Abstract
Chromosomally-integrated arrays of lacO and tetO operator sites visualized by LacI and TetR repressor proteins fused with GFP (or other fluorescent proteins) are widely used to monitor the behavior of chromosomal loci in various systems. Yet, insertion of such arrays and expression of the corresponding proteins is known to perturb genomic architecture. In several cases, juxtaposition of such arrays located on different chromosomes has been inferred to reflect pairing of the corresponding loci. Here, we report that a version of TetR-GFP mutated to disrupt GFP dimerization (TetR-A206KGFP or “TetR-kGFP”), abolishes pairing of tetO-arrays in vivo and brings spatial proximity of chromosomal loci marked with those arrays back to the wild type level. These data argue that pairing of arrays is caused by GFP dimerization and thus presents an example of protein-assisted interaction in chromosomes. Arrays marked with another protein, TetR-tdTomato, which has a propensity to form intra-molecular dimers instead of inter-molecular dimers, also display reduced level of pairing, supporting this idea. TetR-kGFP provides an improved system for studying chromosomal loci with a low pairing background.
INTRODUCTION
Three-dimensional organization of the genome is important for many biological processes including maintenance of genomic integrity1; 2; 3; 4 and gene expression5; 6; 7; 8. Studies in bacteria1, fungi9; 10, insects6 and mammals3; 7; 11; 12 revealed complex and dynamic nature of genomic architecture. A popular approach to studying three-dimensional organization of the genome is FROS (Fluorescent Repressor-Operator System), which involves tagging chromosomal loci with arrays of directly repeated lacO or tetO operator sites13; 14. Expression of the cognate binding proteins from E. coli, lactose repressor (LacI) or tetracycline repressor (TetR) fused with a fluorescent protein (most commonly, GFP) allows direct visualization of the arrays by fluorescence microscopy. These two FROS systems (lacO-array/GFP-LacI and tetO-array/TetR-GFP) have been extensively used to study chromosome dynamics in bacteria15; 16, fungi10; 13; 14; 17; 18; 19; 20; 21, plants22; 23, nematode24 and mammalian cells25. Even though this methodology is a powerful tool to visualize genomic loci, insertion of arrays with concomitant expression of repressor-GFP fusion proteins can perturb 3D-structure of the genome, thus complicating the analysis.
Molecules of GFP are capable of dimerizing in an anti-parallel manner, by way of hydrophobic interactions on the dimerization surface26; 27. Introduction of a single point mutation in one of the hydrophobic amino acids on the dimerization interface drastically reduces protein's ability to form dimers. A number of such mutations have been described including A206K, which replaces hydrophobic Alanine 206 with positively charged Lysine28. Several phenomena related to protein clustering have been attributed to GFP dimerization, and use of non-dimerizing GFP mutants in those cases helped alleviate clustering and allow better analysis28; 29; 30.
We were interested in the possibility that such effects might complicate studies that use arrays to analyze chromosome pairing. If two arrays are present within one cell, they often pair with one another as seen in yeast19; 20; 21 and plants22; 23. One systematic study showed that pairing of arrays occurs regardless of their genomic location31. Such effects could reflect a natural underlying tendency of the corresponding loci to pair with one another and/or pairing mediated by DNA/DNA interactions of the array. However, one systematic study, using FISH instead of fluorescence to monitor array positions, suggested that such pairing can depend on the presence of repressor-GFP fusion protein32. We decided to study the phenomenon of array pairing further by making a mutated version of TetR-GFP with disrupted GFP dimerization, TetR-A206KGFP or “TetR-kGFP”. We report that using this mutated protein eliminates pairing of tetO-arrays in vivo. Our findings argue that pairing of arrays is caused by GFP dimerization and provide an improved tool for tagging chromosomal loci with a low pairing background.
RESULTS
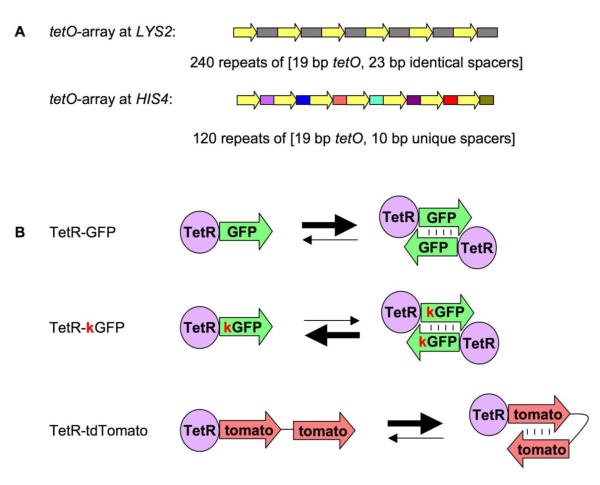
We analyzed a system in which a pair of tetO arrays was integrated at ectopic (unrelated) positions in yeast cells (Figure 1). tetO array at LYS2 locus consists of 240 copies of a DNA fragment, which contains 19 bp tetO binding site and a 23 bp spacer13. tetO array at HIS4 locus consists of 120 DNA fragments, each one of which contains the 19 bp tetO binding site and a unique 10 bp spacer16. We examined the spatial juxtaposition of these arrays in the presence of either TetR-GFP fusion protein encoding wild-type GFP, which has a strong propensity to form dimers, or the same fusion protein in which the GFP portion carried a single mutation, A206K, which is known to reduce GFP/GFP dimerization28.
Figure 1. Arrays and proteins used in this study.
A. Arrays used in this study.
The two arrays used in this study are: tetO array at LYS2 locus and tetO array at HIS4 locus. The former consists of 240 copies of a DNA fragment, which contains 19 bp tetO binding site and a 23 bp spacer (thus all spacers in the array are identical). The latter consists of 120 DNA fragments, each one of which contains the 19 bp tetO binding site and a unique 10 bp spacer (thus all spacers in the array are different).
B. Proteins used in this study.
The three proteins used in this study are: TetR-GFP, TetR-kGFP and TetR-tdTomato. TetR-GFP has a strong tendency to form inter-molecular dimers due to GFP dimerization. TetR-kGFP carries A206K mutation, which disrupts this property. TetR-tdTomato has two units of Tomato attached to TetR, thus it can form intra-molecular dimers instead of inter-molecular dimers.
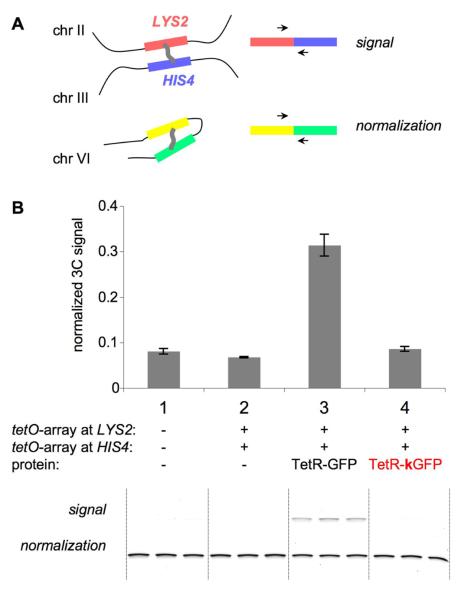
We first assessed pairing of two tetO arrays using 3C (Chromosome Conformation Capture) analysis33, which permits evaluation of array relationships regardless of the presence or nature of any fluorescent fusion protein (Figure 2). We compared the spatial proximities of two genomic loci in a wild type strain carrying no tetO arrays (case 1), and three strains in which both loci are marked with tetO arrays but carried either no repressor-GFP protein (case 2), non-mutated TetR-GFP protein (case 3) or mutated TetR-kGFP protein (case 4). If the non-mutated TetR-GFP protein is present, array-tagged loci exhibit robust pairing, well above the background seen in the absence of any array (compare case 3 and case 1). However, in the absence of any TetR-GFP protein or in the presence of TetR-kGFP (TetR-GFP-A206K), pairing occurred at the same level as in a strain with no arrays.
Figure 2. 3C assay of spatial proximity between chromosomal loci.
A. Schematic representation of the 3C assay.
3C assay relies on chromatin crosslinking and subsequent PCR analysis of ligation junctions between crosslinked segments. Signal PCR assays spatial proximity of LYS2 locus on chromosome II and HIS4 locus on chromosome III, where tetO arrays are integrated. Normalization PCR, which assays spatial proximity between two randomly chosen chromosomal segments located in cis, is used to control for the efficiency of the procedure in all samples.
B. 3C assay of spatial proximity between HIS4 and LYS2 loci.
Below – gel (reactions are done in triplicates, such that 3 PCR reactions were set up using 1 3C template), above – quantification of gel products. Case 1 – control WT strain without arrays and repressor proteins. Cases 2, 3, 4 – tetO arrays are present at HIS4 and LYS2 loci. Case 2 – no repressor protein, case 3 – TetR-GFP, case 4 – TetR-kGFP.
p(1,2) = 8.64 x10−2; p(1,3) = 3.44 x10−3; p(1,4) = 4.09 x10−1; p(2,3) = 4.60 x10−3; p(2,4) = 3.4 x10−2; p(3,4) = 3.92 x10−3 (all p-values that include sample 3 are < 0.01; underlined).
We also compared TetR-GFP and TetR-kGFP strains (cases 3 and 4 in Figure 2) by fluorescence microscopy of fixed whole cells (Figure 3). In the presence of the TetR-GFP protein, most cells exhibited a single fluorescent focus, indicating that the two arrays were usually paired. In contrast, in the presence of the TetR-kGFP mutant protein, most cells had two foci, indicating that arrays were no longer (or less frequently) paired (note that in the case of cells with 2 foci, the difference in brightness of the two foci is due to the difference in the length of the two arrays). Taken together, these results suggest that GFP/GFP dimerization can mediate stable pairing of loci located on different, non-homologous chromosomes in the yeast nucleus.
Figure 3. Microscopic analysis of cells with two ectopic tetO arrays and TetR-GFP, TetR-kGFP or TetR-tdTomato protein.
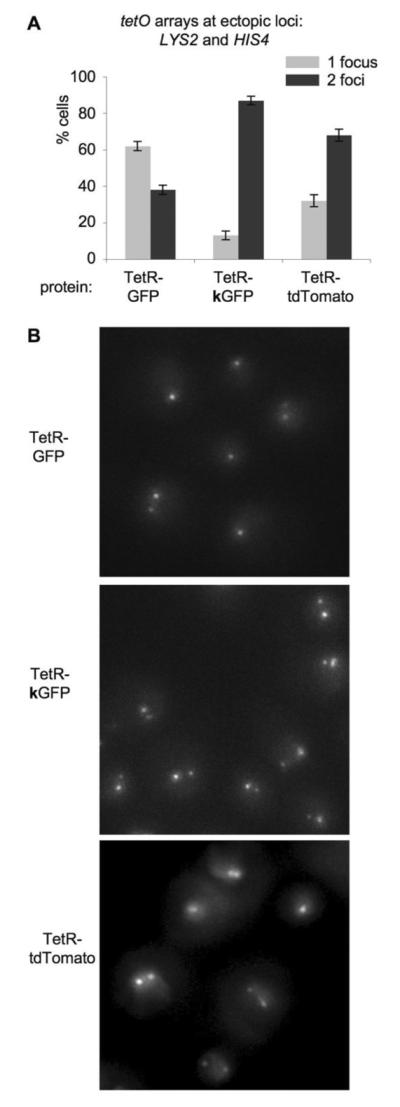
A. Quantitation of the microscopic analysis.
Percentages of cells with 1 focus and 2 foci are plotted for three strains, all of which have two tetO arrays located at HIS4 and LYS2 loci, plus one of the proteins: TetR-GFP, TetR-kGFP or TetR-tdTomato. Light grey bars – percentage of cells with 1 focus, dark grey bars – percentage of cells with 2 foci. p(1,2) = 3.45×10−5; p(1,3) = 7.3×10−4; p(2,3) = 3.38×10−3 (all p-values are < 0.01).
B. Representative images of cells.
In the case of cells with 2 foci, the difference in brightness of the two foci is due to the difference in the length of the two arrays (Figure 1A).
Another variant of FROS involving tetO arrays and TetR fusion protein is TetR-tdTomato34, which has two units of red fluorescent protein attached to TetR, thus it can form intra-molecular dimers rather than inter-molecular dimers (Figure 1B). If fluorescent protein mediated interaction was indeed responsible for pairing of arrays, arrays marked with TetR-tdTomato should exhibit lower level of pairing than those marked with TetR-GFP because of the aforementioned feature of tdTomato. To see if this is true, we included cells with two ectopic tetO arrays and TetR-tdTomato in our cytomogical analysis (Figure 3). As we expected, the majority of cells with TetR-tdTomato had two foci, indicating that the two tetO arrays were not paired. Interestingly, the fraction of cells with 1 focus in the strain with TetR-tdTomato was slightly higher than that of the strain with TetR-kGFP, which might be due to the fact that TetR-tdTomato might have some residual ability to form inter-molecular dimers, whereas positive charge on TetR-kGFP has a repulsive effect on protein-protein interaction.
Arrays are widely used to study pairing of homologous chromosomal loci, e.g. during meiosis17; 19; 21. The above evidence, in accord with earlier studies31; 32, suggests that GFP-mediated pairing could complicate such analysis. We thus used cytological analysis to examine array status in diploid yeast cells carrying tetO arrays integrated at allelic loci on homologous chromosomes in the presence of either non-mutant TetR-GFP or TetR-kGFP protein (Figure 4). Almost all cells expressing the non-mutant TetR-GFP protein exhibited one focus whereas the majority of cells with the mutated TetR-kGFP protein had two foci. Thus, GFP/GFP dimerization creates a significant background of protein-mediated pairing.
Figure 4. Microscopic analysis of cells with two allelic tetO arrays and TetR-GFP or TetR-kGFP protein.
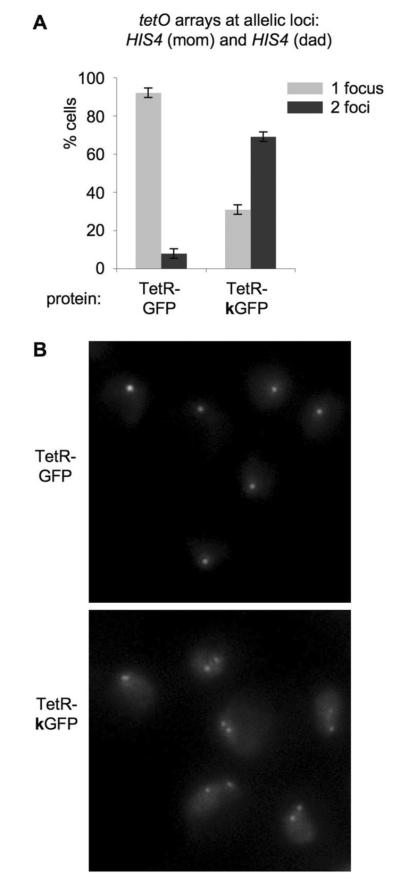
A. Quantitation of the microscopic analysis.
Percentages of cells with 1 focus and 2 foci are plotted for two strains, both of which have two tetO arrays located at HIS4 loci on two homologous chromosomes, plus one of the proteins: TetR-GFP or TetR-kGFP. Light grey bars – percentage of cells with 1 focus, dark grey bars – percentage of cells with 2 foci. p=1.64 x10−5< 0.01.
B. Representative images of cells.
Arrays have also been used to look at the dynamics of sister chromatids in several organisms13; 14; 15; 18. To ask whether GFP/GFP pairing might enhance sister association, we compared diploid yeast strains carrying a single tetO array (on one of the homologous chromosomes), and non-mutant and mutant versions of TetR-GFP, after nocodazole-mediated arrest after replication, at G2/M stage (Figure 5). Sister loci exhibit increased separation in such cells, as compared to untreated cells, as seen by FISH35. Comparing cells with TetR-GFP and TetR-kGFP, we find that the fraction of two-focus cells is significantly lower in the strain carrying the non-mutant TetR-GFP, suggesting that protein-mediated pairing can oppose such separation.
Figure 5. Microscopic analysis of G2/M-arrested cells with tetO array at LYS2 locus and TetR-GFP or TetR-kGFP protein.
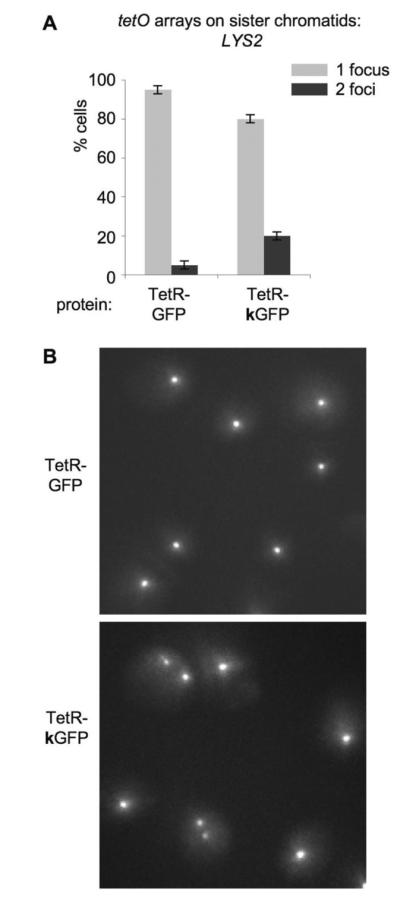
A. Quantitation of the microscopic analysis.
Percentages of cells with 1 focus and 2 foci are plotted for two strains, both of which have tetO array located at LYS2 locus, plus one of the proteins: TetR-GFP or TetR-kGFP. Cells were arrested in G2/M with nocodazole, so each cells contains two sister chromatids, each of which has tetO array at LYS2. Light grey bars – percentage of cells with 1 focus, dark grey bars – percentage of cells with 2 foci. p=2.22 x10−3< 0.01.
B. Representative images of cells.
DISCUSSION
Our data suggests that GFP-mediated dimerization of repressor-GFP fusion proteins can promote substantial levels of in vivo pairing of FROS-marked repetitive arrays, and that this background is substantially reduced by using a fusion protein carrying kGFP instead. These findings are of interest for the following four reasons.
First, we show that GFP dimerization can confer significant pairing of ectopic, allelic and sister loci, thus providing a cautionary note regarding the execution and interpretation of array-based studies of pairing phenomena. We note, however, that our findings do not compromise evidence for pre-meiotic and early meiotic pairing of homologous loci in yeast, which has been detected previously using FISH, Lox/Cre recombination and 3C analysis in the absence of arrays33; 35; 36; 37.
Second, our kGFP system offers an improved tool for array-based analysis of intra-nuclear positions of chromosomal loci that permits use of monomeric green-fluorescent GFP. This system offers a complement to the self-dimerizing red fluorescent protein system provided by tdTomato. Given that all three proteins promote different levels of array pairing, one should exercise caution when comparing GFP, kGFP and tdTomato marked arrays. It is worth mentioning that another way to reduce pairing of arrays is by decreasing their length: in our hands, 80-unit long arrays marked with non-mutated repressor-GFP protein had reduced level of pairing compared to 120-unit long ones, and 30-unit long ones showed no detectable pairing when integrated at ectopic loci, however in the latter case detection becomes more challenging (data not shown).
Third, protein-mediated interactions within and between chromosomes are important for many biological processes (reviewed in7). Pairing of arrays by way of repressor-GFP dimerization provides yet another example of such process.
Fourth, GFP/GFP dimerization could potentially be used as a tool for further dissecting such processes by the introduction of relatively weak artificial tether(s) in chromosomes. Tetramerizing LacI-GFP was used before for this purpose, but the tether created in that case was a much stronger one14.
When the phenomenon of array pairing was first systematically analyzed, it was proposed that the basis for such pairing was DNA-DNA interaction31. Another study suggested that pairing can depend on the presence of repressor-GFP fusion protein, but the molecular mechanism behind the pairing was still not known32. Our observations confirm and extend the latter study by showing that the molecular mechanism behind array pairing is dimerization of GFP. We would like to note that our findings do not extend to all cases of chromosome pairing, which can be protein-dependent such as in the case of pairing centers38, as well as DNA-DNA dependent, such as in the case of genome-wide dsb-independent pairing37.
In the case of ectopic tetO arrays and the new mutated TetR-kGFP protein, vast majority of cells had 2 foci indicating that the two arrays were not paired. However, in the case of allelic tetO arrays and the new mutated TetR-kGFP protein, there was still significant fraction of cells with 1 focus. This difference could arise from residual GFP dimerization being detected in the allelic but not ectopic case perhaps due to more frequent contacts between allelic vs ectopic loci owing to genome-wide dsb-independent pairing of homologous chromosomes37 and/or the fact that allelic loci are located at similar “latitude” in the yeast nucleus10.
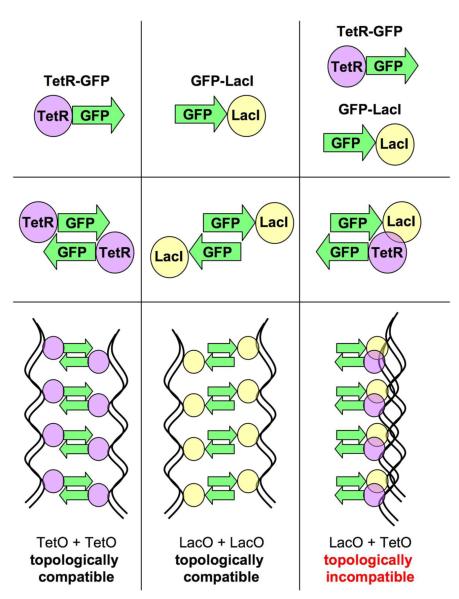
One puzzling observation is that while two tetO arrays pair with one another and two lacO arrays pair with one another, a tetO array and a lacO array don't pair31. How can this fact be explained if GFP dimerization is responsible for pairing of arrays? The explanation might come from the differences in the way the two repressor-GFP fusion proteins were constructed (Figure 6). TetR-GFP fusion protein was made by placing TetR on the N-terminus of GFP13, while GFP-LacI fusion protein was made by placing LacI on the C-terminus of GFP14. Since two molecules of GFP can dimerize in an anti-parallel way26; 27, one can imagine that such dimerization would be permitted in the case of having repressor molecules on the similar ends of two GFP molecules and restricted in the case of having repressor molecules on different ends of two GFP molecules because of steric clash between the two repressor molecules (Figure 6). While we tried to test this explanation by re-making TetR-GFP construct into GFP-TetR (which according to our hypothesis should allow for pairing of a tetO array and a lacO array, being topologically compatible with GFP-LacI), we were unable to produce a functional fusion protein, presumably due to compromised DNA binding.
Figure 6. Model.
Two GFP molecules are capable of forming anti-parallel dimers. Two molecules of TetR-GFP can easily dimerize with each other, thus pairing two tetO arrays. Likewise, two molecules of GFP-LacI can easily dimerize with each other, thus pairing two lacO arrays. However, a TetR-GFP molecule cannot easily dimerize with a GFP-LacI molecule because of steric clash between TetR and LacI, which occurs because TetR and LacI will be on the same side of the dimer (as opposed to TetR-GFP/TetR-GFP or GFP-Lac/GFP-LacI dimers, in which two repressor molecules will be on different sides of the dimer).
MATERIALS AND METHODS
Construction of pTetR-kGFP plasmid
Plasmid pTetR-kGFP was constructed by PCR using plasmid pTetR-GFP, which expresses TetR-GFP fusion protein under the control of URA3 promoter13. GCC codon, which codes for Alanine 206 in GFP was replaced with AAA codon for Lysine. No other changes were made. The plasmid was cut with AflII and integrated into LEU2 locus.
Strains
All strains were diploid derivatives of S. cerevisiae SK1 background ho::hisG/”, ura3/”. KMY52 HIS4::LEU2/his4X::LEU2, leu2/” KMY198 lys2::tetO240::URA3/LYS2, HIS4::LEU2-tetO120/HIS4, leu2/” KMY179 lys2::tetO240::URA3/LYS2, HIS4::LEU2-tetO120/HIS4, LEU2:pTetR-GFP/leu2 KMY248 lys2::tetO240::URA3/LYS2, HIS4::LEU2-tetO120/HIS4, LEU2:pTetR-kGFP/leu2 KMY178 lys2::tetO240::URA3/LYS2, HIS4::LEU2-tetO120/HIS4, LEU2:pTetR-tdTomato/leu2 KMY172 HIS4::LEU2-tetO120/his4X::LEU2-tetO120, LEU2:pTetR-GFP/leu2 KMY247 HIS4::LEU2-tetO120/his4X::LEU2-tetO120, LEU2:pTetR-kGFP/leu2 KMY299 lys2::tetO240::URA3/LYS2, LEU2:pTetR-GFP/leu2 KMY300 lys2::tetO240::URA3/LYS2, LEU2:pTetR-kGFP/leu2
Nocodazole arrest
4 ml YPD was inoculated with a single colony of yeast and grown overnight at 30°C, after which 25 μl of the overnight culture was transferred into 25 ml YPD and grown for 4 hours. 5 ml of culture was transferred into a fresh tube and 7.5 μl of 10 mg/ml nocodazole (Sigma M1404) solution in DMSO was added (15 μg/ml final concentration). After 2 h, G2/M arrest (large budded cells with DAPI-stained body at the neck) was confirmed, and cells were harvested and fixed for microscopy.
3C assay
3C assay was done exactly as in39. For each 3C template, the intensity of signal PCR product was normalized to the intensity of normalization PCR product and averaged among the triplicates. p-values for the two-sample t-test were calculated in R. Signal PCR primers were: LYS2-RX-I TGAAATCAGATTAGTTAGCGTTCGTAACC and KM-5f-1 TAATCGGTCGTCAGCCAACGTGAGAGTGTC; cis PCR primers were KM4a ACACTATCAGACCCTACAGTTAAGGAGAAA and KM9a-2 AAGCAAATGGCGTCCAAAATGTTCGACTTA.
Microscopy
Yeast was grown in YPD media to mid-logarithmic phase and fixed in 40% Ethanol / 0.1M sorbitol (for sister chromatid experiment, nocodazole-arrested cultures [above] were used). Microscopy was performed on an inverted microscope (Nikon Ti-e) with a 100x oil immersion objective. 3D z-stacks were taken at 200nm intervals for a total of 20 z-steps. 3D images were transformed to 2D by maximum intensity projection in Metamorph and spots were manually counted in ImageJ. 3 independent cultures were scored for each case; 500 cells were scored per each sample. Cells with ambiguous number of foci, as well as cells with zero foci or more than 2 foci were ignored (the fraction of such cells did not exceed 5% in all samples). p-values for the two-sample t-test were calculated in R.
Highlights
* Chromosomally-integrated arrays of repetitive sequences pair with each other
* TetR-kGFP (TetR-A206KGFP) abolishes pairing of tetO-arrays in vivo
* Pairing of arrays is caused by protein-protein interactions
ACKNOWLEDGEMENTS
This research was supported by a grant to N.K. from the National Institutes of Health RO1GM044794 and a postdoctoral fellowship to E.M. from the National Institutes of Health F32GM083427. We thank Beth Weiner and Jim Henle for help with the manuscript preparation, Guillaume Witz for discussion and Eugene Gladyshev for help with statistical analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Fisher JK, Bourniquel A, Witz G, Weiner B, Prentiss M, Kleckner N. Four-Dimensional Imaging of E. coli Nucleoid Organization and Dynamics in Living Cells. Cell. 2013;153:882–95. doi: 10.1016/j.cell.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lisby M, Mortensen UH, Rothstein R. Colocalization of multiple DNA double-strand breaks at a single Rad52 repair centre. Nat Cell Biol. 2003;5:572–7. doi: 10.1038/ncb997. [DOI] [PubMed] [Google Scholar]
- 3.Meaburn KJ, Misteli T, Soutoglou E. Spatial genome organization in the formation of chromosomal translocations. Semin Cancer Biol. 2007;17:80–90. doi: 10.1016/j.semcancer.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zickler D, Kleckner N. Meiotic chromosomes: integrating structure and function. Annu Rev Genet. 1999;33:603–754. doi: 10.1146/annurev.genet.33.1.603. [DOI] [PubMed] [Google Scholar]
- 5.Meldi L, Brickner JH. Compartmentalization of the nucleus. Trends Cell Biol. 2011;21:701–8. doi: 10.1016/j.tcb.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKee BD. Homologous pairing and chromosome dynamics in meiosis and mitosis. Biochim Biophys Acta. 2004;1677:165–80. doi: 10.1016/j.bbaexp.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 7.Williams A, Spilianakis CG, Flavell RA. Interchromosomal association and gene regulation in trans. Trends Genet. 2010;26:188–97. doi: 10.1016/j.tig.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taddei A, Van Houwe G, Hediger F, Kalck V, Cubizolles F, Schober H, Gasser SM. Nuclear pore association confers optimal expression levels for an inducible yeast gene. Nature. 2006;441:774–8. doi: 10.1038/nature04845. [DOI] [PubMed] [Google Scholar]
- 9.Duan Z, Andronescu M, Schutz K, McIlwain S, Kim YJ, Lee C, Shendure J, Fields S, Blau CA, Noble WS. A three-dimensional model of the yeast genome. Nature. 2010;465:363–7. doi: 10.1038/nature08973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Therizols P, Duong T, Dujon B, Zimmer C, Fabre E. Chromosome arm length and nuclear constraints determine the dynamic relationship of yeast subtelomeres. Proc Natl Acad Sci U S A. 2010;107:2025–30. doi: 10.1073/pnas.0914187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu M, Cook PR. The role of specialized transcription factories in chromosome pairing. Biochim Biophys Acta. 2008;1783:2155–60. doi: 10.1016/j.bbamcr.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 12.Hubner MR, Spector DL. Chromatin dynamics. Annu Rev Biophys. 2010;39:471–89. doi: 10.1146/annurev.biophys.093008.131348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michaelis C, Ciosk R, Nasmyth K. Cohesins: chromosomal proteins that prevent premature separation of sister chromatids. Cell. 1997;91:35–45. doi: 10.1016/s0092-8674(01)80007-6. [DOI] [PubMed] [Google Scholar]
- 14.Straight AF, Belmont AS, Robinett CC, Murray AW. GFP tagging of budding yeast chromosomes reveals that protein-protein interactions can mediate sister chromatid cohesion. Curr Biol. 1996;6:1599–608. doi: 10.1016/s0960-9822(02)70783-5. [DOI] [PubMed] [Google Scholar]
- 15.Joshi MC, Bourniquel A, Fisher J, Ho BT, Magnan D, Kleckner N, Bates D. Escherichia coli sister chromosome separation includes an abrupt global transition with concomitant release of late-splitting intersister snaps. Proc Natl Acad Sci U S A. 2011;108:2765–70. doi: 10.1073/pnas.1019593108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lau IF, Filipe SR, Soballe B, Okstad OA, Barre FX, Sherratt DJ. Spatial and temporal organization of replicating Escherichia coli chromosomes. Mol Microbiol. 2003;49:731–43. doi: 10.1046/j.1365-2958.2003.03640.x. [DOI] [PubMed] [Google Scholar]
- 17.Brar GA, Hochwagen A, Ee LS, Amon A. The multiple roles of cohesin in meiotic chromosome morphogenesis and pairing. Mol Biol Cell. 2009;20:1030–47. doi: 10.1091/mbc.E08-06-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim KP, Weiner BM, Zhang L, Jordan A, Dekker J, Kleckner N. Sister cohesion and structural axis components mediate homolog bias of meiotic recombination. Cell. 2010;143:924–37. doi: 10.1016/j.cell.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sonntag Brown M, Zanders S, Alani E. Sustained and rapid chromosome movements are critical for chromosome pairing and meiotic progression in budding yeast. Genetics. 2011;188:21–32. doi: 10.1534/genetics.110.125575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bystricky K, Heun P, Gehlen L, Langowski J, Gasser SM. Long-range compaction and flexibility of interphase chromatin in budding yeast analyzed by high-resolution imaging techniques. Proc Natl Acad Sci U S A. 2004;101:16495–500. doi: 10.1073/pnas.0402766101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gladstone MN, Obeso D, Chuong H, Dawson DS. The synaptonemal complex protein Zip1 promotes bi-orientation of centromeres at meiosis I. PLoS Genet. 2009;5:e1000771. doi: 10.1371/journal.pgen.1000771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jovtchev G, Watanabe K, Pecinka A, Rosin FM, Mette MF, Lam E, Schubert I. Size and number of tandem repeat arrays can determine somatic homologous pairing of transgene loci mediated by epigenetic modifications in Arabidopsis thaliana nuclei. Chromosoma. 2008;117:267–76. doi: 10.1007/s00412-007-0146-0. [DOI] [PubMed] [Google Scholar]
- 23.Pecinka A, Kato N, Meister A, Probst AV, Schubert I, Lam E. Tandem repetitive transgenes and fluorescent chromatin tags alter local interphase chromosome arrangement in Arabidopsis thaliana. J Cell Sci. 2005;118:3751–8. doi: 10.1242/jcs.02498. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Serricchio AS, Sternberg PW. Visualization of C. elegans transgenic arrays by GFP. BMC Genet. 2006;7:36. doi: 10.1186/1471-2156-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson SL, Compton DA. Proliferation of aneuploid human cells is limited by a p53-dependent mechanism. J Cell Biol. 2010;188:369–81. doi: 10.1083/jcb.200905057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang F, Moss LG, Phillips GN., Jr. The molecular structure of green fluorescent protein. Nat Biotechnol. 1996;14:1246–51. doi: 10.1038/nbt1096-1246. [DOI] [PubMed] [Google Scholar]
- 27.Shaner NC, Steinbach PA, Tsien RY. A guide to choosing fluorescent proteins. Nat Methods. 2005;2:905–9. doi: 10.1038/nmeth819. [DOI] [PubMed] [Google Scholar]
- 28.Zacharias DA, Violin JD, Newton AC, Tsien RY. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science. 2002;296:913–6. doi: 10.1126/science.1068539. [DOI] [PubMed] [Google Scholar]
- 29.Costantini LM, Fossati M, Francolini M, Snapp EL. Assessing the tendency of fluorescent proteins to oligomerize under physiologic conditions. Traffic. 2012;13:643–9. doi: 10.1111/j.1600-0854.2012.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landgraf D, Okumus B, Chien P, Baker TA, Paulsson J. Segregation of molecules at cell division reveals native protein localization. Nat Methods. 2012;9:480–2. doi: 10.1038/nmeth.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aragon-Alcaide L, Strunnikov AV. Functional dissection of in vivo interchromosome association in Saccharomyces cerevisiae. Nat Cell Biol. 2000;2:812–8. doi: 10.1038/35041055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuchs J, Lorenz A, Loidl J. Chromosome associations in budding yeast caused by integrated tandemly repeated transgenes. J Cell Sci. 2002;115:1213–20. doi: 10.1242/jcs.115.6.1213. [DOI] [PubMed] [Google Scholar]
- 33.Dekker J, Rippe K, Dekker M, Kleckner N. Capturing chromosome conformation. Science. 2002;295:1306–1311. doi: 10.1126/science.1067799. [DOI] [PubMed] [Google Scholar]
- 34.Matos J, Lipp JJ, Bogdanova A, Guillot S, Okaz E, Junqueira M, Shevchenko A, Zachariae W. Dbf4-dependent CDC7 kinase links DNA replication to the segregation of homologous chromosomes in meiosis I. Cell. 2008;135:662–78. doi: 10.1016/j.cell.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 35.Burgess SM, Kleckner N, Weiner BM. Somatic pairing of homologs in budding yeast: existence and modulation. Genes Dev. 1999;13:1627–41. doi: 10.1101/gad.13.12.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burgess SM, Kleckner N. Collisions between yeast chromosomal loci in vivo are governed by three layers of organization. Genes Dev. 1999;13:1871–83. doi: 10.1101/gad.13.14.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiner BM, Kleckner N. Chromosome pairing via multiple interstitial interactions before and during meiosis in yeast. Cell. 1994;77:977–91. doi: 10.1016/0092-8674(94)90438-3. [DOI] [PubMed] [Google Scholar]
- 38.Rog O, Dernburg AF. Chromosome pairing and synapsis during Caenorhabditis elegans meiosis. Curr Opin Cell Biol. 2013 doi: 10.1016/j.ceb.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mirkin EV, Chang FS, Kleckner N. Dynamic trans interactions in yeast chromosomes. PLoS One. 2013;8:e75895. doi: 10.1371/journal.pone.0075895. [DOI] [PMC free article] [PubMed] [Google Scholar]