Summary
Mollusca is an animal phylum with vast morphological diversity and includes worm-shaped aplacophorans, snails, bivalves, and the complex cephalopods [1]. The interrelationships of these class-level taxa are still contentious [2, 3], but recent phylogenomic analyses suggest a dichotomy at the base of Mollusca, resulting in a monophyletic Aculifera (comprising the shell-less, sclerite-bearing aplacophorans and the eight-shelled polyplacophorans) and Conchifera (all other, primarily univalved groups) [4, 5]. The Aculifera concept has recently gained support via description of the fossil Kulindroplax, which shows both aplacophoran- and polyplacophoran-like features and suggests that the aplacophorans originated from a shelled ancestor [6], but the overall morphology of the last common aculiferan ancestor remains obscure. Here we show that larvae of the aplacophoran Wirenia argentea have several sets of muscles previously known only from polyplacophoran mollusks. Most of these are lost during metamorphosis, and we interpret them as ontogenetic remnants of an ancestor with a complex, polyplacophoran-like musculature. Moreover, we find that the first seven pairs of dorsoventral muscles develop synchronously in Wirenia, similar to juvenile polyplacophorans [7], which supports the conclusions based on the seven-shelled Kulindroplax. Accordingly, we argue that the simple body plan of recent aplacophorans is the result of simplification and does not represent a basal molluscan condition.
Highlights
-
•
Myoanatomy of aplacophoran larvae is much more complex than that of adults
-
•
Homologous polyplacophoran and aplacophoran muscles support the Aculifera hypothesis
-
•
The last aculiferan ancestor had seven shell plates and associated dorsoventral muscles
-
•
The simple body plan of adult aplacophorans evolved by secondary simplification
Results and Discussion
The Aculifera Hypothesis and Molluscan Ancestry
The evolutionary origin of Mollusca has been a matter of long-standing debate. Thereby, reconstruction of the last common ancestor (LCA) to all mollusks, the so-called hypothetical ancestral mollusk, has been hampered by difficulties in recovering fossils of unambiguous molluscan stem species and by the lack of a general agreement concerning the relationships of the various molluscan class-level taxa to each other [2]. As a consequence, a broad consensus concerning the deep nodes in molluscan phylogeny is still lacking. Morphology-based analyses have suggested either one or the other of the sclerite-bearing but shell-less aplacophoran clades (Neomeniomorpha or Chaetodermomorpha, respectively) as the earliest molluscan offshoot [8, 9], a monophyletic Aplacophora as sister group to all remaining mollusks (the Testaria) [10], or a polyplacophoran-aplacophoran assemblage (Aculifera) as sister to all other mollusks with a primarily univalved shell (Conchifera) [11, 12]. This controversy may soon be settled, however, since two phylogenomic studies have independently confirmed the latter concept, whereby both recovered a monophyletic Aplacophora as sister group to Polyplacophora (chitons) within Aculifera [4, 5]. In the light of this phylogenetic framework, the recent description of a cylindrical (i.e., worm-shaped) sclerite- and shell-bearing Paleozoic mollusk [6] and the results of integrative molecular-paleontological studies [13, 14] have been considered as evidence for the presence of seven or eight shell plates in the LCA of crown-group aculiferans. If correct, this implies that the body plan of recent aplacophorans is the result of secondary simplification and thus a derived condition [6, 14].
Despite additional descriptions of fossils that exhibit a mixture of polyplacophoran- and aplacophoran-like features [15, 16], the morphology of the LCA of Aculifera remains elusive. This may be due to the overall paucity of well-preserved Paleozoic fossils that undoubtedly can be assigned to the aculiferan lineage, the uncertainty as to whether or not some early (Cambrian or Precambrian) fossils [17, 18] indeed represent crown- or stem-group mollusks, and the fact that solid morphological and developmental evidence from recent aplacophoran representatives is still largely lacking. The few reports of individual aplacophoran larvae and postlarvae have shown that these animals may bear six or seven rows of papillae, sclerites, or sclerite-secreting cells [19–21], but these studies found no further support by gross morphological developmental studies of two neomeniomorph representatives [22, 23].
Myogenesis Suggests that Aplacophorans Have a Secondarily Simplified Body Plan
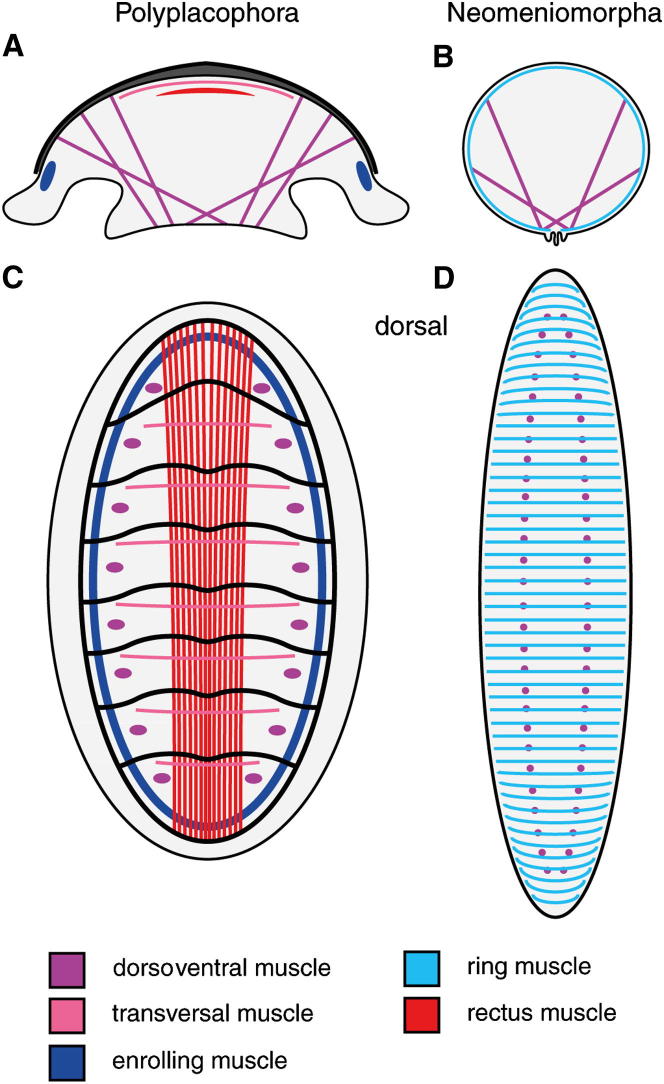
The muscular architecture of mollusks is intimately associated with the existence, number, and arrangement of shells in the respective taxa [24]. In polyplacophorans, the myoanatomy is highly complex [7, 25] (Figures 1A and 1C) and several components, such as a laterally positioned enrolling muscle and a dorsal rectus system that spans the longitudinal axis of the animal, have been widely considered as defining morphological features (autapomorphies) of this taxon [24]. In stark contrast to the sophisticated polyplacophoran myoanatomy, aplacophoran representatives have a much simpler muscular organization that, together with the body wall musculature, mainly comprises serially repeated dorsoventral muscles (Figures 1B and 1D). Accordingly, a scenario that suggests a shell plate-bearing aculiferan LCA with polyplacophoran-like musculature implies drastic secondary simplification of the muscular body plan of aplacophoran mollusks.
Figure 1.
Schematic Representation of Major Muscle Systems of an Adult Polyplacophoran and the Neomeniomorph Aplacophoran Wirenia argentea
Polyplacophoran myoanatomy is highly conserved; thus, the schemes serve as a generalized model for the entire class. Note that in some neomeniomorphs (but not in Wirenia), distinct enrolling muscles are present. Individual muscle units are indicated by color code, with homologous muscles sharing the same color. The body wall musculature (light blue) of Wirenia in (B) and (D), which comprises ring, diagonal, and longitudinal muscles, is represented by only the ring musculature for clarity. The dorsoventral musculature (purple) is depicted as dots to indicate its major dorsal insertion sites in (C) and (D).
(A) Leptochiton asellus, cross-section.
(B) Wirenia argentea, cross-section.
(C) Leptochiton asellus, dorsal view.
(D) Wirenia argentea, dorsal view.
Since ontogenetic data may provide important insights into the evolutionary history of a given taxon [26, 27], we investigated the development of a model neomeniomorph aplacophoran, Wirenia argentea, from hatching of the larvae until after metamorphosis. In comparing myogenesis in Wirenia with that of a polyplacophoran (Leptochiton asellus), we found striking similarities in the muscular organization of both species (see Table 1 for a summary of major larval and adult muscle systems known for the various molluscan lineages), including the presence of a rectus muscle (Figures 2A–2D), so far only known from polyplacophorans, and a laterally positioned enrolling muscle (Figures 2A–2H). Both systems are retained in adult polyplacophorans but are lost during Wirenia postlarval development (note that some neomeniomorphs do exhibit distinct enrolling muscles as adults [28, 29]). Although homology between the polyplacophoran and neomeniomorph enrolling muscles has been questioned [7], their similar position in the respective animals and, in particular, their identical mode of ontogenetic formation as independent muscle system (and not as a thickened derivative of the body wall musculature, as proposed earlier for the aplacophoran taxa [7]) argue strongly for their common evolutionary origin and thus for the presence of such a system in the LCA of both clades.
Table 1.
Molluscan Larval and Adult Muscle Systems
| Polyplacophora | Neomeniomorpha | Chaetodermomorpha | Monoplacophora | Bivalvia | Gastropoda | Scaphopoda | Cephalopoda | ||
|---|---|---|---|---|---|---|---|---|---|
| Larva |
∗Enrolling muscle | + | + | lateral longitudinal muscle? | ? | – | – | – | NA |
| ∗Rectus muscle | + | + | ? | ? | – | – | – | NA | |
| ∗Ventrolateral muscle | + | + | lateral longitudinal muscle? | ? | – | – | – | NA | |
| ∗Ventromedian muscle | + | + | ? | ? | – | – | – | NA | |
| ∗Ring musculature (as element of body wall musculature) | + | + | + | ? | – | – | – | NA | |
| Transversal musculature | + | – | ? | ? | – | – | – | NA | |
| Prototroch muscle ring | + | + | + | ? | + | + | – | NA | |
| Number of sets of dorsoventral muscles | multiple | 7 | ? | ? | 3–8 (?) | 1 | 1–2 | NA | |
| Adult | Enrolling muscle | + | +/– | – | – | – | – | – | – |
| Rectus muscle | + | – | – | – | – | – | – | – | |
| Ring musculature (as element of body wall musculature) | – | + | + | – | – | – | – | – | |
| Transversal musculature | + | – | – | – | – | – | – | – | |
| Number of sets of dorsoventral muscles | 7→8 | multiple | multiple (anteriorly only) or missing | 8 | 3–8 | 1 | 1–2 | 1 | |
Asterisks indicate potential synapomorphies of [Polyplacophora + Neomeniomorpha] or the entire Aculifera. +, present; −, absent; NA, not applicable. Note that in polyplacophorans, seven shell plates are formed simultaneously at first; an eighth forms considerably later in development, after metamorphosis. Note also that cephalopods have direct development and therefore lack molluscan-specific larvae.
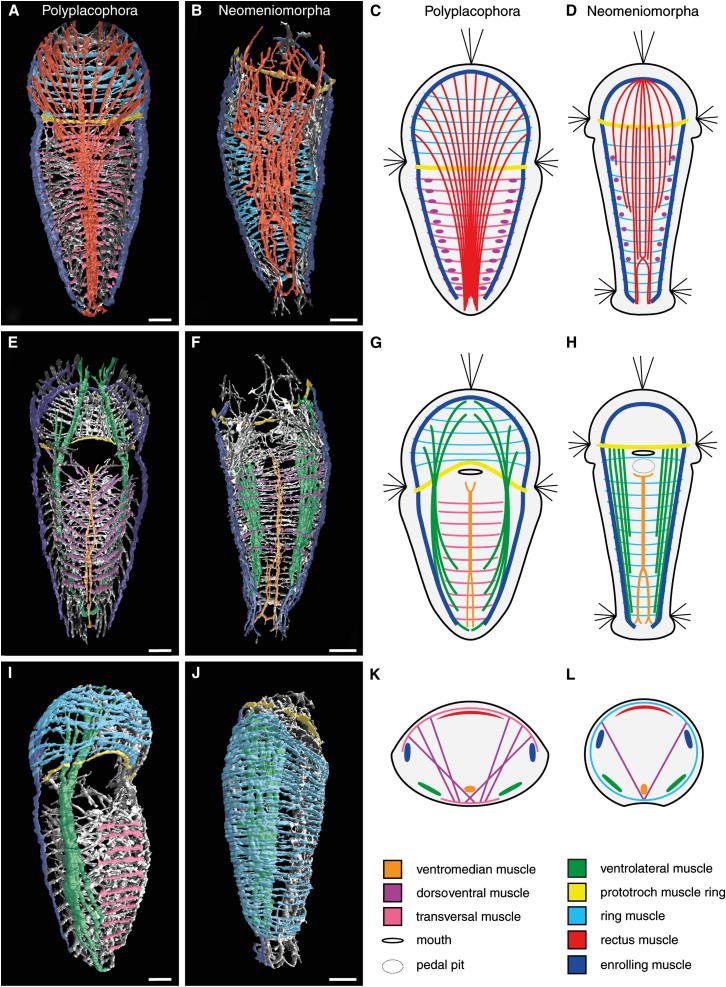
Figure 2.
Myoanatomy of Late-Stage Larvae of the Polyplacophoran Leptochiton asellus and the Neomeniomorph Wirenia argentea
Major homologous muscle units are indicated by shared color code. Scale bars represent 20 μm. The dorsoventral muscles (violet) are easily identified in (E) and (F) since they overlie the ventrolateral muscles (green) due to their more dorsally situated insertion sites. They intercross in the region of the ventromedian muscle (orange) and are depicted as dots to indicate their major dorsal insertion sites in (C) and (D). (A), (B), (E), (F), (I), and (J) are 3D reconstructions based on confocal microscopy data sets. (C), (D), (G), (H), (K), and (L) are schematic representations. For better visualization of individual muscle systems, only ventral or dorsal halves of larvae with section plane in the region of the enrolling muscle are shown in (A)–(H).
(A) Leptochiton asellus, dorsal muscle systems seen from ventral.
(B) Wirenia argentea, dorsal muscle systems seen from ventral.
(C) Leptochiton asellus, dorsal muscle systems seen from ventral.
(D) Wirenia argentea, dorsal muscle systems seen from ventral.
(E) Leptochiton asellus, ventral muscle systems seen from dorsal.
(F) Wirenia argentea, ventral muscle systems seen from dorsal.
(G) Leptochiton asellus, ventral muscle systems seen from dorsal.
(H) Wirenia argentea, ventral muscle systems seen from dorsal.
(I) Leptochiton asellus, ventrolateral right view. Note that the rudimentary body wall musculature is restricted to the anterior body region.
(J) Wirenia argentea, ventrolateral right view.
(K) Leptochiton asellus, cross-section with all muscle systems identified herein. Note that transversal muscles (pink) are found in the dorsal and ventral region. It remains unclear whether or not these have evolved from body wall ring muscles [24].
(L) Wirenia argentea, cross-section with all muscle systems identified herein.
Aside from these muscles, we found two additional muscular systems shared only by polyplacophorans and neomeniomorphs. These are a paired ventrolateral and a single ventromedian muscle (Figures 2E–2L). Both systems are only transiently present in advanced larvae, and neither has a counterpart in either the polyplacophoran or the neomeniomorph adult body plan. As with the rectus and the enrolling muscles, the identical positions of the ventrolateral and the ventromedian muscles in Wirenia and Leptochiton larvae, together with their identical positions relative to other muscles, clearly suggest that these respective muscles are homologous between the two species (see [30] for a recent overview on homology theory and assessment). Earlier, we had already found the ventrolateral system in larvae of another polyplacophoran, Mopalia muscosa [7], but had overlooked the ventromedian muscle. Reinvestigation of the original 3D data set, however, unambiguously revealed such a muscle also in Mopalia (data not shown; available on request). Accordingly, it appears highly likely that the ventrolateral and the ventromedian muscles together with the enrolling and the rectus muscle were all part of the muscular toolkit of the LCA of polyplacophorans and neomeniomorphs, and that the simple myoanatomy of adult Wirenia is a derived, secondary condition.
Formation of the eight sets of dorsoventral muscles in polyplacophorans passes through a transitory stage of multiple individual myocytes that appear synchronously [7] (Figure 2E) and give rise to the first seven paired shell muscle units (while the eighth set forms considerably later, together with the most posterior shell plate [7]). In Wirenia, seven pairs of dorsoventral muscles develop simultaneously (Figures 3A–3E) and differentiate further in later stages (Figure 2F). A gradual numerical increase of the dorsoventral muscle sets was observed only after metamorphosis (Figure 3F). Accordingly, both neomeniomorphs and polyplacophorans exhibit a transient stage of seven-fold seriality in the arrangement of these muscles. Despite the different ontogenetic pathways that lead to this seven-fold seriality (fusion of multiple myocytes in polyplacophorans versus simultaneous formation in Wirenia), this seven-fold seriality appears to be a reoccurring pattern, at least in aculiferan mollusks (unfortunately, the ontogenetic sequence of the formation of the eight pairs of dorsoventral muscles in Monoplacophora is still unknown). This is well in line with the description of the seven-shelled fossil Kulindroplax [6], as well as with the serially arranged sclerites or papillae of some recent aplacophorans [19–21], and lends further support for an aculiferan LCA with a seven-fold seriality of epidermal hardparts and the associated musculature. This implies that the eighth set of dorsoventral muscles of recent polyplacophorans is a derived condition. The late formation of the most posterior shell plate and associated musculature in polyplacophorans [7, 31] may well be considered as ontogenetic testimony of such a scenario.
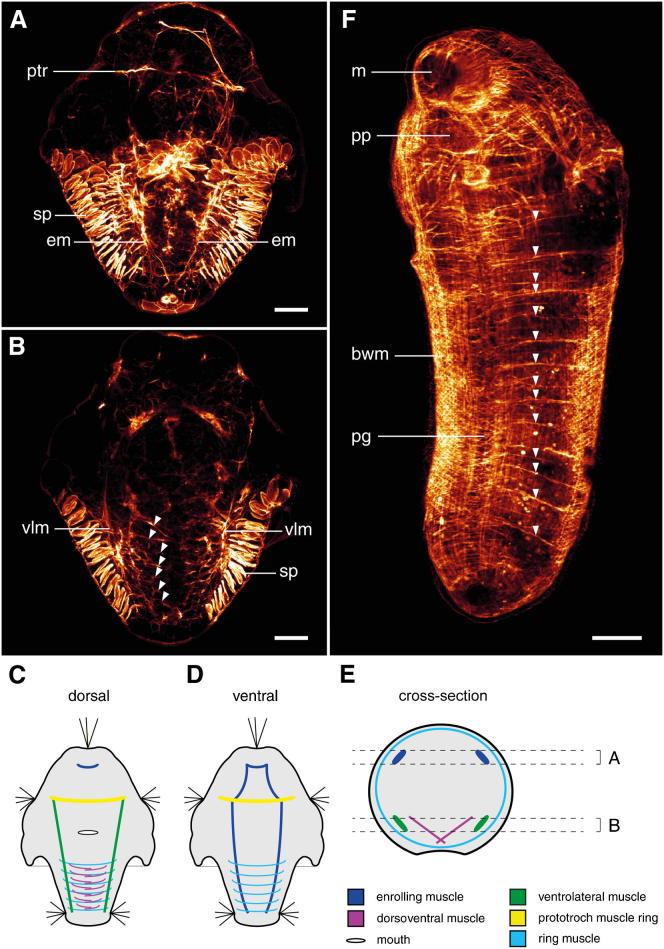
Figure 3.
Myoanatomy of an Early Larva and an Early Postmetamorphic Individual of Wirenia argentea
Scale bars represent 20 μm in (A) and (B) and 100 μm in (F). Apical is up in all panels except (E), which is a cross-section. (A), (B), and (F) are maximum-intensity (MI) z projections of confocal image stacks. For better visualization of the individual muscle systems, only the regions indicated in (E) were used in the MI z projections shown in (A) and (B). (C)–(E) are schematic representations of the musculature in the early-stage larva.
(A) Dorsal third of the larva with early anlage of the prototroch muscle ring (ptr) and enrolling muscle (em). sp, unspecific signal from epidermal spicule-secreting cells.
(B) Ventral region with the paired ventrolateral muscle (vlm) and the first seven pairs of myocytes (arrowheads) of the dorsoventral musculature.
(C) Dorsally positioned musculature seen from ventral.
(D) Ventrally positioned musculature seen from dorsal.
(E) Cross-section with all muscle systems of the early larva.
(F) Ventrolateral view of a postmetamorphic individual caught in the field, showing the body wall musculature (bwm) and the multiple sets of dorsoventral muscles (arrowheads), as well as the mouth (m), pedal pit (pp), and pedal groove (pg).
The rudimentary data on myogenesis (and development in general) [21] of the second aplacophoran taxon, the Chaetodermomorpha, does not allow for definite conclusions concerning the presence of neomeniomorph- and/or polyplacophoran-like features such as the rectus or the ventromedian muscles in the LCA of this taxon. However, the ring musculature in the body wall of both aplacophoran clades, which is also rudimentarily present in the apical region of polyplacophoran larvae [7] (Figure 2), may constitute an aculiferan apomorphy uniting Polyplacophora, Neomeniomorpha, and Chaetodermomorpha (Table 1). This, together with the cylindrical shape of the aplacophorans and the polyplacophoran larva as well as the fossil Kulindroplax, argues for a worm-like body shape of the LCA of Aculifera, rendering the dorsoventrally flattened appearance of recent polyplacophorans a derived condition. The paired lateral longitudinal muscle in the larva of the chaetodermomorph Chaetoderma [21] may correspond to either the enrolling or the ventrolateral muscle of larval polyplacophorans and neomeniomorphs (Table 1), which would further support the inclusion of Chaetodermomorpha within Aculifera. The fact that Kulindroplax shares morphological features not only with polyplacophorans but also with recent chaetodermomorphs, including the absence of a pedal pit and the position of the gills [6], likewise supports such a scenario.
Whether or not a monophyletic Aculifera will stand the test of future phylogenetic analyses or whether Chaetodermomorpha, despite these shared morphological characters, will be proven to have different affinities [32] remains to be seen. The transient expression of typical polyplacophoran-like muscles in the Wirenia larva, however, strongly suggests that at least neomeniomorph aplacophorans stem from an ancestor with polyplacophoran-like features that most likely also included seven shell plates.
Acknowledgments
We thank Henrik Glenner (Department of Biology, University of Bergen) for providing boat time, laboratory space, and logistic support. We are grateful to the crew of the RV Hans Brattström (University of Bergen) for assistance with collection of animals. Thomas Schwaha and Alen Kristof (University of Vienna) provided valuable advice on confocal microscopy and 3D applications. This work was supported by grant number P24276-B22 from the FWF (Austrian Science Fund) to A.W.
Published: October 17, 2013
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplemental Information includes Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2013.08.056.
Supplemental Information
References
- 1.Ponder W.F., Lindberg D.R., editors. Phylogeny and Evolution of the Mollusca. University of California Press; Berkeley: 2008. [Google Scholar]
- 2.Haszprunar G., Wanninger A. Molluscs. Curr. Biol. 2012;22:R510–R514. doi: 10.1016/j.cub.2012.05.039. [DOI] [PubMed] [Google Scholar]
- 3.Todt C. Aplacophoran mollusks—Still obscure and difficult? Am. Malacol. Bull. 2013;31:181–187. [Google Scholar]
- 4.Kocot K.M., Cannon J.T., Todt C., Citarella M.R., Kohn A.B., Meyer A., Santos S.R., Schander C., Moroz L.L., Lieb B., Halanych K.M. Phylogenomics reveals deep molluscan relationships. Nature. 2011;477:452–456. doi: 10.1038/nature10382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith S.A., Wilson N.G., Goetz F.E., Feehery C., Andrade S.C.S., Rouse G.W., Giribet G., Dunn C.W. Resolving the evolutionary relationships of molluscs with phylogenomic tools. Nature. 2011;480:364–367. doi: 10.1038/nature10526. [DOI] [PubMed] [Google Scholar]
- 6.Sutton M.D., Briggs D.E.G., Siveter D.J., Siveter D.J., Sigwart J.D. A Silurian armoured aplacophoran and implications for molluscan phylogeny. Nature. 2012;490:94–97. doi: 10.1038/nature11328. [DOI] [PubMed] [Google Scholar]
- 7.Wanninger A., Haszprunar G. Chiton myogenesis: perspectives for the development and evolution of larval and adult muscle systems in molluscs. J. Morphol. 2002;251:103–113. doi: 10.1002/jmor.1077. [DOI] [PubMed] [Google Scholar]
- 8.Salvini-Plawen L.v., Steiner G. Synapomorphies and plesiomorphies in higher classification of Mollusca. In: Taylor J.D., editor. Origin and Evolutionary Radiation of the Mollusca. Oxford University Press; Oxford: 1996. pp. 29–51. [Google Scholar]
- 9.Haszprunar G. Is the Aplacophora monophyletic? A cladistic point of view. Am. Malacol. Bull. 2000;15:115–130. [Google Scholar]
- 10.Waller T.R. Origin of the molluscan class Bivalvia and a phylogeny of major groups. In: Johnston P.A., Haggart J.W., editors. Bivalves: An Eon of Evolution. University of Calgary Press; Calgary: 1998. pp. 1–45. [Google Scholar]
- 11.Scheltema A.H. Aplacophora as progenetic aculiferans and the coelomate origin of mollusks as the sister taxon of Sipuncula. Biol. Bull. 1993;184:57–78. doi: 10.2307/1542380. [DOI] [PubMed] [Google Scholar]
- 12.Ivanov D.L. Origin of Aculifera and problems of monophyly of higher taxa in molluscs. In: Taylor J.D., editor. Origin and Evolutionary Radiation of the Mollusca. Oxford University Press; Oxford: 1996. pp. 59–65. [Google Scholar]
- 13.Vinther J., Sperling E.A., Briggs D.E.G., Peterson K.J. A molecular palaeobiological hypothesis for the origin of aplacophoran molluscs and their derivation from chiton-like ancestors. Proc. Biol. Sci. 2012;279:1259–1268. doi: 10.1098/rspb.2011.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vinther J., Jell P., Kampouris G., Carney R., Racicot R.A., Briggs D.E.G. The origin of multiplacophorans—convergent evolution in aculiferan molluscs. Palaeontology. 2012;55:1007–1019. [Google Scholar]
- 15.Sutton M.D., Briggs D.E.G., Siveter D.J., Siveter D.J. An exceptionally preserved vermiform mollusc from the Silurian of England. Nature. 2001;410:461–463. doi: 10.1038/35068549. [DOI] [PubMed] [Google Scholar]
- 16.Sutton M.D., Sigwart J.D. A chiton without a foot. Palaeontology. 2012;55:401–411. [Google Scholar]
- 17.Fedonkin M.A., Waggoner B.M. The Late Precambrian fossil Kimberella is a mollusc-like bilaterian organism. Nature. 1997;388:868–871. [Google Scholar]
- 18.Caron J.-B., Scheltema A., Schander C., Rudkin D. A soft-bodied mollusc with radula from the Middle Cambrian Burgess Shale. Nature. 2006;442:159–163. doi: 10.1038/nature04894. [DOI] [PubMed] [Google Scholar]
- 19.Pruvot G. Sur le développement d’un Solénogastre. C. R. Acad. Sci. Paris. 1890;111:689–692. [Google Scholar]
- 20.Scheltema A.H., Ivanov D.L. An aplacophoran postlarva with iterated dorsal groups of spicules and skeletal similarities to Paleozoic fossils. Invertebr. Biol. 2002;121:1–10. [Google Scholar]
- 21.Nielsen C., Haszprunar G., Ruthensteiner B., Wanninger A. Early development of the aplacophoran mollusc Chaetoderma. Acta Zool. 2007;88:231–247. [Google Scholar]
- 22.Okusu A. Embryogenesis and development of Epimenia babai (Mollusca Neomeniomorpha) Biol. Bull. 2002;203:87–103. doi: 10.2307/1543461. [DOI] [PubMed] [Google Scholar]
- 23.Todt C., Wanninger A. Of tests, trochs, shells, and spicules: Development of the basal mollusk Wirenia argentea (Solenogastres) and its bearing on the evolution of trochozoan larval key features. Front. Zool. 2010;7:6. doi: 10.1186/1742-9994-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haszprunar G., Wanninger A. Molluscan muscle systems in development and evolution. J. Zool. Syst. Evol. Res. 2000;38:157–163. [Google Scholar]
- 25.Wingstrand K.G. On the anatomy and relationships of recent Monoplacophora. Galathea Rep. 1985;16:7–94. [Google Scholar]
- 26.Haeckel E. Engelmann; Leipzig: 1874. Anthropogenie. [Google Scholar]
- 27.Kristof A., Wollesen T., Wanninger A. Segmental mode of neural patterning in sipuncula. Curr. Biol. 2008;18:1129–1132. doi: 10.1016/j.cub.2008.06.066. [DOI] [PubMed] [Google Scholar]
- 28.Hoffman S. Studien über das Integument der Solenogastren nebst Bemerkungen über die Verwandtschaft zwischen den Solenogastren und Placophoren. Zool. Bidrag Uppsala. 1949;27:293–427. [Google Scholar]
- 29.Scheltema A.H., Tscherkassy M., Kuzirian A.M. Aplacophora. In: Harrison F.W., Kohn A.J., editors. Microscopic Anatomy of Invertebrates, Volume 5: Mollusca I. Wiley; New York: 1994. pp. 13–54. [Google Scholar]
- 30.Minelli A., Fusco G. Homology. In: Kampourakis K., editor. The Philosophy of Biology: A Companion for Educators (History, Philosophy and Theory of the Life Sciences, Volume 1) Springer; Dordrecht: 2013. pp. 289–322. [Google Scholar]
- 31.Jacobs D.K., Wray C.G., Wedeen C.J., Kostriken R., DeSalle R., Staton J.L., Gates R.D., Lindberg D.R. Molluscan engrailed expression, serial organization, and shell evolution. Evol. Dev. 2000;2:340–347. doi: 10.1046/j.1525-142x.2000.00077.x. [DOI] [PubMed] [Google Scholar]
- 32.Giribet G., Okusu A., Lindgren A.R., Huff S.W., Schrödl M., Nishiguchi M.K. Evidence for a clade composed of molluscs with serially repeated structures: monoplacophorans are related to chitons. Proc. Natl. Acad. Sci. USA. 2006;103:7723–7728. doi: 10.1073/pnas.0602578103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.