Abstract
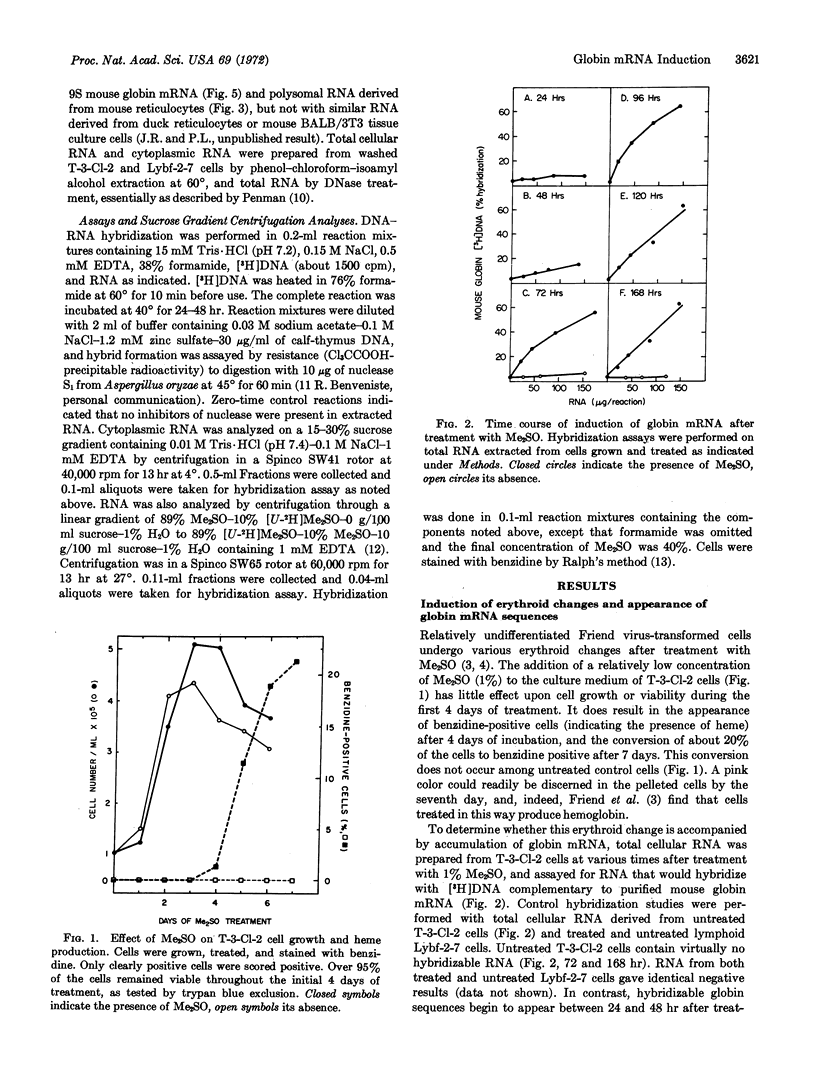
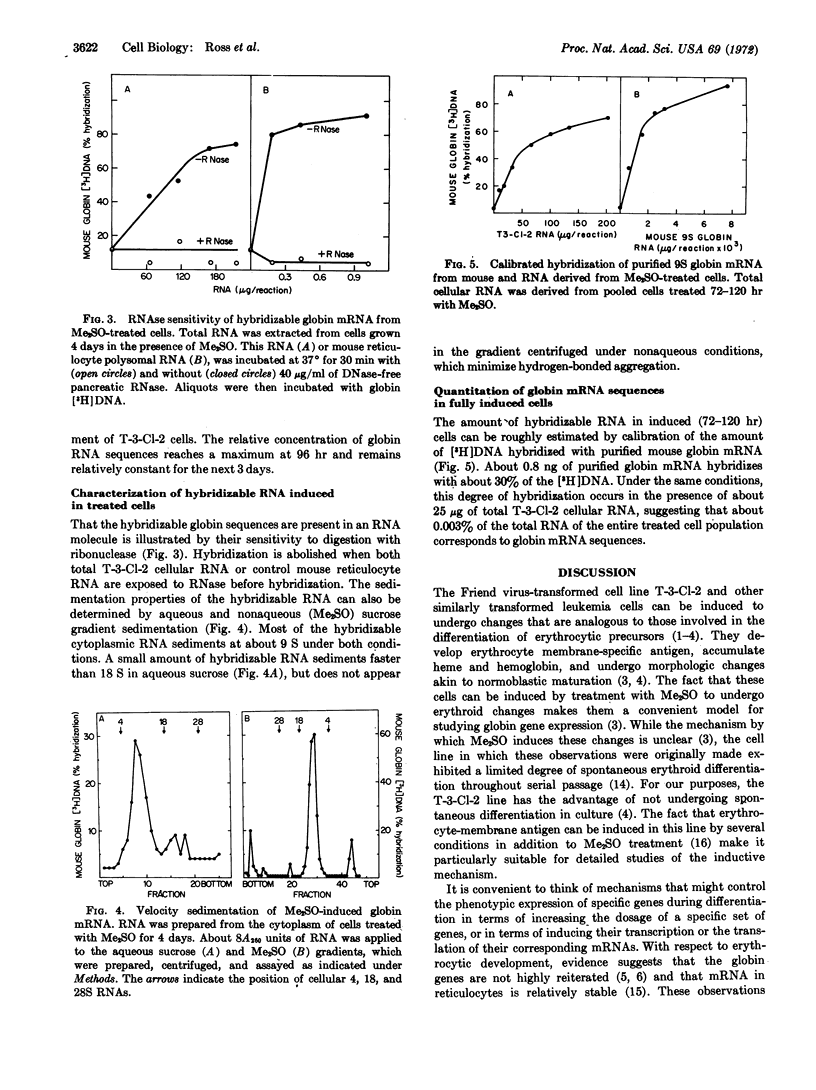
A cloned line of murine proerythroblastoid cells (T-3-Cl-2), transformed by Friend leukemia virus, undergoes changes associated with erythroid differentiation when treated with dimethylsulfoxide in culture. This line, which does not undergo spontaneous differentiation, develops specific erythrocyte-membrane antigen and accumulates detectable amounts of heme within four days of dimethylsulfoxide treatment. In the present study, we have followed the phenotypic expression of the globin genes by measuring globin mRNA in differentiating cells. Our hybridization probe for this purpose is [3H]DNA, which is complementary to purified globin mRNA, synthesized by viral RNA-directed DNA polymerase. This probe is sufficiently sensitive to detect less than 1 ng of globin mRNA. Using it, we find little or no hybridizable globin mRNA in either uninduced cells or in treated control lymphoid cells. In contrast, globin mRNA can be detected in T-3-Cl-2 cell 2 days after induction by dimethylsulfoxide; it reaches a maximum concentration four days after induction. At this time, cells that stain positively for heme appear. The hybridizable cytoplasmic RNA induced in these cells has the sedimentation properties of 9S globin mRNA. Considering the stable character of globin mRNA, our results are most readily explained in terms of a transcriptional activation of the globin genes.
Keywords: Friend leukemia virus, synthetic globin DNA, DNA-RNA hybridization
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson N. H., Buetti E., Scherrer K., Weil R. Transcription of the polyoma virus genome: synthesis and cleavage of giant late polyoma-specific RNA. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2231–2235. doi: 10.1073/pnas.68.9.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank A., Terada M., Metafora S., Dow L., Marks P. A. In vitro synthesis of DNA components of human genes for globins. Nat New Biol. 1972 Feb 9;235(58):167–169. doi: 10.1038/newbio235167a0. [DOI] [PubMed] [Google Scholar]
- Bishop J. O., Pemberton R., Baglioni C. Reiteration frequency of haemoglobin genes in the duck. Nat New Biol. 1972 Feb 23;235(60):231–234. doi: 10.1038/newbio235231a0. [DOI] [PubMed] [Google Scholar]
- Friend C., Patuleia M. C., De Harven E. Erythrocytic maturation in vitro of murine (Friend) virus-induced leukemic cells. Natl Cancer Inst Monogr. 1966 Sep;22:505–522. [PubMed] [Google Scholar]
- Friend C., Scher W., Holland J. G., Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971 Feb;68(2):378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRASSO J. A., WOODARD J. W., SWIFT H. Cytochemical studies of nucleic acids and proteins in erythrocytic development. Proc Natl Acad Sci U S A. 1963 Jul;50:134–140. doi: 10.1073/pnas.50.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. R., Hell A., Birnie G. D., Paul J. Evidence for single copies of globin genes in the mouse genome. Nature. 1972 Sep 22;239(5369):219–221. doi: 10.1038/239219a0. [DOI] [PubMed] [Google Scholar]
- Ikawa Y., Sugano H. An ascites tumor derived from early splenic lesion of Friend's disease: a preliminary report. Gan. 1966 Dec;57(6):641–643. [PubMed] [Google Scholar]
- LOBUE J., DORNFEST B. S., GORDON A. S., HURST J., QUASTLER H. Marrow distribution in rat femurs determined by cell enumeration and Fe59 labeling. Proc Soc Exp Biol Med. 1963 Apr;112:1058–1062. doi: 10.3181/00379727-112-28250. [DOI] [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
- Ross J., Aviv H., Scolnick E., Leder P. In vitro synthesis of DNA complementary to purified rabbit globin mRNA (RNA-dependent DNA polymerase-reticulocyte-hemoglobin-density gradient centrifugation-oligo(dT) primer). Proc Natl Acad Sci U S A. 1972 Jan;69(1):264–268. doi: 10.1073/pnas.69.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher W., Holland J. G., Friend C. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro. I. Partial purification and identification of hemoglobins. Blood. 1971 Apr;37(4):428–437. [PubMed] [Google Scholar]
- Sutton W. D. A crude nuclease preparation suitable for use in DNA reassociation experiments. Biochim Biophys Acta. 1971 Jul 29;240(4):522–531. doi: 10.1016/0005-2787(71)90709-x. [DOI] [PubMed] [Google Scholar]
- Verma I. M., Temple G. F., Fan H., Baltimore D. In vitro synthesis of DNA complementary to rabbit reticulocyte 10S RNA. Nat New Biol. 1972 Feb 9;235(58):163–167. doi: 10.1038/newbio235163a0. [DOI] [PubMed] [Google Scholar]



