Abstract
Objective To document whether elements of a structured history and examination predict adverse outcome of acute sore throat.
Design Prospective clinical cohort.
Setting Primary care.
Participants 14 610 adults with acute sore throat (≤2 weeks’ duration).
Main outcome measures Common suppurative complications (quinsy or peritonsillar abscess, otitis media, sinusitis, impetigo or cellulitis) and reconsultation with new or unresolving symptoms within one month.
Results Complications were assessed reliably (inter-rater κ=0.95). 1.3% (177/13 445) of participants developed complications overall and 14.2% (1889/13 288) reconsulted with new or unresolving symptoms. Independent predictors of complications were severe tonsillar inflammation (documented among 13.0% (1652/12 717); odds ratio 1.92, 95% confidence interval 1.28 to 2.89) and severe earache (5% (667/13 323); 3.02, 1.91 to 4.76), but the model including both variables had modest prognostic utility (bootstrapped area under the receiver operator curve 0.61, 0.57 to 0.65), and 70% of complications (124/177) occurred when neither was present. Clinical prediction rules for bacterial infection (Centor criteria and FeverPAIN) also predicted complications, but predictive values were also poor and most complications occurred with low scores (67% (118/175) scoring ≤2 for Centor; 126/173 (73%) scoring ≤2 for FeverPAIN). Previous medical problems, sex, temperature, and muscle aches were independently but weakly associated with reconsultation with new or unresolving symptoms.
Conclusion Important suppurative complications after an episode of acute sore throat in primary care are uncommon. History and examination and scores to predict bacterial infection cannot usefully identify those who will develop complications. Clinicians will need to rely on strategies such as safety netting or delayed prescription in managing the uncertainty and low risk of complications.
Introduction
Respiratory tract infections are the commonest acute illnesses managed in everyday practice, and most patients receive antibiotics,1 whether for a lower respiratory tract infection or acute sore throat. Prescribing of antibiotics in the community is a key driver of antibiotic resistance, which may lead to many untreatable serious infections,2 3 and since new classes of antibiotics are not being developed, the key to maintaining the precious resource of antibiotics is to reduce antibiotic use for those who will receive no meaningful clinical benefit.
Although systematic reviews and randomised trials of antibiotics in acute sore throat have shown a modest effect on symptoms, prescribing antibiotics prevents both suppurative complications (quinsy, otitis media, sinusitis, cellulitis) and non-suppurative complications, although non-suppurative complications are probably currently rare in resource rich settings.4 5 6 Thus it is important not to deny those patients at major risk of severe illness or complications the benefit from antibiotics. Both patients and general practitioners are concerned about the danger of severe illness from upper respiratory tract infections, and in the absence of evidence general practitioners currently use a range of ad hoc clinical criteria to justify prescribing antibiotics.7 A better understanding of those patients who are or are not at risk of poorer outcomes could help tackle such concerns.7
A key question is therefore how to better target antibiotics. Management of sore throat has traditionally been advocated based on the risk of infection with group A β haemolytic streptococci,8 9 10 including the simple Centor criteria—three out of four of pus, cervical nodes, a history of fever and no history of cough—which are widely advocated in guidance.11 12 13 14 15 However, these criteria have low specificity12 for bacterial infection, leading to high antibiotic use.12 Furthermore, small studies in typical primary care settings have suggested other features might be useful in refining the criteria, such as short previous duration and pain severity.10 16 We previously reported evidence that group C and G streptococci present in a similar manner to group A streptococci,17 and in combination with another diagnostic cohort we proposed a five item score, FeverPAIN (Fever, Pus, rapid Attendance (illness ≤3 days), severe Inflammation, and No cough or coryza). However, it is unclear whether those who are more likely to have streptococcal infection are also more likely to have worse symptoms, prolonged illness, or complications. To our knowledge only one case-control study has used routine data on the suppurative complications of acute sore throat, which showed a higher risk for middle aged men who smoke,18 but no prospective clinical study has addressed whether elements of standard history and examination can usefully predict complications.
We assessed the clinical predictors of complications after presentation of acute sore throat, and investigated whether clinical prediction scores for bacterial infection predict common complications such as quinsy (peritonsillar abscess), otitis media, sinusitis, and cellulitis.
Methods
Overall study design
The concept of the study was to develop a simple one page paper or web based clinical proforma documenting clinical features to facilitate the generation of a large prospective cohort to predict adverse outcomes, among patients recruited consecutively where time for consent in busy winter clinics allowed. Within the observational study, we nested smaller studies to develop and trial a clinical scoring method for bacterial infection. All the studies used the same baseline clinical proforma and the same outcomes measures. The nested studies concerned two consecutive diagnostic cohorts (n=1107) where a clinical score to predict bacterial infection was developed, and a randomised trial (n=1781) that compared the use of the clinical score and the targeted use of a rapid antigen detection test with delayed antibiotic prescribing (see supplementary appendix 1 for details).
Practitioner inclusion criteria—So that the impact of antibiotics could be potentially explored we recruited general practitioners who reported prescribing immediate antibiotics in 50% or less of patients with tonsillitis. General practitioners had the option of using either paper or web based clinical proformas. Initial recruitment was among six local networks (Southampton, Bristol, Birmingham, Oxford, Cardiff, and Exeter) but was extended nationally during the last 18 months of recruitment.
Patient inclusion criteria—We included previously well patients aged 16 or more with acute uncomplicated illness (≤14 days), who presented with sore throat as the main symptom and had an abnormal examination result of the pharynx (identical criteria to our previous studies19). Exclusion criteria were severe mental health problems (for example, cognitive impairment associated with being unable to consent or assess history) and complicated illness (for example, complication at presentation or incipient complication; immune suppression).
Baseline clinical proforma—This consisted of a single clinical sheet documenting age, sex, current smoking status, previous duration of illness, and the presence and severity of baseline symptoms (sore throat, difficulty swallowing, fever during the illness, runny nose, cough, feeling unwell, diarrhoea, vomiting, abdominal pain, headache, muscle aches, sleep disturbance, earache). Symptoms were recorded using 4 point Likert scales (none, a slight problem, a moderately bad problem, a severe problem), and the presence of signs (pus, nodes, cervical nodes, temperature, fetor, palatal oedema, difficulty speaking due to sore throat) to include those used in previous clinical scores.5 10 20 21
Documentation of outcomes
We defined suppurative complications (the primary outcome) as a new diagnosis recorded in the clinical record in the month after the index presentation of otitis media, sinusitis, quinsy, cellulitis (the major complications based on previous systematic review and trial evidence4 5 6), assessed using a standardised proforma by staff in general practices or by primary care research network staff. The structured proforma was developed iteratively following initial attempts at proformas that were difficult to operationalise reliably. No formal training was provided: the proformas were designed to be used with minimal training across a wide geographical area, and came with a manual of instructions, supported by study staff at the main centre if there were queries.
Where information about complications was not available from notes review we used information from a freepost card returned directly to the study centre by patients. To minimise subjective judgments by the reviewing staff we separated the notes review proforma into a wide variety of terms reflecting the possible consultation diagnosis or symptom presentation. We documented inter-rater reliability of the assessment of complications and progression of illness by second raters blind to the first rating among 153 patients. The patients for this reliability study were selected from two of the sites, in urban practices close to each study centre, chosen for ease of access to the study centres.
Progression/non-resolution of illness (secondary outcome)—This was defined as reconsultation with unresolving symptoms or development of a new respiratory diagnosis, symptom, or sign within a month of the index presentation—similar to outcomes used previously in a cohort of children22 and in a large trial of antibiotics for lower respiratory tract infection in adults.23
Selection bias—We asked general practitioners or nurses to document when eligible patients were not entered into the study, the reason why, treatment, and clinical characteristics, but completion of this information was poor, since the reasons patients were not recruited (time pressures at the busiest times of year) made it difficult to document additional information.
Sample size—For the sample size calculations we assumed 5% two sided significance and 80% power, using the NQuery sample size program (Statistical Solutions). We assumed that a clinically useful variable would be likely to predict complications with an odds ratio of at least 2.5, that important predictive variables would have a prevalence among those with complications of 35-75%,5 6 24 and that complications (quinsy, otitis media, cellulitis, sinusitis) occur 1:150 times among unselected patients.24 On the assumption that the group not receiving immediate antibiotics might be the most appropriate group in which to develop a model, we determined that 6749 data forms would be needed among that group. Our previous data suggested minimal clustering by general practitioner but, assuming an intracluster correlation coefficient of 0.01, we estimated that we might need to recruit up to 17 412 patients to allow both for up to 50% of patients receiving immediate antibiotics and for clustering.
Statistical analysis
We assessed the individual predictors of complications or non-resolution or progression of symptoms using logistic regression for binary outcomes. The multivariate models controlled for the potential confounding effect of antibiotic prescribing, clustering by practice, and other significant individual predictors. The analysis was undertaken on the complete dataset, without imputing missing data. We used backward selection and retained variables in the final model only if they were significant predictors at the 5% level. The primary analysis used variables that had been found to be predictive of bacterial infection in previous cohorts and the nested diagnostic study (fever, absence of cough, absence of coryza, difficulty swallowing, previous duration, muscle aches, headache, temperature, and purulence), but following peer review a secondary analysis included the full range of variables—diarrhoea, vomiting, abdominal pain, earache, generally unwell, fetor, and palatal oedema. We developed a clinical score based on a simple sum of the variables that independently predicted complications in multivariate analysis. We documented the sensitivity, specificity, and positive and negative predictive values of the score for predicting complications. To overcome the problem of over-fitting of a score derived from the same cohort as the component variables, we used bootstrapped estimates of the area under the receiver operator curve to document the overall prognostic value of each predictive variables and the score.25 We also assessed whether clinical scores previously developed to predict bacterial infections also predicted complications. The clinical scores we used were a modified version of the Centor criteria, which were developed to predict the presence of group A streptococci (fever during the previous 24 hours, purulent tonsils, the absence of cough, and cervical nodes), and a score developed to predict the presence of Lancefield groups A, C, and G streptococci (FeverPAIN: Fever, Purulent tonsils, rapid Attendance (illness ≤3 days), severely Inflamed tonsils, and No cough or coryza). We also assessed a modified version of the FeverPAIN score but excluding previous rapid attendance (owing to concerns about the generalisability to other clinical settings of the variable “rapid attendance”).
We agreed the final analysis plan based on a review of the characteristics of patients in the cohort who did and did not receive antibiotics: given the important differences between such patients, we judged that developing a model among those who were not prescribed antibiotics (our original plan) would be unrepresentative of the key patient groups, so we revised our primary analysis to include the whole cohort. Antibiotic prescribing would only be expected to partially attenuate the observed risk of complications,18 hence multivariate analyses controlled for both antibiotic prescribing strategy and clustering by recruiting clinician. We performed a secondary analysis among those who were not prescribed antibiotics and checked for interactions with prescribing strategy—that is, we assessed a differential estimate for predictors according to prescribing strategy.
Results
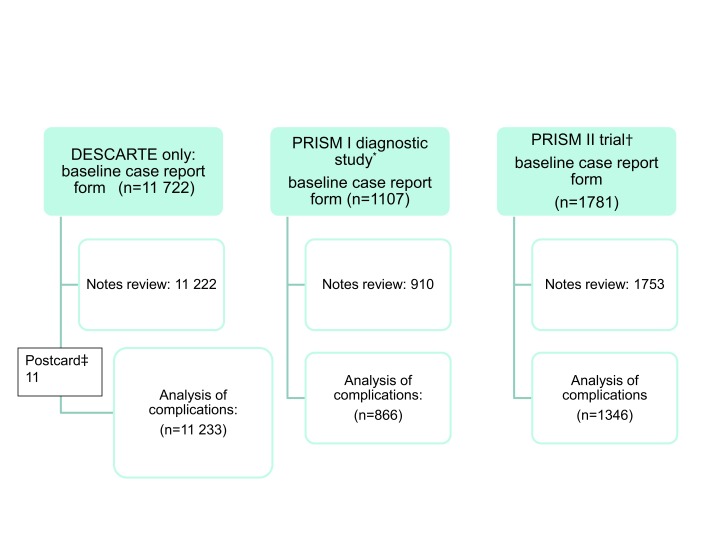
Overall, 14 610 adolescents and adults were recruited between 10 November 2006 and 1 June 2009 from 616 practices (figure). Table 1 shows the clinical characteristics of the participants. The inter-rater reliability for assessing complications was good (κ=0.95; of 11 patients with complications only one was judged not to have a complication on the second assessment), as was return with non-resolution of symptoms (κ=0.84; of 29 patients documented as reconsulting with new or worsening symptoms only one required modification of the result on the second assessment). Few variables had lots of missing data (see supplementary appendix 4).
Patient recruitment and follow-up. *Baseline case report form and notes review as in DESCARTE (Decision rule for the Symptoms and Complications of Acute Red Throat in Everyday practice) but also throat swab sent for microbiological diagnostic study. †As in DESCARTE but patients randomised to delayed antibiotics, antibiotics targeted by clinical score, or antibiotics targeted by clinical score and rapid test for streptococci. 407 children in the PRISM trial were not included in the analysis of complications. ‡Self completed postcards were used for 87 people with no data on complications in notes review, and a further 11 people were included in notes review but had no baseline data
Table 1.
Characteristic of patients at index consultation. Values are numbers (percentages) unless stated otherwise
| Characteristics | Not given antibiotics | Given antibiotics | Delayed antibiotics |
|---|---|---|---|
| Clinical assessment: | |||
| Mean (SD) severity of sore throat/difficulty swallowing (4 point Likert scale) | 2.93 (0.7) | 3.32 (0.6) | 3.06 (0.7) |
| Mean (SD) previous duration in days | 5.0 (6.5) | 4.6 (4.1) | 4.3 (3.3) |
| Mean (SD) age (years) | 34.7 (15.4) | 32.7 (14.2) | 34.1 (14.6) |
| Women | 3610/5243 (68.9) | 4147/6269 (66.2) | 1770/2501 (70.8) |
| Smoker | 1016/5212 (19.5) | 1445/6240 (23.2) | 481/2484 (19.4) |
| Fever in past 24 hours | 2279/4852 (47.0) | 4109/5704 (72.0) | 1268/2317 (54.7) |
| Mean (SD) temperature (°C) | 36.7 (0.6) | 37.0 (0.8) | 36.8 (0.6) |
| Pus on tonsils | 376/5213 (7.2) | 3751/6232 (60.2) | 654/2495 (26.2) |
| Severely inflamed tonsils | 86/4923 (1.8) | 1418/5855 (24.282) | 178/2344 (7.6) |
| Mean (SD) No of previous medical problems | 0.2 (0.5) | 0.2 (0.5) | 0.2 (0.4) |
| Return <4 weeks with new or worsening symptoms | 803/4974 (16.1) | 864/5932 (14.6) | 222/2382 (9.5) |
| Return <4 weeks with complications | 75/4974 (1.5) | 78/5932 (1.3) | 21/2382 (0.9) |
| Individual complications: | |||
| Quinsy | 11/4974 (0.2) | 30/5932 (0.5) | 6/2382 (0.3) |
| Sinusitis | 23/4974 (0.5) | 12/5932 (0.2) | 3/2382 (0.1) |
| Otitis media | 31/4974 (0.6) | 27/5932 (0.5) | 11/2382 (0.5) |
| Celluliltis or impetigo | 10/4974 (0.2) | 9/5932 (0.2) | 1/2382 (0.0) |
Denominators vary due to missing data.
Complications
The major suppurative complications of acute sore throat (quinsy, otitis media, sinusitis, impetigo or cellulitis) occurred in approximately 1% of patients regardless of whether they were given antibiotics, not given antibiotics, or given delayed antibiotics (table1). We recruited 7161 patients who did not receive immediate antibiotics (more than we required based on our minimal sample size calculation), but clinical differences were noticeable between those not prescribed antibiotics, those prescribed antibiotics immediately, and those given delayed antibiotics (for example, purulence, fever, tonsillar inflammation, table 1).
In multivariate analysis, predictors of complications were severe tonsillar inflammation (documented among 13.0% (1652/12 717); odds ratio 1.92, 95% confidence interval 1.28 to 2.89) and severe earache (documented among 5.0% (667/13 323), 3.02, 1.91 to 4.76) (table 2). However, the model with both variables had modest prognostic utility (bootstrapped area under the receiver operator curve 0.61, 0.57 to 0.65), and 70% of complications (124/177) occurred where neither was present. The bootstrapped area under the receiver operator curve for a simple clinical score generated using the sum of these two variables (a score taking the value 0, 1, or 2) was little different (0.61, 0.57 to 0.65, table 3). The predictive value of severe earache was not likely to be simply an incorrect initial diagnosis (that is, missed diagnosis of otitis media) because severe earache significantly predicted all complications, including quinsy and otitis media (otitis media 5.27, 2.91 to 9.51; P<0.001; quinsy 3.04, 1.35, 6.84; P=0.007; sinusitis 1.73, 0.54 to 5.54; P=0.354, cellulitis or impetigo 1.40, 0.19 to 10.55; P=0.742).
Table 2.
Predictors of suppurative complications (quinsy, otitis media, sinusitis, impetigo or cellulitis) in month after index consultation
| Variables | No (%) with no complications | No (%) with complications | Univariate odds ratio (95% CI) | P value | Multivariate odds ratio* (95% CI) | P value |
|---|---|---|---|---|---|---|
| Predictors: | ||||||
| Previous duration ≤3 days | 6503/13 164 (49.4) | 89/177 (50.3) | 1.04 (0.77 to 1.39) | 0.82 | 0.94 (0.70 to 1.26) | 0.68 |
| Fever (during past 24 hours) | 7825/13 182 (59.4) | 119/173 (67.2) | 1.40 (1.02 to 1.93) | 0.04 | 1.33 (0.92 to 1.92) | 0.13 |
| Muscle aches | 7938/ 13 175 (60.3) | 117/177 (66.1) | 1.26 (0.93 to 1.72) | 0.14 | 1.26 (0.90 to 1.77) | 0.18 |
| Headache | 8560/13 173 (65.0) | 120/177 (67.8) | 1.13 (0.83 to 1.56) | 0.44 | 1.06 (0.78 to 1.44) | 0.72 |
| Sore throat† | 4962/12 325 (40.3) | 73/167 (43.7) | 1.15 (0.85 to 1.57) | 0.37 | 0.95 (0.67 to 1.34) | 0.76 |
| Absence of cough | 5770/13 187 (43.8) | 81/177 (45.8) | 1.08 (0.81 to 1.46) | 0.59 | 1.09 (0.79 to 1.50) | 0.62 |
| Absence of runny nose | 7493/12 771 (58.7) | 99/173 (57.2) | 0.94 (0.70 to 1.28) | 0.70 | 0.94 (0.70 to 1.27) | 0.70 |
| Absence of cough and runny nose | 6200/12 767 (48.6) | 83/173 (48.00) | 0.98 (0.72 to 1.32) | 0.88 | 0.95 (0.72 to 1.27) | 0.75 |
| Generally unwell† | 1780/13 188 (13.5) | 31/177 (17.5) | 1.36 (0.92 to 2.01) | 0.12 | 1.21 (0.77 to 1.89) | 0.41 |
| Diarrhoea | 1087/13 179 (8.3) | 12/177 (6.8) | 0.77 (0.43 to 1.39) | 0.39 | 0.83 (0.44 to 1.56) | 0.56 |
| Disturbed sleep† | 3246/13 177 (24.6) | 55/177 (31.6) | 1.43 (1.03 to 1.97) | 0.03 | 1.32 (0.89 to 1.95) | 0.17 |
| Difficulty swallowing† | 4204/12 323 (34.1) | 64/167 (38.3) | 1.20 (0.88 to 1.64) | 0.26 | 0.98 (0.69 to 1.37) | 0.89 |
| Earache† | 642/13 163 (4.9) | 25/177 (14.2) | 3.22 (2.10 to 4.96) | <0.01 | 3.02 (1.91. to 4.76) | <0.01 |
| Vomiting† | 70/13 146 (0.5) | 1/177 (0.6) | 1.07 (0.15 to 7.73) | 0.95 | 0.98 (0.13 to 7.10) | 0.98 |
| Abdominal pain† | 142/13 149 (1.1) | 4/177 (2.3) | 2.13 (0.78 to 5.82) | 0.14 | 1.90 (0.67 to 5.41) | 0.23 |
| Examination: | ||||||
| Temperature >37.5°C | 1981/12 704 (15.6) | 31/174 (17.8) | 1.17 (0.79 to 1.74) | 0.423 | 1.18 (0.79 to 1.75) | 0.41 |
| Purulent tonsils | 4495/13 162 (34.2) | 60/177 (33.9) | 0.99 (0.72 to 1.35) | 0.94 | 0.86 (0.57 to 1.31) | 0.49 |
| Cervical glands | 9320/12 560 (74.2) | 131/171 (76.6) | 1.13 (0.80 to 1.63) | 0.48 | 1.20 (0.82 to 1.76) | 0.35 |
| Tender cervical glands | 5460/13 119 (41.6) | 63/175 (36.0) | 0.79 (0.58 to 1.08) | 0.14 | 0.78 (0.56 to 1.08) | 0.13 |
| Severely inflamed tonsils | 1615/12 544 (12.9) | 37/173 (21.4) | 1.84 (1.28 to 2.66) | <0.01 | 1.92 (1.28 to 2.89) | <0.01 |
| Fetor | 1930/13 081 (14.8) | 27/176 (15.3) | 1.05 (0.69 to 1.58) | 0.83 | 0.97 (0.62 to 1.51) | 0.90 |
| Palatal oedema | 3168/13 106 (24.2) | 45/176 (25.6) | 1.08 (0.77 to 1.52) | 0.67 | 1.04 (0.69 to 1.57) | 0.85 |
*Controlling for clustering, antibiotic prescribing, and other independently predictive covariates (severely inflamed tonsils and very bad earache).
†Defined as very bad.
Table 3.
Predictive value of clinical score using severely inflamed tonsils and earache
| Clinical score | No (%) with no complications (n=13 187) | No (%) with complications (n=177) | Univariate odds ratio (95% CI) | P value | Multivariate odds ratio* (95% CI) | P value |
|---|---|---|---|---|---|---|
| 0 | 11 093 (84.1) | 124 (70.1) | 1.00 | — | 1.00 | — |
| 1 | 1954 (14.8) | 45 (25.4) | 2.12 (1.51 to 2.99) | <0.01 | 2.41 (1.62 to 3.58) | <0.01 |
| 2 | 140 (1.1) | 8 (4.5) | 5.15 (2.47 to 10.74) | <0.01 | 5.44 (2.51 to 11.79) | <0.01 |
*Controlling for clustering and antibiotic prescribing.
Supplementary appendix 3 shows the predictive values of individual variables. The sensitivity of a Centor score of 4 or more for complications was 17/175 (9.7%), and the positive predictive value was 1.7% (17/995). For FeverPAIN, a score of 4 or more also had a low sensitivity (12.7%: 22/173) and positive predictive value (2.1%: 22/1064). Most complications occurred with low scores, with 67% (118/175) scoring 2 or less for the Centor criteria and 73% (126/173) scoring 2 or less for FeverPAIN (table 4).
Table 4.
Predictive value of bacterial clinical scoring methods for complications
| Scoring methods | No (%) with no complications | No (%) with complications | Univariate odds ratio (95% CI) | P value | Multivariate odds ratio* (95% CI) | P value |
|---|---|---|---|---|---|---|
| Centor criteria: | n=12 863 | n=175 | ||||
| 0/1 | 4364 (33.9) | 57 (32.6) | 1.00 | — | 1.00 | — |
| 2 | 4408 (34.3) | 61 (34.9) | 1.06 (0.74 to 1.53) | 0.76 | 1.33 (0.91 to 1.96) | 0.14 |
| 3 | 3113 (24.2) | 40 (22.9) | 0.98 (0.65 to 1.48) | 0.94 | 1.39 (0.88 to 2.19) | 0.15 |
| 4 | 978 (7.6) | 17 (9.7) | 1.33 (0.77 to 2.30) | 0.31 | 1.94 (1.13 to 3.35) | 0.02 |
| FeverPAIN: | n=12 363 | n=173 | ||||
| 0/1 | 6391 (51.7) | 84 (48.6) | 1.00 | — | 1.00 | — |
| 2 | 3139 (25.4) | 42 (24.3) | 1.02 (0.70 to 1.48) | 0.93 | 1.26 (0.85 to 1.86) | 0.25 |
| 3 | 1791 (14.5) | 25 (14.5) | 1.06 (0.68 to 1.66) | 0.79 | 1.36 (0.82 to 2.23) | 0.23 |
| 4/5 | 1042 (8.4) | 22 (12.7) | 1.61 (1.00 to 2.58) | 0.05 | 2.09 (1.18 to 3.70) | 0.01 |
| FeverP(A)IN†: | n=12 398 | n=173 | ||||
| 0/1 | 8592 (69.3) | 111 (64.2) | 1.00 | — | 1.00 | — |
| 2 | 2381 (19.2) | 34 (19.7) | 1.11 (0.75 to 1.63) | 0.61 | 1.40 (0.92 to 2.12) | 0.11 |
| 3 | 1105 (8.9) | 20 (11.6) | 1.40 (0.87 to 2.26) | 0.17 | 1.87 (1.10 to 3.16) | 0.02 |
| 4 | 320 (2.6) | 8 (4.6) | 1.94 (0.94 to 4.00) | 0.08 | 2.57 (1.22 to 5.43) | 0.01 |
*Controlling for clustering and antibiotic prescribing.
†Not including attendance.
Secondary analysis of complications in no antibiotic prescription group
There was no evidence that either severe tonsillar inflammation or severe earache predicted outcome significantly differently for the different antibiotic prescribing strategies (likelihood ratio test for interaction terms P=0.753 and P=0.304, respectively). Selecting just the no antibiotic group, severe tonsillar inflammation similarly predicted the development of complications but with wide confidence intervals owing to the few complications and lower power (odds ratio 1.63, 95% confidence interval 0.37 to 7.22; P=0.52), and severe earache also predicted complications (3.11, 1.47 to 6.58; P<0.01). We also found similar predictive values for both Centor and FeverPAIN score in the no antibiotic group.
Reconsultations with unresolving or new symptoms
In multivariate analysis, reconsultations with unresolving or new symptoms was predicted by sex, number of medical problems, age, a history of fever, temperature, and muscle aches (table 5 and supplementary appendix 2). Predictive values were, however, mostly weak (odds ratios <1.5) and the bootstrapped area under the receiver operator curve for the model using the exact coefficients was modest (0.58, 95% confidence interval 0.57 to 0.60), so it was not possible to develop a useful clinical prediction rule. A modified Centor score of 4 or more or modified FeverPAIN score (when excluding previous rapid attendance) predicted new or unresolving symptoms only at high scores, again with modest odds ratios (table 6). The clinical score developed to predict complications again had low prognostic utility for reconsultations (bootstrapped area under the receiver operator curve 0.55, 95% confidence interval 0.53 to 0.57) (table 7).
Table 5.
Predictive value for new or unresolving symptoms
| Variables | No (%) with no new or unresolving symptoms | No (%) with new or unresolving symptoms | Univariate odds ratio (95% CI) | P value | Multivariate odds ratio* (95% CI) | P value |
|---|---|---|---|---|---|---|
| Predictors: | ||||||
| Previous duration ≤3 days | 5665/11 441 (49.5) | 927/1900 (48.8) | 0.97 (0.88 to 1.07) | 0.56 | 0.99 (0.88 to 1.12) | 0.90 |
| Sore throat† | 4273/10 712 (39.9) | 762/1780 (42.8) | 1.13 (1.02 to 1.25) | 0.02 | 1.09 (0.92 to 1.29) | 0.34 |
| Fever (during past 24 hours) | 6745/11 465 (58.8) | 1199/1894 (63.3) | 1.21 (1.09 to 1.33) | <0.01 | 1.21 (1.08 to 1.36) | <0.01 |
| Muscle aches | 6848/11 459 (59.9) | 1207/1893 (64.1) | 1.19 (1.08 to 1.32) | <0.01 | 1.14 (1.02 to 1.27) | 0.03 |
| Headache | 7426/11 456 (64.8) | 1254/1894 (66.2) | 1.06 (0.96 to 1.18) | 0.24 | 1.00 (0.89 to 1.14) | 0.94 |
| Absence of cough | 5063/11 104 (44.2) | 788/1895 (41.6) | 0.90 (0.82 to 0.99) | 0.04 | 0.93 (0.82 to 1.05) | 0.25 |
| Absence of runny nose | 6532/11 104 (58.8) | 1060/1840 (57.6) | 0.95 (0.86 to 1.05) | 0.33 | 0.98 (0.85 to 1.13) | 0.79 |
| Absence of cough and runny nose | 5421/11 100 (48.8) | 862/1840 (46.9) | 0.92 (0.84 to 1.02) | 0.11 | 0.97 (0.84 to 1.11) | 0.63 |
| Generally unwell† | 1518/11 470 (13.2) | 293/1895 (15.5) | 1.20 (1.05 to 1.38) | 0.01 | 1.13 (0.95 to 1.33) | 0.16 |
| Diarrhoea | 928/11 463 (8.1) | 171/1893 (9.0) | 1.12 (0.95 to 1.34) | 0.18 | 1.08 (0.89 to 1.30) | 0.46 |
| Disturbed sleep† | 2700/11 097 (24.3) | 499/1837 (27.2) | 1.16 (1.04 to 1.30) | 0.01 | 1.10 (0.94 to 1.30) | 0.23 |
| Difficulty swallowing† | 3638/10 708 (34.00) | 630/1782 (35.4) | 1.06 (0.96 to 1.18) | 0.26 | 1.06 (0.89 to 1.26) | 0.49 |
| Earache† | 556 /11 435 (4.9) | 111/1888 (5.9) | 1.22 (0.99 to 1.51) | 0.06 | 1.21 (0.96 to 1.54) | 0.11 |
| Vomiting† | 62/11 434 (0.5) | 9/1888 (0.5) | 0.88 (0.44 to 1.77) | 0.72 | 0.85 (0.44 to 1.63) | 0.62 |
| Abdominal pain† | 116/11 437 (1.0) | 30/1888 (1.56) | 1.58 (1.05 to2.36) | 0.03 | 1.33 (0.89 to 1.99) | 0.16 |
| Examination: | ||||||
| Temperature >37.5°C | 1687/11 050 (15.3) | 325/1828 (17.8) | 1.20 (1.05 to 1.37) | 0.01 | 1.18 (1.01 to 1.38) | 0.03 |
| Purulent tonsils | 3907/11 448 (34.1) | 648/1891 (34.3) | 1.01 (0.91 to 1.11) | 0.91 | 1.06 (0.92 to 1.22) | 0.42 |
| Cervical glands | 8111/10 930 (74.2) | 1340/1801 (74.4) | 1.01 (0.90 to 1.13) | 0.86 | 1.08 (0.94 to 1.25) | 0.28 |
| Tender cervical glands | 4775/11 411 (41.9) | 748/1883 (39.7) | 0.92 (0.83 to 1.01) | 0.08 | 0.89 (0.78 to 1.01) | 0.06 |
| Severely inflamed tonsils | 1408/10 925 (12.9) | 244/1792 (13.6) | 1.07 (0.92 to 1.23) | 0.40 | 1.08 (0.89 to 1.31) | 0.44 |
| Fetor | 1650/11 380 (14.5) | 307/1877 (16.4) | 1.15 (1.01 to 1.32) | 0.04 | 1.13 (0.96 to 1.34) | 0.15 |
| Palatal oedema | 2760/11 404 (24.2) | 453/1878 (24.1) | 1.00 (0.89 to 1.12) | 0.94 | 1.03 (0.85 to 1.26) | 0.74 |
*Controlling for clustering, antibiotic prescribing and other independently predictive covariates (sex, age, number of medical problems, fever in past 24 hours, high temperature, and muscle aches).
†Defined as very bad.
Table 6.
Predictive value of bacterial clinical scoring methods for reconsultation with new or unresolving symptoms
| Clinical scoring methods | No (%) with no new or unresolving symptoms | No (%) with new or unresolving symptoms | Univariate odds ratio (95% CI) | P value | Multivariate odds ratio* (95% CI) | P value |
|---|---|---|---|---|---|---|
| Centor criteria: | n=11 199 | n=1836 | ||||
| 0/1 | 3771 (33.7) | 650 (35.4) | 1.00 | — | 1.00 | — |
| 2 | 3873 (34.6) | 596 (32.4) | 0.89 (0.79 to1.01) | 0.06 | 0.91 (0.80 to1.04) | 0.16 |
| 3 | 2733 (24.4) | 420 (22.8) | 0.89 (0.78 to1.02) | 0.09 | 0.96 (0.82 to1.13) | 0.65 |
| 4 | 822 (7.3) | 173 (9.4) | 1.22 (1.02 to1.47) | 0.03 | 1.33 (1.06 to1.66) | 0.01 |
| FeverPAIN: | n=10 772 | n=1764 | ||||
| 0/1 | 5549 (51.5) | 926 (52.5) | 1.00 | — | 1.00 | — |
| 2 | 2750 (25.5) | 431 (24.4) | 0.94 (0.83 to1.06) | 0.32 | 0.95 (0.83 to1.10) | 0.53 |
| 3 | 1570 (14.6) | 246 (14.00) | 0.94 (0.81 to1.09) | 0.42 | 0.98 (0.82 to1.17) | 0.84 |
| 4/5 | 903 (8.4) | 161 (9.1) | 1.07 (0.89 to1.28) | 0.48 | 1.12 (0.92 to1.37) | 0.26 |
| FeverP(A)IN†: | n=10 804 | n=1767 | ||||
| 0/1 | 7481 (69.2) | 1222 (69.2) | 1.00 | — | 1.00 | — |
| 2 | 2084 (19.3) | 331 (18.7) | 0.97 (0.85 to1.11) | 0.67 | 1.01 (0.86 to1.18) | 0.92 |
| 3 | 972 (9.0) | 153 (8.7) | 0.96 (0.80 to1.15) | 0.69 | 0.98 (0.81 to1.18) | 0.82 |
| 4 | 267 (2.5) | 61 (3.5) | 1.40 (1.05 to1.86) | 0.02 | 1.56 (1.10 to2.22) | 0.01 |
*Controlling for clustering and antibiotic prescribing.
†Not including attendance.
Table 7.
Predictive value of clinical score using severely inflamed tonsils and earache
| Clinical score | No (%) with no new or unresolving symptoms (n=11 467) | No (%) with new or unresolving symptoms (n=1897) | Univariate odds ratio (95% CI) | P value | Multivariate odds ratio* (95% CI) | P value |
|---|---|---|---|---|---|---|
| 0 | 9655 (84.2) | 1561 (82.3) | 1.00 | — | 1.00 | — |
| 1 | 1686 (14.7) | 314 (16.6) | 1.15 (1.01 to 1.31) | 0.04 | 1.19 (1.00 to 1.41) | 0.048 |
| 2 | 126 (1.1) | 22 (1.2) | 1.08 (0.68 to 1.70) | 0.74 | 1.08 (0.66 to 1.79) | 0.76 |
*Controlling for clustering and antibiotic prescribing.
Discussion
Severe earache and tonsillar inflammation in acute sore throat predict the development of complications, but most cases of complications of acute sore throat occur in the absence of either variable, so history taking and examination in primary care are of limited predictive value. The scores used to assess risk of bacterial infection are also not useful in predicting complications. The most important suppurative complications are uncommon in a resource rich setting.
Strengths and weaknesses of this study
The study was designed for rapid recruitment to minimise selection bias using a simple clinical proforma to generate a large generalisable prospective cohort. Although a few practices also recruited patients for more intensive substudies (two diagnostic studies and a randomised trial), patients could be recruited to the current study if they declined those studies, so there were minimal barriers to recruitment. We adjusted for antibiotic prescribing in the analysis, and most of the variables predicting antibiotic prescriptions did not predict complications, Nevertheless, we may not have accurately controlled for the impact of antibiotics, since compliance in routine settings can be poor,26 and although evidence of confounding by indication was minimal (estimates changed little when controlling for antibiotic prescribing), residual confounding by indication may have attenuated predictive values. Similarly, the use of steroids and non-steroidal anti-inflammatory drugs may be relevant to outcome, but steroids were not used in this dataset and since most use of non-steroidal anti-inflammatory drugs is from over the counter purchases rather than as prescribed drugs, the impact of non-steroidal anti-inflammatory drugs cannot be estimated. The type of antibiotic might have modified the outcome, but the study had limited power to assess this since most of the prescriptions were for phenoxymethylpenicillin. We recruited fewer patients than we originally anticipated owing to funding limitations, and so type 2 error is possible. As it transpired, we recruited more patients not receiving immediate antibiotics than the minimum we initially anticipated. Furthermore, the inclusion of participants prescribed antibiotics in the primary analysis provided considerably more power. Patients were recruited at the busiest times of year, and as with other studies of acute infection,23 27 documentation of the details of those not approached was poor owing to time pressures (since time pressure to recruit also meant time pressure to document non-recruitment). Although the diagnosis of quinsy and cellulitis is relatively straightforward, the clinical diagnosis of otitis media (www.sign.ac.uk/guidelines/fulltext/66) or sinusitis (www.cks.nhs.uk/sinusitis) is likely to be more variable, which will have reduced the power to find associations. However, the systematic review of trials4 reviewed clinical diagnoses made in medical records so the data in the current study was comparable, if not preferable, because we used a highly structured record review proforma that was reliable for both complications and progression of symptoms. Outcome was assessed in routine clinical records, and although the original clinical reports forms were not available, since these were clinical records, baseline criteria will have also been (variably) recorded in the notes, and so some bias was possible. However, neither clinicians nor those who reviewed the notes (mostly not the clinicians who had recruited patients) had any basis for knowing what variables might predict complications. The most plausible bias would be that those clinicians who were familiar with the individual Centor criteria might be more likely to record complications where individual features of the Centor criteria were present. However, since none of the features of the Centor criteria were found to predict complications, even this tenuous series of links seem implausible. The fact that at least two variables predicted complications (tonsillar inflammation, earache), there was a progressive increase in the odds ratios for FeverPAIN, and the impact of antibiotic strategies in line with the trial evidence suggests outcome ascertainment was probably adequate. Using notes review for the broader definition of return to the surgery with unresolving or new symptoms—which is a much more inclusive outcome, and shown to be a useful outcome in a previous large international trial23—demonstrated even less predictive value for key variables, with lower odds ratios. Errors in exposure ascertainment will also have occurred: however, the study used the kind of clinical assessment of symptoms and signs used in everyday practice, which has proved successful in identifying predictors of bacterial infection,17 and since this was designed as a pragmatic study to assess the value of routine clinical history taking and examination, the results reflect more faithfully the utility of predictor variables in routine care. The complication rate in the current study was also of a similar magnitude to that of the trial data4 and to other previous studies in routine practice (for example, complication rate 1:400 for quinsy, and 0.8% including other suppurative complications in a previous UK pragmatic trial,19 24 1:1000 for quinsy in routine observational studies1), which supports the likely generalisability of the results, as does the wide range of general practitioners and practices involved. In the United Kingdom, rapid antigen detection tests are rarely used, so it is possible that such tests might predict complications, but since the bacterial scores were not predictive, and since rapid antigen detection tests do not detect group C and G streptococci, it is unclear whether these tests would be useful in predicting complications.
Main results in context of other literature
Complications were uncommon: no non-suppurative complications occurred, and less than 2% of patients developed complications overall. Only severe tonsillar inflammation and severe earache (among 13.0% and 5.0% of the cohort, respectively) significantly predicted the development of complications in both univariate and multivariate analysis, but the predictive value was limited. Severe earache predicted complications but did not significantly predict bacterial infections in the previous nested diagnostic studies. The finding that severe earache is a predictor is not simply due to misdiagnosis at presentation, because severe earache also predicted complications other than otitis media. One possibility is that severe earache may be an indicator of severe pharyngeal inflammation around the opening to the eustachean tubes or inflammation of the eustachean tubes, with referred ear pain. This explanation would be consistent with the finding that severe tonsillar inflammation is predictive, and raises the possibility that inflammatory markers such as C reactive protein might be helpful. Previous clinical scores designed to predict bacterial infection (Centor criteria, FeverPAIN) also predicted complications but only at high scores and again with low sensitivity and positive predictive values. To date, validation of the Centor score in clinical datasets has concentrated on predicting the presence of group A streptococci, and not assessed the prediction of clinical outcomes such as complications.28 A previous study of routine data from practice documented that middle aged men who smoke were most likely to develop complications,18 which we could not confirm in the current study. The number of previous medical problems, sex, temperature, muscle aches, and previous bacterial scores weakly predicted reconsultation with new or non-resolving symptoms.
In the absence of good clinical prediction of complications, clinicians and patients are therefore left in some uncertainty. Patients are not, however, just concerned about complications but also about poorly controlled symptoms, and even though the FeverPAIN score did not usefully predict complications, using this score can help both in the management of symptoms and the reduction in antibiotic use compared with an empirical delayed prescription strategy.29 Furthermore, clinicians can reassure their patients that clinically important complications are uncommon and that many complications are uncomfortable but self limiting (sinusitis, otitis media) and in most cases can be treated with analgesics.11 Those that are mostly not self limiting (cellulitis, quinsy) can be treated either with antibiotics (cellulitis) in an outpatient setting or, in the case of quinsy, with a short hospital admission. Attempts to avoid complications by liberal prescribing for a broad group of patients has been modelled by the National Institute for Health and Care Excellence and shown clearly not to be cost effective,11 and complications were of a similar order of magnitude among the patients who were and were not prescribed antibiotics. Uncertainty in clinical practice in primary care is an everyday reality and can be managed in this case either by sensible safety netting, including clear advice to patients about what to look for (particularly continuing fever, progressive difficulty swallowing, or the appearance of spreading erythema in the skin), the expected time course, and actions to be taken.30 Alternatively, the delayed prescription strategy could be used (where antibiotics are advised only if progression or non-resolution of symptoms is significant).11 The group most likely to benefit from either immediate or delayed antibiotic prescription in preventing complications are the small number with both severe tonsillar inflammation and severe earache (1% of the cohort), 1 in 20 of whom will develop complications.
Clinical implications
The most important suppurative complications of acute sore throat in primary care are uncommon, and history and examination are not useful in facilitating the prediction of complications or of reconsultations. Since a policy of liberal antibiotic prescription for sore throat to prevent complications is highly unlikely to be cost effective, and clinicians cannot rely on clinical targeting to predict most complications, clinicians will need to rely on strategies such as safety netting or delayed prescription in managing the low risk of suppurative complications.
What is already known on this topic
Antibiotic prescription rates are rising again and have exceeded the peak in the late 1990s
General practitioners currently use several clinical criteria to justify prescribing to prevent complications in the absence of evidence
The only case-control study to date showed a higher risk for middle aged men who smoke
What this study adds
The most important suppurative complications of acute sore throat in primary care are uncommon
These complications cannot usefully be predicted by either history and examination findings, or the previously developed scores for bacterial infection
Clinicians cannot rely on their own ad hoc clinical criteria to justify antibiotic prescribing to prevent complications and will need to rely on strategies such as safety netting or delayed prescription
We thank the local GP champions who promoted the study; the doctors, practices, and patients who participated; and those running the project in each centre: in Oxford Sue Smith managed day to day data collection; in Cardiff Eleri Owen-Jones managed the centre and Amanda Iles provided administrative support; in Exeter Joy Choules was the research administrator and Emily Fletcher helped with notes review; and in Bristol the research administrator was Catherine Derrick.
Contributors: DESCARTE (Decision rule for the Symptoms and Complications of Acute Red Throat in Everyday practice) investigators: CCB developed the protocol for funding, supervised the running of the study in the Cardiff Network and contributed to the drafting of the paper. PB and SB developed the protocol, provided day to day overall management of the study, coordinated recruitment in the lead study centre and coordination of other centres, and commented on drafts of the paper. JC developed the protocol for funding, lead the running of the study in the Exeter Network, and contributed to the drafting of the paper. BD developed the protocol for funding, coordinated the development and management of the web resource, and contributed to drafting of the paper. HE developed the protocol, with SB led the reliability study, supervised data collection for the reliability study, and contributed to analysis and drafting the paper. ADRH developed the protocol for funding, led the Bristol study centrem and contributed to the analysis and the drafting of the paper. FDRH developed the protocol for funding, led the Birmingham study centre, and contributed to the drafting of the paper. PL had the original idea for the protocol, led protocol development and the funding application, supervised the running of the lead study centre and coordination of centres, contributed to the analysis, led the drafting of the paper, and is the guarantor for the paper. DM developed the protocol for funding, supervised the running of clinical studies in the Oxford centre, and contributed to the analysis and the drafting of the paper. KM provided administrative support, developed data management protocols, coordinated data entry, and commented on drafts of the paper. MM developed the protocol for funding, contributed to the management of the study, and contributed to the drafting of the paper. MMullee (director Research Design Service, University of Southampton) developed the protocol for funding, contributed to study management, supervised data management, shared the quantitative analysis with BS and PL, and contributed to the drafting of the paper. BS developed the protocol, led the quantitative analysis with MM and PL, and with PL drafted the initial versions of the paper. IW developed the protocol for funding, and contributed to the management of the study and drafting of the paper. KH contributed to protocol development, supervised the running of the study in the Cardiff Network, and contributed to the drafting of the paper. PL is a senior investigator for the National Institute for Health Research in the Primary Care and Population Sciences Division at Aldermoor Health Centre, University of Southampton.
Funding: This work was sponsored by the University of Southampton, funded by the Medical Research council (grant No G0500977) and through costs by the National Institute for Health Research Service Support. Neither sponsor nor funder had any role in specifying the analysis or in the write up.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any other organisations for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: This study was approved by the South West Multicentre Research Ethics Committee (No 06/MRE06/17).
Data sharing: No additional data available.
Transparency: PL is the guarantor of the paper and affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Cite this as: BMJ 2013;347:f6867
Web Extra. Extra material supplied by the author
Appendices 1-5
Protocol version of study
References
- 1.Petersen I, Johnson A, Islam A, Duckworth G, Livermore D, Hayward A. Protective effect of antibiotics against serious complications of common respiratory tract infections: retrospective cohort study with the UK General Practice Research Database. BMJ 2007;335:982 doi:10.1136/bmj.39345.405243.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.House of Lords. House of Lords Select Committee on Science and Technology: 7th report. Occasional Report 1998.
- 3.Standing Medical Advisory Committee (SMAC) Sub-Group on Antimicrobial resistance. The path of least resistance. Occasional Report. Department of Health, 1998.
- 4.Spinks A, Glasziou PP, Del Mar CB. Antibiotics for sore throat. Cochrane Database Syst Rev 2013;(11):CD000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zwart S, Sachs A, Ruijs G, Hoes A, DeMelker R. Penicillin for acute sore throat: randomised double blind trial of seven days versus three days treatment or placebo in adults. BMJ 2000;320:150-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dagnelie CF, Van der Graf Y, De Melker R, Touw-Otten FWMM. Do patients with sore throat benefit from penecillin? A randomised double blind placebo controlled clinical trial with penicillin V in general practice. Br J Gen Pract 1996;46:589-93. [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar S, Little P, Britten N. Why do GPs prescribe antibiotics for sore throat? A grounded theory interview study of general practitioners. BMJ 2003;326:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Del Mar C. Managing sore throat: a literature review I: making the diagnosis. Med J Austr 1992;156:572-5. [PubMed] [Google Scholar]
- 9.Dagnelie C, Bartelink M, Van Der Graaf Y, Goessens W, De Melker R. Towards better diagnosis of throat infections with GABHS in general practice. Br J Gen Pract 1998;48:959-62. [PMC free article] [PubMed] [Google Scholar]
- 10.Dobbs F. A scoring system for predicting Group A streptococcal throat infection. Br J Gen Pract 1996;46:461-4. [PMC free article] [PubMed] [Google Scholar]
- 11.NICE guideline development group. Prescribing of antibiotics for self-limiting respiratory tract infections in adults and children in primary care. (Clinical guideline 69.) 2008. http://guidance.nice.org.uk/CG69. [PubMed]
- 12.McIsaac W, Kellner J, Aufricht P, Vanjaka A, Low D. Empirical validation of guidelines for the management of pharyngitis in children and adults. JAMA 2004;291:1587-95. [DOI] [PubMed] [Google Scholar]
- 13.Cooper R, Hoffman J, Bartlett J, Besser R, Gonzales R, Hickner JM. Principles of appropriate antibiotic use for acute pharyngitis in adults. Ann Intern Med 2001;134:509-17. [DOI] [PubMed] [Google Scholar]
- 14.Principles of appropriate antibiotic use for acute pharyngitis in adults. 2001. www.acponline.org/clinical_information/guidelines. [DOI] [PubMed]
- 15.Vincent M, Celestin N, Hussain A. Pharyngitis. Am Fam Phys 2004;69:1465-70. [PubMed] [Google Scholar]
- 16.Lindbaek M, Hoiby E, Steinsholt I, Hjortdahl P. Clinical symptoms and signs in sore throat patients with large colony variant β-haemolytic streptococci groups C or G versus group A. Br J Gen Pract 2005;55:615-9. [PMC free article] [PubMed] [Google Scholar]
- 17.Little P, Hobbs R, Mant D, McNulty C, Mullee M. Incidence and clinical variables associated with streptococcal throat infections:a prospective diagnostic cohort study. Br J Gen Pract 2012;62:e787-94. doi:10.3399/bjgp12X658322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunn N, Lane D, Everitt H, Little P. Use of antibiotics for sore throat and incidence of quinsy. Br J Gen Pract 2007;57:45-9. [PMC free article] [PubMed] [Google Scholar]
- 19.Little PS, Gould C, Williamson I, Warner G, Gantley M, Kinmonth AL. Reattendance and complications in a randomised trial of prescribing strategies for sore throat: the medicalising effect of prescribing antibiotics. BMJ 1997;315:350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centor RM, Witherspoon JM, Dalton HP. The diagnosis of strep throat in the emergency room. Med Decis Making 1981;1:239-46. [DOI] [PubMed] [Google Scholar]
- 21.Breese B. A simple scorecard for the tentative diagnosis of streptococcal pharyngitis. Am J Dis Child 1977;131:514-7. [DOI] [PubMed] [Google Scholar]
- 22.Hay A, Fahey T, Peters T, Wilson A. Predicting complications from acute cough in pre-school children in primary care: a prospective cohort study. Br J Gen Pract 2004;54:9-14. [PMC free article] [PubMed] [Google Scholar]
- 23.Little P, Stuart B, Moore M, Coenen S, Butler C, Godycki-Cwirko M, et al. Amoxicillin for acute lower respiratory tract infection in primary care when pneumonia is not suspected: a 12 country, randomised, placebo controlled trial in primary care. Lancet Infect Dis 2012;13:123-9. http://dx.doi.org/10.1016/S1473-3099(12)70300-6. [DOI] [PubMed] [Google Scholar]
- 24.Little PS, Williamson I, Warner G, Gould C, Gantley M, Kinmonth AL. An open randomised trial of prescribing strategies for sore throat. BMJ 1997;314:722-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrel FE. Regression modeling strategies. With applications to linear models, logistic regression, and survival analysis. Springer Verlag, 2001.
- 26.Francis N, Gillespie D, Nuttall J, Hood K, Little P, Verheij T, et al. Antibiotics for acute cough: an international observational study of patient adherence in primary care. Br J Gen Pract 2012;62:e429-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Little P, Rumsby K, Kelly J, Watson L, Moore M, Warner G, et al. Information leaflet and antibiotic prescribing strategies for acute lower respiratory tract infection: a randomised controlled trial. JAMA 2005;293:3029-35. [DOI] [PubMed] [Google Scholar]
- 28.Fine A, Nizet V, Mandl K. Large-scale validation of the Centor and McIsaac scores to predict group A streptococcal pharyngitis. Arch Intern Med 2012;172:847-52. doi:10.1001/archinternmed.2012.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Little P, Hobbs FD, Moore M, Mant D, Williamson I, McNulty C, et al. Clinical score and rapid antigen detection test to guide antibiotic use for sore throats: randomised controlled trial of PRISM (primary care streptococcal management). BMJ 2013;347:f5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Almond S, Mant D, Thompson M. Diagnostic safety-netting. Br J Gen Pract 2009;59:872-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendices 1-5
Protocol version of study