Abstract
Disseminated mucormycosis is a rare entity most frequently seen in neutropenic patients with hematologic malignancies, post transplants or in patients on deferoxamine therapy. We report a 64-year-old immunocompetent male with an acute pneumonia and a generalized jaundice who died within 24 h. In the autopsy, extensive perforations of spleen and multiple hemorrhage foci on the pancreas were two significant findings. Histopathological study of tissue sections revealed typical zygomycetes hyphae in the left lung, pancreas, spleen and brain. Involvement of pancreas in this patient was one of the rare features of mucormycosis reported occasionally in the literature. Our case implies an unusual clinical presentation of disseminated mucormycosis and highlights that disseminated mucormycosis should be regarded even in the immunocompetent patients.
Keywords: Autopsy, immune system, mucormycosis
INTRODUCTION
Mucormycosis is a serious infection caused by fungi belonging to class zygomycetes, order Mucorales.[1] The fungal agents of mucormycosis are common in the environment and may contaminate the human host via inhalation, ingestion or direct skin inoculation.[2,3] However, due to the effect of the immune system, mucormycosis is a rare human infection. This rare infection primarily occurs in patients with some underlying conditions such as poorly controlled diabetes mellitus, neutropenia, hematological malignancy, iron chelation therapy, severe malnutrition and primary breakdown in the integrity of the cutaneous barrier.[4] Mucormycosis may be manifested by various patterns such as rhino-orbito-cerebral, pulmonary, cutaneous, gastrointestinal, arteries and disseminated infections.[5,6] Disseminated form, which indicates the involvement of two or more noncontiguous organ systems with zygomycetes is extremely rare and generally occurs in severely immunocompromised patients.[7,8] The authors describe a case of immunocompetent patient with disseminated mucormycosis discovered in the autopsy.
CASE REPORT
A 64-year-old, previously healthy male was transferred to Alzahra Hospital (Isfahan University of Medical Sciences, Isfahan, Iran) in August 2011. 15 days before, he had a stroke following a cardiac surgery (mitral valve replacement and coronary artery bypass graft). The patient had no history of diabetes mellitus, alcohol abuse and malignancy. Upon admission physical examination revealed confusion, dysarthria, left side hemiparesis and extensive bedsores in the sacral area. Initial laboratory study showed leukocyte count: 15,000/mcl, hemoglobin: 10 mg/dl. During hospitalization, the neurological state of the patient gradually ameliorated; however, in the 7th day of admission, he developed dyspnea, productive cough and jaundice. Physical examination revealed fever, tachypnea, generalized jaundice and course crackles in the base of the left lung. New laboratory study showed leukocyte count 9700/mcl, hemoglobin 10 mg/dl, total bilirubin 6 mg/dl, (direct bilirubin 1 mg/dl), aspartate aminotransferase 154 U/L, alanine aminotransferase 122 U/L and alkaline phosphatase 1150 U/L. Human immunodeficiency virus antibody test was negative. Chest X-ray showed focal pneumonic infiltration in the left lower lobe. Abdominal ultrasonography showed no abnormality. Considering the probability of pneumonia, empirical antibiotic therapy was started. However, his condition deteriorated and without any response to the treatment he died within 24 h.
In the autopsy, multiple hemorrhagic foci on the pancreas and extensive perforations of spleen were two significant findings. Following the autopsy, each organ was separately put into 10% buffered formalin and abnormal macroscopic findings for each organ were recorded by the pathologist. Then, small slices with at least 0.5 cm were cut and the formalin solution was changed for the fixing overnight. The next day, proper cut for paraffin embedded tissue blocks were prepared. Two routine staining procedures including hematoxylin and eosin and periodic acid schiff were performed for tissue sections.
Histopathological study of tissue sections revealed neutrophilic infiltration, massive coagulative necrosis and thrombotic vessels that some of them contained fungal hyphae in the left lung, pancreas, spleen and brain. On high power microscopic evaluation these hyphae appeared as ribbons which were often twisted and collapsed with a variable width. Septa in the most structures were absent. Some hyphae revealed swollen segments in cross-section [Figures 1–5]. Hence, the histological findings were compatible with the diagnosis of mucormycosis. Despite making several cuts, no considerable finding was discovered in the other organs.
Figure 1.
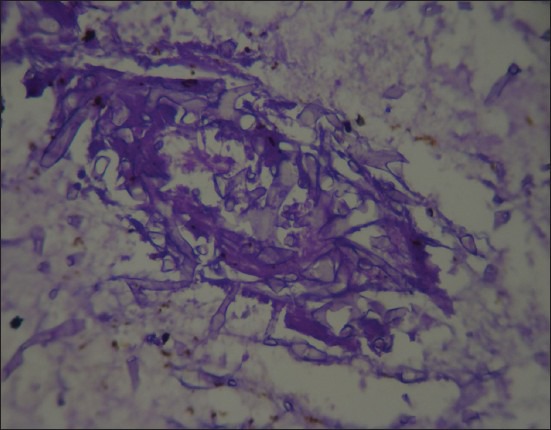
Pancreatic tissue revealed fungal hypha (periodic acid schiff, ×400)
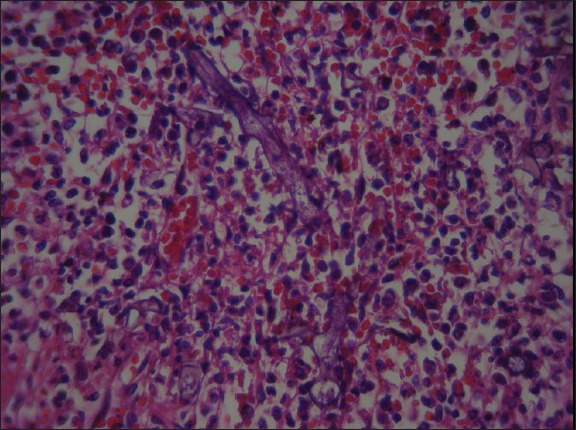
Figure 5.
Dural vascular spaces revealed fungal hypha (H and E, ×400)
Figure 2.
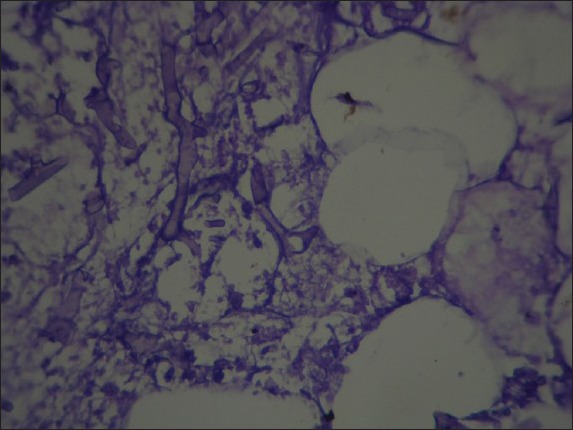
Peripancreatic fatty tissue revealed fat necrosis with fungal hypha (periodic acid schiff, ×400)
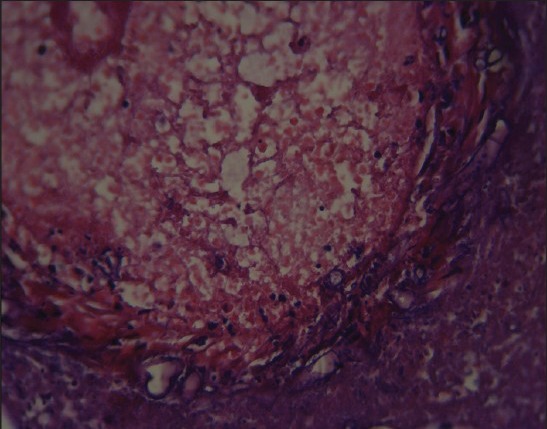
Figure 3.
Splenic tissue revealed ribbon like fungal hypha (H and E, ×400)
Figure 4.
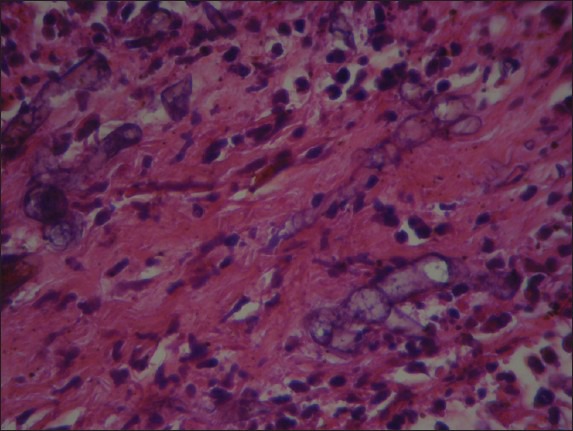
Splenic fibrous septa revealed non-setated and right angled fungal hypha (H and E, ×400)
DISCUSSION
In this report, we presented a case of disseminated mucormycosis with lung, pancreas, spleen and brain involvement discovered in the autopsy. Although disseminated mucormycosis generally occurs in severely immunocompromised patients or individuals who have received deferoxamine, but, our patient has none of these underlying factors.[3]
Disseminated mucormycosis has nonspecific manifestation, which causes to hamper the diagnosis.[8] In our case, non-specific presentation in addition to rapid and virulent course of the disease impeded a proper diagnosis.
Direct inoculation of organisms of mucormycosis is a common pathway of infection in patients without any immunocompromising condition. The resulted infection may invade cutaneous, subcutaneous, fat, muscle and skeletal tissues. In rare cases, it may even disseminate to deep organs.[4,8] In our patient, extensive bed sores in the sacral area might be major risk-factor and a route for initiation and dissemination of an infection.
Lung and brain are usually involved in disseminated mucormycosis, as were detected in our patient.[9] Involvement of pancreas in this patient was one of the rare features of mucormycosis reported occasionally in the disseminated infection.[8]
Tissue culture and histology are still the most important diagnostic approach for mucormycosis.[10] In this case, we used histological features, as the tissue culture is often negative. Moreover, as a positive culture in mycology laboratory considered due to common contaminants;[11] therefore, demonstration of hyphae in the tissue sample is essential for diagnosis of this organism.
The important challenge in the histopathological diagnosis is differentiating the mucormycosis from aspergillus species. Zygomycetes have a characteristic morphology; they have larger hyphae (5-20 μ in width) in comparison with aspergillus species hyphae (which are 3-5 μ in width). The zygomycetes hyphae have few septation and few branching. These few branches are at random angles, including right angles and other non-acute angles, in contrast with most other pathogenic fungi which have acute-angle branching. Zygomycete hyphae frequently collapse in tissue section and creating a characteristic twisted ribbon appearance.[12]
Fungal angioinvasion and the presence of neutrophilic infiltration in necrotic tissue are another characteristic features of mucormycosis,[13] which seen in histopathologic study of the specimens of our case.
Although disseminated infection is associated with the mortality rate of approximately 100%, but, successful treatments have been reported.[14,15,16] The only hope for treatment is rapid diagnosis, correction of predisposing factors, surgical debridement of necrotic tissue and antifungal therapy.[17] Rapid diagnosis needs a high index of suspicious and our case demonstrated that this suspicious should not be confined to immunocompromised patients. This case may imply that disseminated mucormycosis should also be regarded in the immunocompetent individuals especially those with cutaneous lesions.
ACKNOWLEDGMENT
We are thankful to the technicians of Legal Medicine Center of Isfahan for their assistances in the process of autopsy and preparation of samples for pathology.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Chander J, Kaur J, Attri A, Mohan H. Primary cutaneous zygomycosis from a tertiary care centre in north-west India. Indian J Med Res. 2010;131:765–70. [PubMed] [Google Scholar]
- 2.Bitar D, Van Cauteren D, Lanternier F, Dannaoui E, Che D, Dromer F, et al. Increasing incidence of zygomycosis (mucormycosis), France, 1997-2006. Emerg Infect Dis. 2009;15:1395–401. doi: 10.3201/eid1509.090334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horger M, Hebart H, Schimmel H, Vogel M, Brodoefel H, Oechsle K, et al. Disseminated mucormycosis in haematological patients: CT and MRI findings with pathological correlation. Br J Radiol. 2006;79:e88–95. doi: 10.1259/bjr/16038097. [DOI] [PubMed] [Google Scholar]
- 4.Petrikkos G, Drogari-Apiranthitou M. Zygomycosis in Immunocompromised non-Haematological Patients. Mediterr J Hematol Infect Dis. 2011;3:e2011012. doi: 10.4084/MJHID.2011.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzalez CE, Couriel DR, Walsh TJ. Disseminated zygomycosis in a neutropenic patient: Successful treatment with amphotericin B lipid complex and granulocyte colony-stimulating factor. Clin Infect Dis. 1997;24:192–6. doi: 10.1093/clinids/24.2.192. [DOI] [PubMed] [Google Scholar]
- 6.Meidani M, Soheilipour S, Barzegar F, Heidarpour M, Shakeri H. A case of temporal arteritis associated with mucoromycosis. J Isfahan Med Sch. 2009;27:56–61. [Google Scholar]
- 7.Lee DG, Choi JH, Choi SM, Yoo JH, Kim YJ, Min CK, et al. Two cases of disseminated mucormycosis in patients following allogeneic bone marrow transplantation. J Korean Med Sci. 2002;17:403–6. doi: 10.3346/jkms.2002.17.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta KL, Joshi K, Pereira BJ, Singh K. Disseminated mucormycosis presenting with acute renal failure. Postgrad Med J. 1987;63:297–9. doi: 10.1136/pgmj.63.738.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pak J, Tucci VT, Vincent AL, Sandin RL, Greene JN. Mucormycosis in immunochallenged patients. J Emerg Trauma Shock. 2008;1:106–13. doi: 10.4103/0974-2700.42203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morace G, Borghi E. Invasive mold infections: Virulence and pathogenesis of mucorales. Int J Microbiol. 2012;2012:349278. doi: 10.1155/2012/349278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verma GR, Lobo DR, Walker R, Bose SM, Gupta KL. Disseminated mucormycosis in healthy adults. J Postgrad Med. 1995;41:40–2. [PubMed] [Google Scholar]
- 12.Nichols L, Ocque RZ, Daly I. Zygomycosis associated with HIV infection and liver transplantation. Patholog Res Int 2011. 2011 doi: 10.4061/2011/545981. 545981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dizbay M, Adisen E, Kustimur S, Sari N, Cengiz B, Yalcin B, et al. Fungemia and cutaneous zygomycosis due to Mucor circinelloides in an intensive care unit patient: Case report and review of literature. Jpn J Infect Dis. 2009;62:146–8. [PubMed] [Google Scholar]
- 14.Mantadakis E, Samonis G. Clinical presentation of zygomycosis. Clin Microbiol Infect. 2009;15(Suppl 5):15–20. doi: 10.1111/j.1469-0691.2009.02974.x. [DOI] [PubMed] [Google Scholar]
- 15.Rickerts V, Atta J, Herrmann S, Jacobi V, Lambrecht E, Bialek R, et al. Successful treatment of disseminated mucormycosis with a combination of liposomal amphotericin B and posaconazole in a patient with acute myeloid leukaemia. Mycoses. 2006;49(Suppl 1):27–30. doi: 10.1111/j.1439-0507.2006.01299.x. [DOI] [PubMed] [Google Scholar]
- 16.Maury S, Leblanc T, Feuilhade M, Molina JM, Schaison G. Successful treatment of disseminated mucormycosis with liposomal amphotericin B and surgery in a child with leukemia. Clin Infect Dis. 1998;26:200–2. doi: 10.1086/517067. [DOI] [PubMed] [Google Scholar]
- 17.Alacacioglu I, Kargi A, Ozcan MA, Piskin O, Solak C, Secil M, et al. Fatal disseminated mucormycosis in a patient with mantle cell non-Hodgkin's lymphoma: An autopsy case. Braz J Infect Dis. 2009;13:238–41. doi: 10.1590/s1413-86702009000300017. [DOI] [PubMed] [Google Scholar]


