almond dietary supplementation on coronary heart disease lipid risk factors and serum lipid oxidation parameters in men with mild hyperlipidemia. J Altern Complement Med. 2010;16:1279–1283. doi: 10.1089/acm.2009.0693. [DOI] [PubMed] [Google Scholar]
- Spiller GA, Jenkins DAJ, Bosello O, Gates JE, Cragen LN, Bruce B. Nuts and plasma lipids: an almond-based diet lowers LDL-C while preserving HDL-C. J Am Coll Nutr. 1998;17:285–590. doi: 10.1080/07315724.1998.10718761. [DOI] [PubMed] [Google Scholar]
- Spiller GA, Miller A, Olivera K, Reynolds J, Miller B, Morse SJ, et al. Effects of plant-based diets high in raw or roasted almonds, or roasted almond butter on serum lipoproteins in humans. J Am Coll Nutr. 2003;22:195–200. doi: 10.1080/07315724.2003.10719293. [DOI] [PubMed] [Google Scholar]
- Damasceno NRT, Perez-Heras A, Serra M, Cofan M, Sala-Vila A, Salas-Salvado J, et al. Crossover study of diets enriched with virgin olive oil, walnuts or almonds. Effects on lipids and other cardiovascular risk markers. Nutr Metab Cardiovasc Dis. 2011;21:S14–S20. doi: 10.1016/j.numecd.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Wien MA, Bleich SN, Raghuwanshi M, Gould-Forgerite S, Gomes J, Monahan-Couch L, et al. Almond consumption and cardiovascular risk factors in adults with prediabetes. J Am Coll Nutr. 2010;29:189–197. doi: 10.1080/07315724.2010.10719833. [DOI] [PubMed] [Google Scholar]
- Li S-C, Liu Y-H, Liu J-F, Chang W-H, Chen C-M, Chen C-YO. Almond consumption improved glycemic control and lipid profiles in patients with type 2 diabetes mellitus. Metabolism. 2011;60:474–479. doi: 10.1016/j.metabol.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Houchins JA, Tan S-Y, Campbell WW, Mattes RD. Effects of fruit and vegetable, consumed in solid vs beverage forms, on acute and chronic appetitive responses in lean and obese adults. Int J Obes. 2012;37:1109–1115. doi: 10.1038/ijo.2012.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jambazian PR, Haddad E, Rajaram S, Tanzman J, Sabate J. Almonds in the diet simultaneously improve plasma a-tocopherol cencentrations and reduce plasma lipids. J Am Diet Assoc. 2005;105:449–454. doi: 10.1016/j.jada.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Li N, Jia X, Chen C-YO, Blumberg JB, Song Y, Zhang W, et al. Almond consumption reduces oxidative DNA damage and lipid peroxidation in male smokers. J Nutr. 2007;137:2717–2722. doi: 10.1093/jn/137.12.2717. [DOI] [PubMed] [Google Scholar]