Abstract
RNA from encephalomyocarditis virus was injected into oocytes of Xenopus laevis. After incubation of the oocytes in [35S]methionine, polyacrylamide gel electrophoresis showed that a new series of polypeptides had been synthesized. They were identical in size to the polypeptides that appeared in ascites cells after infection with this virus. Electrophoretic and chromatographic analysis of the methionine-containing tryptic peptides from three of the induced polypeptides confirmed that they were virus-specific. All of the bands that appeared in ascites cells after infection also appeared in oocytes after injection of RNA from the virus. We conclude that Xenopus oocytes translate a mammalian viral mRNA faithfully and extensively, and perform normal post-translational modifications.
Keywords: picornavirus, frog oocytes, protein synthesis, gel electrophoresis, peptide analysis
Full text
PDF
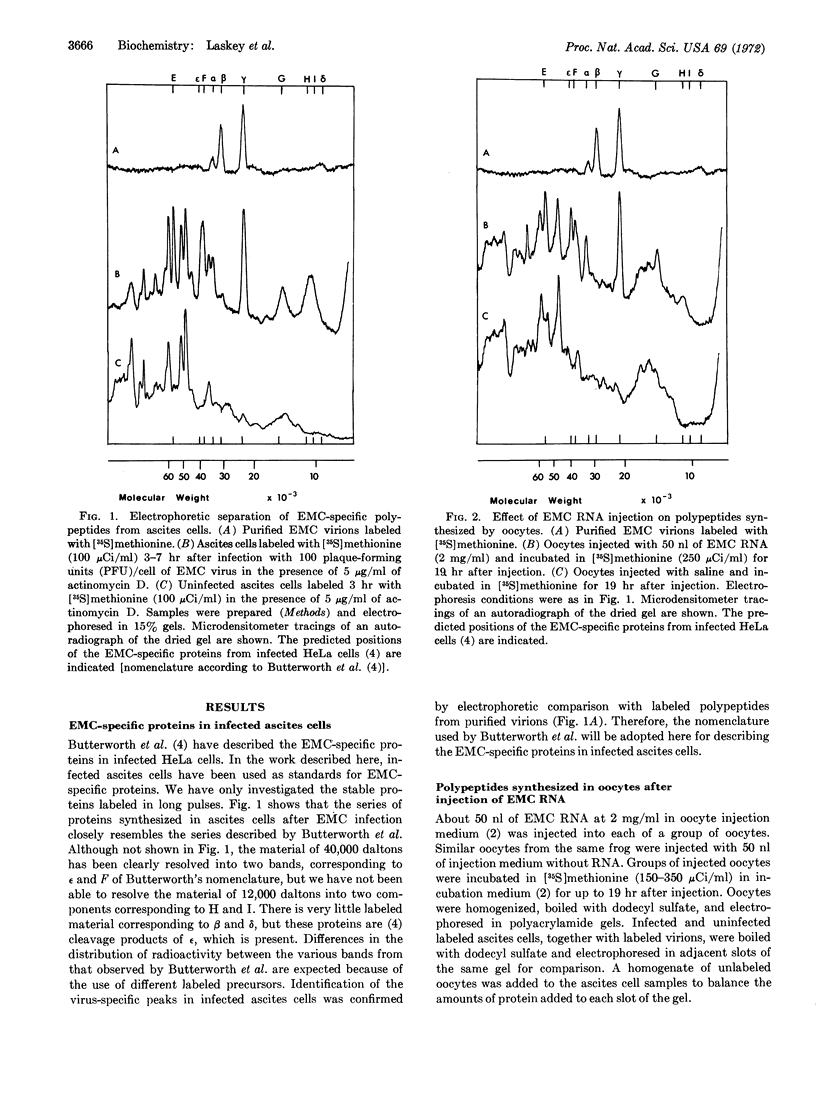

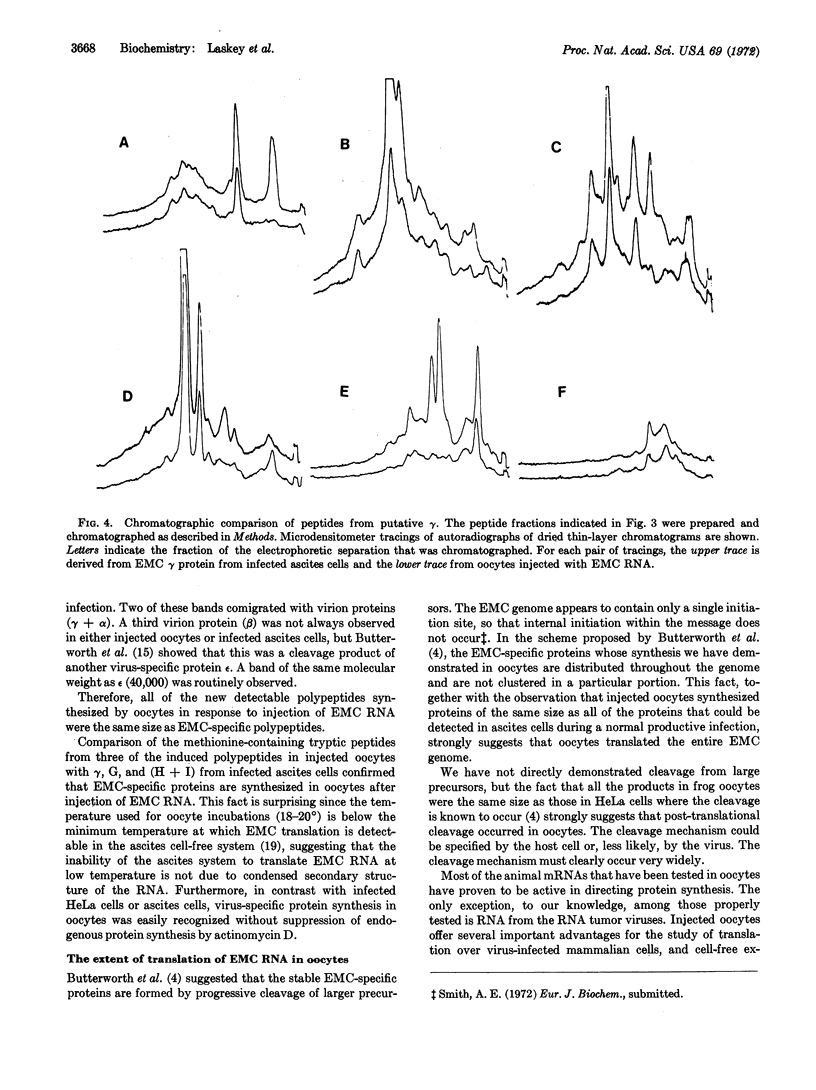

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BELLETT A. J., BURNESS A. T. Intracellular sites of synthesis of encephalomyocarditis virus components in Krebs-2 ascites tumour cells. J Gen Microbiol. 1963 Jan;30:131–140. doi: 10.1099/00221287-30-1-131. [DOI] [PubMed] [Google Scholar]
- Berns A. J., van Kraaikamp M., Bloemendal H., Lane C. D. Calf crystallin synthesis in frog cells: the translation of lens-cell 14S RNA in oocytes. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1606–1609. doi: 10.1073/pnas.69.6.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. S., Smith A. E. Biosynthesis of 35 S-L-methionine of very high specific activity. Anal Biochem. 1972 May;47(1):310–312. doi: 10.1016/0003-2697(72)90308-9. [DOI] [PubMed] [Google Scholar]
- Burness A. T. Purification of Encephalomyocarditis virus. J Gen Virol. 1969 Sep;5(2):291–303. doi: 10.1099/0022-1317-5-2-291. [DOI] [PubMed] [Google Scholar]
- Burns D. J., Turner N. A. Peptide mapping on cellulose thin layers. J Chromatogr. 1967 Oct;30(2):469–475. doi: 10.1016/s0021-9673(00)84179-5. [DOI] [PubMed] [Google Scholar]
- Butterworth B. E., Hall L., Stoltzfus C. M., Rueckert R. R. Virus-specific proteins synthesized in encephalomyocarditis virus-infected HeLa cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3083–3087. doi: 10.1073/pnas.68.12.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon J. B. Changes in somatic cell nuclei inserted into growing and maturing amphibian oocytes. J Embryol Exp Morphol. 1968 Nov;20(3):401–414. [PubMed] [Google Scholar]
- Gurdon J. B., Lane C. D., Woodland H. R., Marbaix G. Use of frog eggs and oocytes for the study of messenger RNA and its translation in living cells. Nature. 1971 Sep 17;233(5316):177–182. doi: 10.1038/233177a0. [DOI] [PubMed] [Google Scholar]
- HOSKINS J. M., SANDERS F. K. Propagation of mouse encephalomyocarditis virus in ascites tumour cells maintained in vitro. Br J Exp Pathol. 1957 Jun;38(3):268–272. [PMC free article] [PubMed] [Google Scholar]
- Kerr I. M., Cohen N., Work T. S. Factors controlling amino acid incorporation by ribosomes from krebs II mouse ascites-tumour cells. Biochem J. 1966 Mar;98(3):826–835. doi: 10.1042/bj0980826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr I. M., Martin E. M. Simple method for the isolation of encephalomyocarditis virus ribonucleic acid. J Virol. 1972 Mar;9(3):559–561. doi: 10.1128/jvi.9.3.559-561.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr I. M., Martin E. M. Virus protein synthesis in animal cell-free systems: nature of the products synthesized in resonse to ribonucleic acid of encephalomyocarditis virus. J Virol. 1971 Apr;7(4):438–447. doi: 10.1128/jvi.7.4.438-447.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mathews M. B. Further studies on the translation of globin mRNA and encephalomyocarditis virus RNA in a cell-free system from Krebs II ascites cells. Biochim Biophys Acta. 1972 Jun 22;272(1):108–118. doi: 10.1016/0005-2787(72)90038-x. [DOI] [PubMed] [Google Scholar]
- Mathews M. B., Korner A. The inhibitory action of a mammalian viral RNA on the initiation of protein synthesis in a reticulocyte cell-free system. Eur J Biochem. 1970 Dec;17(2):339–343. doi: 10.1111/j.1432-1033.1970.tb01171.x. [DOI] [PubMed] [Google Scholar]
- Mathews M., Korner A. Mammalian cell-free protein synthesis directed by viral ribonucleic acid. Eur J Biochem. 1970 Dec;17(2):328–338. doi: 10.1111/j.1432-1033.1970.tb01170.x. [DOI] [PubMed] [Google Scholar]
- Smith A. E., Marcker K. A., Mathews M. B. Translation of RNA from encephalomyocarditis virus in a mammalian cell-free system. Nature. 1970 Jan 10;225(5228):184–187. doi: 10.1038/225184a0. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]