Abstract
Background
This study was aimed at the evaluation of the relationship between genetic polymorphisms of catechol-O-methyltransferase (COMT) (rs4680:A > G—Val158Met, rs6269:A > G, rs4633:C > T, rs4818:C > G) and pain sensitivity after lumbar discectomy.
Methods
All patients had one-level symptomatic disc herniation from L3 to S1. The primary data recorded included visual analogue pain scales assessing back and leg pain, Oswestry Disability Questionnaire assessing quality of life and pain intensity, received/filled pre- and postoperatively. Each subject was genotyped for single-nucleotide polymorphism in the COMT gene. Clinical outcome was measured by difference between pre- and postoperative values and those results were analyzed with genetics findings.
Results
Pain intensity was associated with the COMT polymorphism. Carriers of rs6269 AA, rs4633 TT, rs4818 CC, and rs4680 AA genotypes were characterized by the lowest preoperative scores related to pain intensity and lower pain intensity at 1 year after the surgery. The rs4633 CC, rs4680 GG genotypes demonstrated significant clinical improvement in VASBACK score at 1 year after the surgery. Patients with COMT haplotype associated with low metabolic activity of enzyme (A_C_C_G) showed better clinical outcome measured by ODI score and VASBACK score 1 year after surgery. We did not observe any significant correlation between leg pain and single-nucleotide polymorphisms in the COMT gene.
Conclusions
The results of our study indicate that polymorphism in the COMT gene may play an important role in the mechanism of pain perception, which may have a potential implication for clinical decision-making in the future.
Keywords: COMT, Lumbar discectomy, Clinical outcome, Genetic variations, Lumbar disk herniation, Pain
Introduction
Disc herniation is a classic cause of spinal and radicular pain. Isolated lower back pain is a common symptom and has been estimated to affect up to 80 % of individuals at any time of their lives [2]. In 98 % of cases, lower back pain is thought to be caused by degenerative changes in the lumbar spine, whereas the remaining 2 % of cases are attributed to other disorders. However, there is no clear relationship between clinical symptoms and radiological findings [14, 22, 28]. Lumbar discectomy is commonly offered for treatment of sciatica due to disc herniation. However, literature reports on resolving back pain are non-conclusive, i.e., some results show significant improvement in reducing back pain [3, 11, 25, 29], whereas other papers present contradictory observations [4, 30].
One of the potential factors affecting pain perception is genetic diversity [19]. Polymorphisms in the coding regions of the COMT gene result in altered enzymatic activity, and entails different pain-associated catecholamine and encephalin levels [20]. Studies show that reduced COMT activity results in increased pain sensitivity in animal models and in humans [8, 20]. Clinical studies demonstrate also less prominent facial pain in patients characterized by lower COMT activity [16]. Similarly, some studies reported that polymorphisms of the COMT gene resulting in reduced enzyme activity were associated with fibromyalgia [10], temporomandibular disorder [8, 20], and affected morphine efficacy. Dai et al. [7] postulated in their study of patients subjected to degenerative disc disease that genetic variation in the COMT gene might be one of the factors associated with pain sensitivity. The authors were able to reveal a borderline association (not statistically significant) between certain COMT haplotypes and pain intensity measured by VAS score after surgery.
Since there is no clear statement on the role of COMT polymorphism in pain perception in subjects after lumbar spine surgery, the present study was designed. It is aimed at the search for an association between COMT SNPs (single-nucleotide polymorphism) and haplotypes and pain sensitivity in patients with a history of leg and back pain, diagnosed with lumbar disc herniation (LDH), who subsequently underwent lumbar discectomy.
Materials and methods
A total of 176 patients of Caucasian origin diagnosed with lumbar disc herniation and subjected to lumbar discectomy were recruited for the prospective study carried out at the Neurosurgery Department of Medical University of Gdansk, Poland, in 2009–2010. The main inclusion criterion was persistent back and leg pain resistant to conservative therapy for at least 6 months with lumbar disc herniation on CT or MRI scans. Surgery was performed on one of the levels from L3 to S1 by one of four neurosurgeons. The same midline standard posterior approach was used with the assistance of an intra-operative microscope. Exclusion criteria included: patients receiving narcotic pain medications, patients who had progression of symptoms and required immediate surgery, re-operation, fixed motor deficit, spinal stenosis, cauda equina syndrome, and spine deformities. Patients diagnosed with conditions which could influence their perception of pain, like mental disorder (depression) or polyneuropathy, were not eligible for this study. Any other comorbidities were not analyzed.
Patient clinical status was recorded by means of self-reported questionnaires: (Oswestry Disability Index (ODI) and visual analogue pain scale (VAS) separately for lower back pain—VASBACK, and leg pain—VASLEG), all completed before surgery and 12 months post-operatively with the aim to concentrate on chronic pain. For the purpose of this study, the first question from ODI was extracted. The study protocol was approved by the local ethics committee, and each patient signed informed consent forms.
Genotyping
SNPs within the COMT gene were evaluated using Taqman genotyping assays. Genomic DNA was extracted from 200 μl of whole blood samples using GeneMATRIX Quick Blood DNA Purification Kit (EURx, Poland). Pre-validated allelic discrimination TaqMan real-time PCR assays were used for detection of rs6269:G > A, rs4633:C > T, rs4818:C > G, and rs4680:G > A SNPs (respective assay IDs: C___2538746_1, C___2538747_20, C___2538750_10, and C__25746809_50) (Applied Biosystems, California, USA). Fluorescence data was captured using 7500 FAST Real-Time PCR System (Applied Biosystems), after 40 cycles of PCR. Haplotypes were classified in according to COMT activity, A_C_C_G – low, A_T_C_A – medium and G_C_G_G – high activity. Diplotype was assigned to each patient and data were further analyzed.
Statistical analysis
Associations between categorical variables were assessed by Chi-square test. Analysis of associations between COMT genotypes and clinical parameters was performed by means of Mann–Whitney U test. General linear model was used for multivariate analysis. A p value of less than 0.05 was considered statistically significant. Calculations were performed using the Statistica 7.1 software package (StatSoft, Oklahoma, USA). The EH program (Rockefeller University) was used to estimate haplotype frequencies.
Results
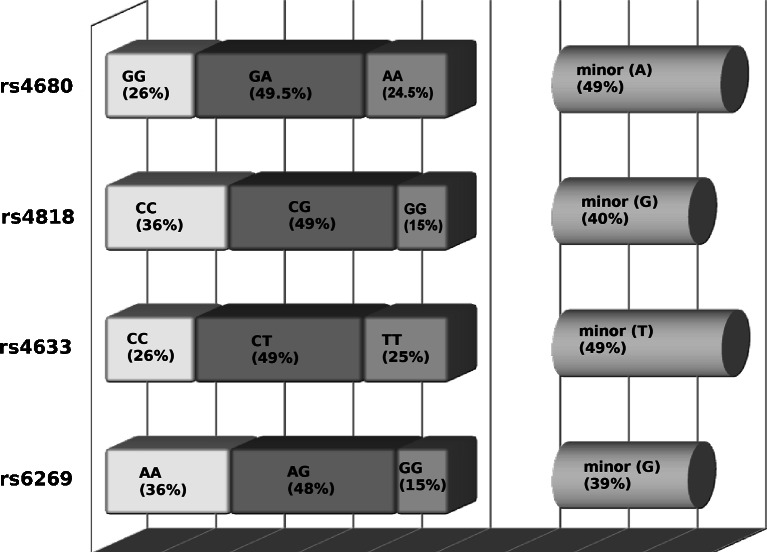
The data from 176 patients, 73 females (41.5 %) and 103 males (58.5 %), aged 20 to 80 years (mean age, 46.7 ± 13.2 years) was collected. Distribution of COMT SNPs rs6269:A > G, rs4633:C > T, rs4818:C > G and rs4680:A > G were in agreement with Hardy-Weinberg equilibrium (p > 0.7). The frequencies of COMT “low” A_C_C_G, “medium” A_T_C_A and “high” G_C_G_G activity haplotypes were calculated (Fig. 1). The most common COMT haplotypes were these of “medium” (49.1 %) and “high” (39.5 %) activity, the “low” activity haplotype A_C_C_G was found in 11.1 % of patients. No associations between COMT genotypes or haplotypes with gender were found.
Fig. 1.
Illustration of incidence of different SNPs, depicted on the right are minor allele frequencies
Homozygous COMT rs6269 AA patients demonstrated a trend towards lower pain intensity preoperatively (pain intensity in Oswestry score) (p = 0.056) as well as lower pain intensity at 1 year after the surgery p = 0.091 in comparison with G allele carries. Significantly lower postoperative pain intensity was experienced by rs6269 G subjects (p = 0.02 GG + GA vs. AA). Patients who were homozygous for COMT rs6269 A demonstrated also a significantly lower preoperative back pain VASBACK (p = 0.045) compared with the GG genotype subjects. Non-significant trend was observed for VASBACK improvement in GG subjects in comparison with other genotypes (p = 0.053) (Tables 1 and 2).
Table 1.
Distress amplitude and magnitude of recovery by genotype group
| Genotype rs6269 | Genotype rs4633 | Genotype rs4818 | Genotype rs4680 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Traits | GG | GA | AA | TT | TC | CC | GG | GC | CC | AA | AG | GG |
| Preoperative ODI | 29.0 ± 8.6 | 26.14 ± 9.4 | 25.4 ± 9.4 | 23.65 ± 9.7 | 26.47 ± 9.3 | 28.14 ± 8.9 | 29.03 ± 8.6 | 26.13 ± 9.4 | 25.07 ± 9.5 | 23.60 ± 9.8 | 26.40 ± 9.2 | 28.13 ± 8.8 |
| Postoperative ODI | 18.96 ± 10.40 | 17.62 ± 11.5 | 15.2 ± 9.2 | 15.20 ± 8.6 | 17.54 ± 11.7 | 17.5 ± 10.1 | 18.96 ± 10.4 | 17.54 ± 11.5 | 15.26 ± 9.3 | 15.30 ± 8.7 | 17.47 ± 11.7 | 17.5 ± 10.1 |
| ODI change | −10.10 ± 12.19 | −8.51 ± 12.6 | −9.9 ± 9.6 | −8.45 ± 8.6 | −8.93 ± 12.9 | −10.63 ± 11.1 | −10.07 ± 12.1 | −8.59 ± 12.5 | −9.80 ± 9.7 | −8.31 ± 8.6 | −9.0 ± 12.9 | −10.63 ± 11.1 |
| Preoperative pain intensity | 3.55 ± 0.75 | 3.6 ± 1.13 | 2.9 ± 1.4 | 2.68 ± 1.5 | 3.16 ± 1.1 | 3.41 ± 0.9 | 3.55 ± 0.8 | 3.15 ± 1.1 | 2.85 ± 1.4 | 2.67 ± 1.5 | 3.16 ± 1.1 | 3.41 ± 0.9 |
| Postoperative pain intensity | 2.25 ± 1.65 | 1.92 ± 1.7 | 1.4 ± 1.4 | 1.22 ± 1.3 | 1.96 ± 1.7 | 2.0 ± 1.5 | 2.25 ± 1.6 | 1.90 ± 1.7 | 1.41 ± 1.4 | 1.25 ± 1.3 | 1.93 ± 1.7 | 2.0 ± 1.5 |
| Pain change | −1.29 ± 1.8 | −1.22 ± 1.8 | −1.5 ± 1.7 | −1.49 ± 1.7 | −1.20 ± 1.8 | −1.41 ± 1.7 | −1.29 ± 1.8 | −1.24 ± 1.8 | −1.44 ± 1.7 | −1.49 ± 1.7 | −1.22 ± 1.8 | −1.41 ± 1.7 |
| Preoperative VASBACK | 7.74 ± 1.91 | 6.65 ± 2.7 | 6.39 ± 2.9 | 6.13 ± 3.0 | 6.65 ± 2.7 | 7.39 ± 2.1 | 6.33 ± 2.9 | 6.69 ± 2.7 | 7.74 ± 1.9 | 6.04 ± 3.0 | 6.71 ± 2.7 | 7.39 ± 2.1 |
| Postoperative VASBACK | 4.44 ± 2.48 | 4.71 ± 3.0 | 4.07 ± 2.8 | 4.29 ± 2.5 | 4.72 ± 3.17 | 4.06 ± 2.6 | 4.11 ± 2.8 | 4.68 ± 3.0 | 4.44 ± 2.5 | 4.34 ± 2.5 | 4.68 ± 3.2 | 4.06 ± 2.5 |
| VASBACK change | −3.29 ± 2.65 | −1.94 ± 2.6 | −2.31 ± 3.3 | −1.84 ± 3.0 | −1.95 ± 2.9 | −3.32 ± 2.6 | −2.22 ± 3.3 | −2.01 ± 2.6 | −3.29 ± 2.7 | −1.69 ± 2.9 | −2.02 ± 3.0 | −3.32 ± 2.6 |
| Preoperative VASLEG | 7.66 ± 1.83 | 7.03 ± 2.4 | 6.75 ± 3.0 | 6.48 ± 3.3 | 7.16 ± 2.4 | 7.28 ± 2.1 | 6.69 ± 3.0 | 7.06 ± 2.4 | 7.66 ± 1.8 | 6.40 ± 3.3 | 7.20 ± 2.4 | 7.28 ± 2.1 |
| Postoperative VASLEG | 3.74 ± 2.8 | 4.44 ± 3.4 | 3.46 ± 2.9 | 3.40 ± 2.7 | 4.22 ± 3.5 | 4.08 ± 2.9 | 3.52 ± 2.9 | 4.39 ± 3.4 | 3.74 ± 2.8 | 3.48 ± 2.7 | 4.17 ± 3.5 | 4.08 ± 2.9 |
| VASLEG change | −3.92 ± 3.31 | −2.58 ± 3.7 | −3.28 ± 3.5 | −3.07 ± 3.3 | −2.94 ± 3.9 | −3.19 ± 3.4 | −3.17 ± 3.5 | −2.67 ± 3.7 | −3.92 ± 3.4 | −2.91 ± 3.2 | −3.02 ± 3.9 | −3.19 ± 3.4 |
ODI Oswestry Disability Index, VASLEG,VASBACK visual analog scale for sciatica and low back pain
Table 2.
Statistical significances of differences in clinical factors between clinical factors in separate genetic groups. Numbers in bold indicate p < 0.05
| Preoperative ODI | Postoperative ODI | ODI change | Preoperative pain intensity | Postoperative pain intensity | Pain change | Preoperative VAS BACK | Postoperative VAS BAC | VAS BACK change | Preoperative VAS LEG | Postoperative VAS LEG | VAS LEG change | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs6269 | ||||||||||||
| GG vs. AA + GA | 0.14 | 0.23 | 0.99 | 0.056 | 0.091 | 0.82 | 0.045 | 0.91 | 0.053 | 0.27 | 0.75 | 0.16 |
| AA vs. GG + GA | 0.29 | 0.14 | 0.54 | 0.093 | 0.020 | 0.38 | 0.28 | 0.21 | 0.87 | 0.68 | 0.15 | 0.51 |
| rs4633 | ||||||||||||
| TT vs. TC + CC | 0.068 | 0.34 | 0.63 | 0.044 | 0.010 | 0.47 | 0.15 | 0.78 | 0.27 | 0.48 | 0.26 | 0.97 |
| CC vs. TC + TT | 0.14 | 0.58 | 0.56 | 0.051 | 0.24 | 0.78 | 0.082 | 0.36 | 0.0042 | 0.76 | 0.67 | 0.799 |
| rs4818 | ||||||||||||
| GG vs. GC + CC | 0.147 | 0.23 | 0.99 | 0.055 | 0.09 | 0.82 | 0.18 | 0.26 | 0.91 | 0.52 | 0.22 | 0.68 |
| CC vs. GC + GG | 0.31 | 0.16 | 0.61 | 0.10 | 0.031 | 0.47 | 0.045 | 0.91 | 0.053 | 0.27 | 0.75 | 0.16 |
| rs4680 | ||||||||||||
| AA vs. AG + GG | 0.071 | 0.39 | 0.55 | 0.048 | 0.018 | 0.59 | 0.092 | 0.91 | 0.16 | 0.34 | 0.38 | 0.79 |
| GG vs. AG + AA | 0.14 | 0.58 | 0.56 | 0.051 | 0.24 | 0.78 | 0.083 | 0.36 | 0.0042 | 0.76 | 0.67 | 0.79 |
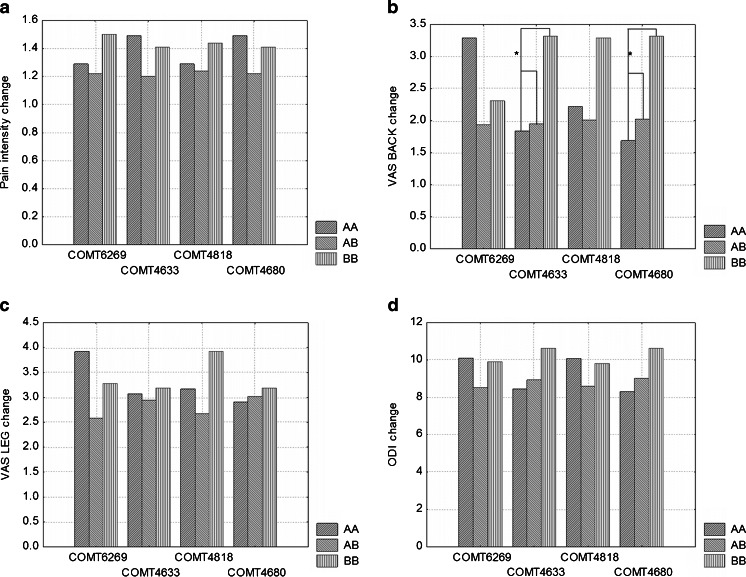
Statistical analysis of COMT rs4633 polymorphism point out significantly lower pain intensity in both pre- (p = 0.044) and postoperative (p = 0.010) period in TT genotype carriers vs. TC + CC genotypes. In homozygous CC patients, higher scores in VASBACK scale before surgery were recorded. Significantly higher VASBACK improvement in CC carriers in comparison with other genotypes (p = 0.0042) was noted. No association between SNP COMT rs4818 and improvement in ODI or VAS changes was found. However, patients with CC genotype demonstrated significantly lower pain intensity in postoperative ODI (p = 0.031). Additionally, carriers of CC genotype showed significantly higher preoperative VASBACK score compared with GC + GG patients. CC subjects tended to have better clinical outcome one year after surgery (p = 0.055) (Tables 1 and 2). Association of COMT rs4680 polymorphism with ODI and VAS scores is presented in Table 1 with p values shown in Table 2. Carriers of AA genotype presented lower pain intensity in ODI pre- (p = 0.048) and postoperatively (p = 0.018), though improvement rate was not significant (p = 0.59). In patients with GG genotype, a trend towards higher preoperative pain intensity in ODI and significantly better clinical outcome recorded in VASBACK score in comparison with other genotypes were observed (p = 0.0042) (Fig. 2).
Fig. 2.
a–d Plots of changes in different pain scales after surgery and 1-year follow-up, grouped by distinct genotypes. Asterisk (*) depicts differences with p < 0,05. (A)—denotes minor allele, (B)—major allele
The carrier status of at least one copy of COMT A_C_C_G haplotype determines low metabolic activity of COMT enzyme, and patients with at least one copy of A_C_C_G haplotype show better clinical outcome measured by ODI score, compared with non-carriers, who inherited a combination of G_C_G_G, A_T_C_A or A_T_G_G haplotypes (i.e., subjects without A_C_C_G haplotype), although the recorded improvement in ODI score was only borderline significant (p = 0.079, Table 3). However, the effect of A_C_C_G haplotype was reflected by the improvement in VASBACK score as compared with non-carriers (p = 0.017). Because of the presence of COMT G_C_G_G (high activity) haplotype is equivalent to the presence of rs6269 G allele and the presence of A_T_C_A (medium) haplotype is equivalent to the presence of rs4680 A allele, associations of these haplotypes with the evaluated parameters are equal to the respective genotypes. However, haplotype “low” is not equivalent to the presence of any of the studied genotypes (the respective results are presented in Table 3).
Table 3.
Patients’ clinical evaluation in patients classified based on the presence of COMT A_C_C_G haplotype, determining low enzyme activity
| Haplotype L (A_C_C_G) | Present vs. absent | ||
|---|---|---|---|
| Absent (n = 138) | Present (n = 38) | p value | |
| Preoperative ODI | 25.79 ± 9.49 | 27.68 ± 8.82 | 0.27 |
| Postoperative ODI | 17.36 ± 10.69 | 15.42 ± 10.20 | 0.28 |
| ODI change | −8.42 ± 11.6 | −12.26 ± 10.44 | 0.079 |
| Preoperative VAS BACK | 6.68 ± 2.73 | 6.89 ± 2.42 | 0.81 |
| Postoperative VAS BACK | 4.65 ± 2.8 | 3.65 ± 2.97 | 0.052 |
| VAS BACK change | −2.08 ± 2.78 | −3.23 ± 3.12 | 0.017 |
| Preoperative VAS LEG | 7.01 ± 2.65 | 7.06 ± 2.23 | 0.77 |
| Postoperative VAS LEG | 3.97 ± 3.17 | 4.07 ± 3.2 | 0.92 |
| VAS LEG change | −3.04 ± 3.56 | −3.03 ± 3.73 | 0.98 |
Significance for bold numbers in all the text (including tables) is p < 0.05
Multivariate general linear models adjusted for patients’ age and gender and COMT genotypes (Table 4) showed that the presence of at least one copy of rs4633 T or rs4626 A allele or COMT L A_C_C_G haplotype (due to a strong linkage disequilibrium, these variables could not be analyzed in one model) are independent predictors of better clinical response (by 1.2 and 1.45 in VASBACK, p = 0.0032 and p = 0.046, respectively). Haplotype “low” activity was also markedly, but statistically insignificantly, associated with clinical improvement in ODI (by 4.1, p = 0.051).
Table 4.
General linear regression models of factors potentially associated with back pain intensity
| Independent variables | VASBACK change | p value |
| Regression coefficient | ||
| Age | 0.014 | 0.38 |
| Male sex | 0.59 | 0.17 |
| Presence of rs4633 allele T | −1.45 | 0.0032 |
| Independent variables | VASBACK change | p value |
| Regression coefficient | ||
| Age | 0.012 | 0.44 |
| Male sex | 0.53 | 0.22 |
| Presence of rs4680 allele A | −1.2 | 0.046 |
| Independent variables | VASBACK change | p value |
| Regression coefficient | ||
| Age | 0.01 | 0.47 |
| Male sex | 0.66 | 0.13 |
| Presence of COMT haplotype L | −1.3 | 0.014 |
| Independent variables | ODI change | p value |
| Regression coefficient | ||
| Age | 0.009 | 0.89 |
| Male sex | 2.36 | 0.18 |
| Presence of COMT haplotype L | −4.14 | 0.051 |
Significance for bold numbers in all the text (including tables) is p < 0.05
Discussion
In spite of recent advances in surgical technique, effective pain treatment in patients suffering from lumbar spine pathology still remains a considerable clinical problem. Surgical intervention criteria in those patients are clear-cut in case of cauda equina syndrome or progressive motor deficit, but elective surgery is based on individual decision [6, 13, 23, 26, 27]. The latter approach is partly caused by the use of various pain-assessment tools, and even those considered as a “gold standard” in evaluation of surgery results still lack validation [6]. Problems with decision-making in patient classification for surgery or conservative treatment may also result from interindividual differences in pain threshold that may be determined at least partly by genotype.
The first direct evidence that COMT polymorphism affects neural processing of pain was reported by Zubieta et al. [31], who noted that COMT 158met (rs4680) homozygotes were characterized by higher pain sensitivity, diminished regional μ-opioid system responses to pain, as well as a higher μ-opioid receptor binding potential, in comparison with heterozygotes and 158 val homozygotes. Additionally, Loggia et al. [15] provided information on COMT val158met polymorphism involvement in pain perception in response to heat stimuli. The authors found that met/met subjects exhibited stronger pain-related fMRI signals than val/val in several brain structures. Therefore, met/met patients might be characterized by lower pain threshold and higher risk of chronic pain development. Our own observations in patients suffering from chronic lumbar pain did not support the aforementioned reports, since no significant associations between COMT val158met polymorphism and VAS/ODI score have been found, except for better response to treatment in GG (val/val) cases.
However, analyzing solely pre- and postoperative pain intensity it can be seen, that AA subjects were characterized by markedly lower pain intensity measures vs. G allele carriers. Contribution of other COMT genotypes to pain perception does also support a modulatory effect of these genetic parameters. Significantly lower preoperative pain intensity was experienced by 4633 TT, 4680 AA and higher in 4818 GG subjects, whereas postoperative pain intensity was markedly decreased in 4633 TT, 4818 CC, 4680 AA and 6269 AA carriers. Also, local pain perception in LDH patients was associated with COMT polymorphism, and preoperative VASBACK was the most prominent in patients characterized by 6269 GG and 4818 CC genotypes. Likewise, COMT polymorphism significantly modulated response to surgery (VASBACK change), i.e., the best outcomes were observed in 6269 CC, 4633 CC, and 4680 GG carriers. These observations are supported by linear regression analysis, which demonstrated that COMT rs4633 T allele and rs4680 A allele are independent risk factors for VASBACK change (Table 4). The observed differences in pain perception in patients of various genetic constitutions may enable identification of subjects particularly predisposed to chronic pain development, and thus requiring more intensive analgesic treatment. Our findings are in agreement with the report by Jacobsen et al. [12], who revealed that COMT val158met polymorphism could contribute to discogenic subacute low back pain and sciatica in degenerative disc disease, since val/val patients (like in the present study) were characterized by the largest reduction in postoperative pain in comparison with met/val and met/met carriers. Associations between COMT gene SNPs and pain were also evaluated by Dai et al. [7], using ODI and VAS, in a group of patients subjected to surgical treatment for LDH. The authors reported that patients homozygous for COMT rs4633 T allele were characterized by most prominent mean improvement in ODI score one year after surgery, which was not observed in our study (response measured by ODI change was similar in all rs4633 genotypes). Contrary to the present results Dai et al. [7] did not reveal significant impact of other studied SNPs, i.e., rs6269, rs4818 and rs4680 on pain perception.
Recent studies suggest that COMT haplotypes, rather than individual SNPs, would be more appropriate for the prediction of COMT biological effects [8, 31]. Even silent mutations that usually do not change the amino-acid sequence may affect function through alterations in COMT mRNA structure and stability, thus influencing disease risk or treatment efficacy. Diatchenko et al. [8] revealed a strong association between a specific COMT haplotype - G_C_G_G and pain sensitivity, and an inverse correlation between pain sensitivity and COMT activity in healthy subjects. Low sensitivity to experimental pain was associated with G_C_G_G haplotype (high activity haplotype), whereas A_C_C_G haplotype (low activity) was associated with high pain sensitivity. Dai et al. [7] also reported that COMT haplotype status was associated with pain perception in patients undergoing surgical treatment for lumbar degenerative disc disease. Patients with A_T_C_A haplotype responded most effectively to the surgical procedure, and were followed by subjects carrying G_C_G_G haplotype and A_C_C_G, haplotype, as measured by ODI score. Our findings are contrary to observations of Dai et al., who found that A_C_C_G haplotype patient were characterized by significantly higher postoperative VASBACK pain, and responded less effectively to treatment (VASBACK change). Likewise, linear regression analysis applied in the present study indicated that COMT A_C_C_G haplotype was an independent risk factor for VASBACK and possibly ODI changes (Table 3). Recently, a study of McLean et al. [17] has confirmed the opinion that individuals with COMT pain vulnerable genotype A_C_C_G were at increased risk of acute pain and needed preventive interventions. The authors evaluated an association between COMT genotype and acute neck pain intensity in individuals after motor vehicle collision, and found that individuals characterized by COMT A_C_C_G haplotype were more likely to report moderate-to-severe neck pain, headache, and dizziness. Moreover, patients with genotype coding low COMT enzyme activity were found to have longer time to physical and emotional recovery. Likewise, Tchivileva et al. [24], studying an association between common COMT haplotypes and response to β-blocker treatment in a common chronic musculoskeletal pain disorder, found that carriers of low and medium COMT activity haplotype showed the most prominent response to propranolol in all pain measures except for thermal threshold. Patients carrying COMT A_C_C_G haplotype demonstrated not only more efficient response to pharmacological treatment but also better surgical treatment outcome.
Pain analysis is problematic due to its subjective nature and broad range of factors modulating it, such as ethnicity as evidenced by known differences in both pain perception and reporting between African Americans and Caucasians [5, 9]. This may be related to COMT rs4680 158met frequency in various populations, ranging from 0.01 to 0.62 [21]. This fact creates difficulties in developing reliable scales characterizing pain. As Polish population in 98 % consists in Caucasian and additionally ethnic differences in catechol-O-methyltransferase activity is confirmed [1, 18] we found it reasonable to focus only on one ethnic group. Undoubtedly, to confirm our results, further studies in Asian or African American populations should be designed.
In conclusion, the aim of this study was to find a potential correlation between genetic polymorphism of the COMT enzyme and pain perception. This association was observed in patients with the COMT SNP rs4633 CC, rs4680 GG, who demonstrated greater reduction of low back pain in 1 year after surgery. This fact may have potential clinical implication in decision-making, e.g., selection of those subjects for earlier surgery. We believe that the lack of correlation between COMT activity and reduction of leg pain (VASLEG) after surgery can be explained by the pivotal role of direct nerve root decompression during surgery. As for the present study, the studied group of patients is too small to draw arbitrary conclusions regarding genetic diversity in COMT and its role in predicting clinical outcome after lumbar discectomy. The observed associations suggest that it is not possible to make a clinical decision on operation or conservative treatment based solely on the COMT gene polymorphism. However, the COMT genotype may serve as a tool indicating patients who will benefit more from surgery, being one of the factors behind decision-making, along with other parameters (like radiological findings, clinical status); to some extent, pharmacogenetics may play a supportive role as a prognostic factor. However, given a relatively small group size, larger studies are needed in order to confirm the value of the present study observations.
Acknowledgments
This study was supported by a grant from the Polish State Committee for Scientific Research of the Ministry of Scientific Research and Information Technology, No. N402386433.
Conflicts of interest
None.
References
- 1.Ameyaw MM, Syvänen AC, Ulmanen I, Ofori-Adjei D, McLeod HL. Pharmacogenetics of catechol-O-methyltransferase: frequency of low activity allele in a Ghanaian population. Hum Mutat. 2000;16(5):445–446. doi: 10.1002/1098-1004(200011)16:5<445::AID-HUMU13>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 2.An H, Thomas A. Spinal Stenosis. Princ. Tech. Spine Surg. Baltimore: Williams & Wilkins; 1997. pp. 443–460. [Google Scholar]
- 3.Asch HL, Lewis PJ, Moreland DB, Egnatchik JG, Yu YJ, Clabeaux DE, Hyland AH. Prospective multiple outcomes study of outpatient lumbar microdiscectomy: should 75 to 80% success rates be the norm? J Neurosurg. 2002;96(1 Suppl):34–44. doi: 10.3171/spi.2002.96.1.0034. [DOI] [PubMed] [Google Scholar]
- 4.Atlas SJ, Keller RB, Chang Y, Deyo RA, Singer DE. Surgical and nonsurgical management of sciatica secondary to a lumbar disc herniation: five-year outcomes from the Maine Lumbar Spine Study. Spine. 2001;26(10):1179–1187. doi: 10.1097/00007632-200105150-00017. [DOI] [PubMed] [Google Scholar]
- 5.Campbell CM, Edwards RR, Fillingim RB. Ethnic differences in responses to multiple experimental pain stimuli. Pain. 2005;113(1–2):20–26. doi: 10.1016/j.pain.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Chapman JR, Norvell DC, Hermsmeyer JT, Bransford RJ, DeVine J, McGirt MJ, Lee MJ. Evaluating common outcomes for measuring treatment success for chronic low back pain. Spine. 2011;36(21 Suppl):S54–68. doi: 10.1097/BRS.0b013e31822ef74d. [DOI] [PubMed] [Google Scholar]
- 7.Dai F, Belfer I, Schwartz CE, Banco R, Martha JF, Tighioughart H, Tromanhauser SG, Jenis LG, Kim DH. Association of catechol-O-methyltransferase genetic variants with outcome in patients undergoing surgical treatment for lumbar degenerative disc disease. Spine J Off J North Am Spine Soc. 2010;10(11):949–957. doi: 10.1016/j.spinee.2010.07.387. [DOI] [PubMed] [Google Scholar]
- 8.Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, Goldman D, Xu K, Shabalina SA, Shagin D, Max MB, Makarov SS, Maixner W. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14(1):135–143. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- 9.Edwards CL, Fillingim RB, Keefe F. Race, ethnicity and pain. Pain. 2001;94(2):133–137. doi: 10.1016/S0304-3959(01)00408-0. [DOI] [PubMed] [Google Scholar]
- 10.Gürsoy S, Erdal E, Herken H, Madenci E, Alaşehirli B, Erdal N. Significance of catechol-O-methyltransferase gene polymorphism in fibromyalgia syndrome. Rheumatol Int. 2003;23(3):104–107. doi: 10.1007/s00296-002-0260-5. [DOI] [PubMed] [Google Scholar]
- 11.Häkkinen A, Ylinen J, Kautiainen H, Airaksinen O, Herno A, Tarvainen U, Kiviranta I. Pain, trunk muscle strength, spine mobility and disability following lumbar disc surgery. J Rehabil Med Off J UEMS Eur Board Phys Rehabil Med. 2003;35(5):236–240. doi: 10.1080/16501970306096. [DOI] [PubMed] [Google Scholar]
- 12.Jacobsen LM, Schistad EI, Storesund A, Pedersen LM, Rygh LJ, Røe C, Gjerstad J (2012) The COMT rs4680 Met allele contributes to long-lasting low back pain, sciatica and disability after lumbar disc herniation. Eur J Pain Lond Engl. http://onlinelibrary.wiley.com/doi/10.1002/j.1532-2149.2011.00102.x/abstract [DOI] [PubMed]
- 13.Jacobs WCH, van Tulder M, Arts M, Rubinstein SM, van Middelkoop M, Ostelo R, Verhagen A, Koes B, Peul WC. Surgery versus conservative management of sciatica due to a lumbar herniated disc: a systematic review. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2011;20(4):513–522. doi: 10.1007/s00586-010-1603-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen MC, Brant-Zawadzki MN, Obuchowski N, Modic MT, Malkasian D, Ross JS. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331(2):69–73. doi: 10.1056/NEJM199407143310201. [DOI] [PubMed] [Google Scholar]
- 15.Loggia ML, Jensen K, Gollub RL, Wasan AD, Edwards RR, Kong J. The catechol-O-methyltransferase (COMT) val158met polymorphism affects brain responses to repeated painful stimuli. PLoS ONE. 2011;6(11):e27764. doi: 10.1371/journal.pone.0027764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marbach JJ, Levitt M. Erythrocyte catechol-O-methyltransferase activity in facial pain patients. J Dent Res. 1976;55(4):711. doi: 10.1177/00220345760550043801. [DOI] [PubMed] [Google Scholar]
- 17.McLean SA, Diatchenko L, Lee YM, Swor RA, Domeier RM, Jones JS, Jones CW, Reed C, Harris RE, Maixner W, Clauw DJ, Liberzon I. Catechol-O-methyltransferase haplotype predicts immediate musculoskeletal neck pain and psychological symptoms after motor vehicle collision. J Pain Off J Am Pain Soc. 2011;12(1):101–107. doi: 10.1016/j.jpain.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLeod HL, Fang L, Luo X, Scott EP, Evans WE. Ethnic differences in erythrocyte catechol-O-methyltransferase activity in black and white Americans. J Pharmacol Exp Ther. 1994;270(1):26–29. [PubMed] [Google Scholar]
- 19.Mogil JS. The genetic mediation of individual differences in sensitivity to pain and its inhibition. Proc Natl Acad Sci U S A. 1999;96(14):7744–7751. doi: 10.1073/pnas.96.14.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nackley AG, Tan KS, Fecho K, Flood P, Diatchenko L, Maixner W. Catechol-O-methyltransferase inhibition increases pain sensitivity through activation of both beta2- and beta3-adrenergic receptors. Pain. 2007;128(3):199–208. doi: 10.1016/j.pain.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmatier MA, Kang AM, Kidd KK. Global variation in the frequencies of functionally different catechol-O-methyltransferase alleles. Biol Psychiatry. 1999;46(4):557–567. doi: 10.1016/S0006-3223(99)00098-0. [DOI] [PubMed] [Google Scholar]
- 22.Powell MC, Wilson M, Szypryt P, Symonds EM, Worthington BS. Prevalence of lumbar disc degeneration observed by magnetic resonance in symptomless women. Lancet. 1986;2(8520):1366–1367. doi: 10.1016/S0140-6736(86)92008-8. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro S. Cauda equina syndrome secondary to lumbar disc herniation. Neurosurgery. 1993;32(5):743–746. doi: 10.1227/00006123-199305000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Tchivileva IE, Lim PF, Smith SB, Slade GD, Diatchenko L, McLean SA, Maixner W. Effect of catechol-O-methyltransferase polymorphism on response to propranolol therapy in chronic musculoskeletal pain: a randomized, double-blind, placebo-controlled, crossover pilot study. Pharmacogenet Genomics. 2010;20(4):239–248. doi: 10.1097/FPC.0b013e328337f9ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toyone T, Tanaka T, Kato D, Kaneyama R. Low-back pain following surgery for lumbar disc herniation. A prospective study. J Bone Joint Surg Am. 2004;86-A(5):893–896. doi: 10.2106/00004623-200405000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Weber H. The effect of delayed disc surgery on muscular paresis. Acta Orthop Scand. 1975;46(4):631–642. doi: 10.3109/17453677508989245. [DOI] [PubMed] [Google Scholar]
- 27.Weber H. Lumbar disc herniation. A controlled, prospective study with ten years of observation. Spine. 1983;8(2):131–140. doi: 10.1097/00007632-198303000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Weinreb JC, Wolbarsht LB, Cohen JM, Brown CE, Maravilla KR. Prevalence of lumbosacral intervertebral disk abnormalities on MR images in pregnant and asymptomatic nonpregnant women. Radiology. 1989;170(1 Pt 1):125–128. doi: 10.1148/radiology.170.1.2521192. [DOI] [PubMed] [Google Scholar]
- 29.Wenger M, Mariani L, Kalbarczyk A, Gröger U. Long-term outcome of 104 patients after lumbar sequestrectomy according to Williams. Neurosurgery. 2001;49(2):329–334. doi: 10.1097/00006123-200108000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Yorimitsu E, Chiba K, Toyama Y, Hirabayashi K. Long-term outcomes of standard discectomy for lumbar disc herniation: a follow-up study of more than 10 years. Spine. 2001;26(6):652–657. doi: 10.1097/00007632-200103150-00019. [DOI] [PubMed] [Google Scholar]
- 31.Zubieta J-K, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, Koeppe RA, Stohler CS, Goldman D. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299(5610):1240–1243. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]