Abstract
Chronic kidney disease is a major public health problem globally. Despite this, there are fewer high-quality, high-impact clinical trials in nephrology than other internal medicine specialties, which has led to large gaps in evidence. To address this deficiency, the Australasian Kidney Trials Network, a Collaborative Research Group, was formed in 2005. Since then, the Network has provided infrastructure and expertise to conduct patient-focused high-quality, investigator-initiated clinical trials in nephrology. The Network has not only been successful in engaging the nephrology community in Australia and New Zealand but also in forming collaborations with leading researchers from other countries. This article describes the establishment, development, and functions of the Network. The article also discusses the current and future funding strategies to ensure uninterrupted conduct of much needed clinical trials in nephrology to improve the outcomes of patients affected by kidney diseases with cost-effective interventions.
Keywords: chronic kidney disease, clinical trials, collaborative research, health policy, investigator initiated, randomized controlled trial
Chronic kidney disease (CKD) is a major public health problem in Australia and throughout the world.1 The prevalence of CKD has been estimated at between 10 and 15% in industrialized countries.2, 3, 4 End-stage kidney disease (ESKD) consumes disproportionate health-care resources. In 2011–2012, the Commonwealth of Australia spent 1.6% of its annual health-care budget to provide treatment to patients with ESKD, who constitute <0.1% of the entire population.5 The disparate spending of government funds on ESKD care is mirrored in other developed countries. Annually, the United States spends 6% of its Medicare budget on its ESKD program, despite the Medicare ESKD population representing <0.2% of the country's population,6 whereas Taiwan, another country with a comparatively high prevalence of ESKD (0.24%),7 spends 7.7% of its annual health-care budget on the provision of dialysis.8 The presence of CKD is one of the most potent risk factors for cardiovascular disease. CKD and ESKD are associated with substantially increased risk of all-cause and cardiovascular mortality and poor quality of life.9
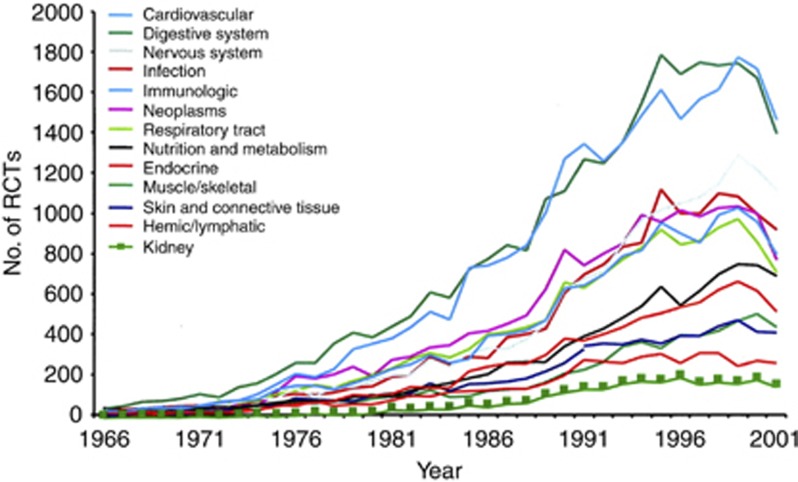
Despite the burden of morbidity and mortality and high health-care costs associated with CKD, the overall number of published clinical trials in nephrology is the lowest of any internal medicine specialty (Figure 1).10, 11 Moreover, the quality and coverage of renal clinical trials lag behind other medical fields.12 Many industry-sponsored trials routinely exclude patients with significant CKD to maintain a homogeneous study population and to exclude a potential confounding effect of CKD on renally excreted agents; those that do include CKD patients are often designed to address commercial imperatives rather than clinically important questions. This contributes to the under-representation of CKD patients in commercial trials, and has the implication that outcomes from such trials cannot easily be extrapolated to a CKD population. Such questions are usually answered by investigator-initiated trials, which, because of funding constraints and limited infrastructural support, are frequently small, short-duration studies limited to one or a few centers. A recent evaluation of interventional trials registered on ClinicalTrials.gov between 2007 and 2010 found that 50% of studies enrolled fewer than 70 participants, raising significant questions around their ability to establish conclusively the safety and effectiveness of treatments and enable better decision-making in clinical practice.13 These factors led the peak nephrology body in Australasia to recognize the need for infrastructure to support investigator-initiated trials to address clinically significant issues specific to a CKD population.
Figure 1.
Number of randomized controlled trials (RCT) published in nephrology and 12 other specialties of internal medicine from 1966 to 2002. Reproduced from J Am Soc Nephrol with permission.10
This article provides an overview of the establishment, development, and functions of the Australasian Kidney Trials Network (AKTN), a clinical trials network in nephrology involving centers from Australia and New Zealand.
ESTABLISHMENT OF THE AKTN
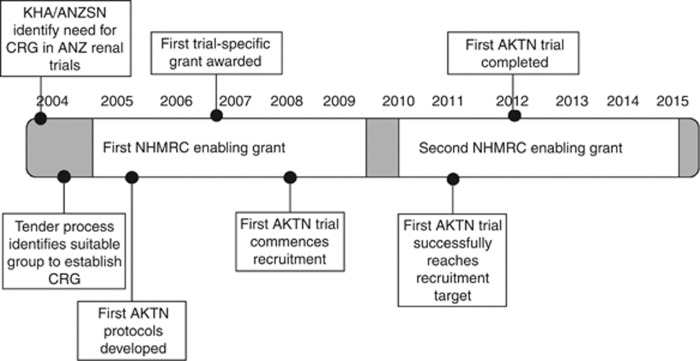
The Initiating Dialysis Early and Late study14 was conducted across 32 centers in Australia and New Zealand and was the first large-scale, investigator-initiated collaborative clinical research effort in Australasia in nephrology. A subsequent cost-effectiveness evaluation involving Initiating Dialysis Early and Late study participants revealed important cost-saving information relating to delaying dialysis commencement, which confirms that well-conducted clinical trials can provide significant economic benefits to research-active institutions.9 At the time of trial inception, limited infrastructure existed to support Australasian nephrologists in the conduct of clinical trials. To address the dearth of clinical studies in nephrology, to ensure that the lessons learnt and momentum generated by the success of the Initiating Dialysis Early and Late trial were not lost, and to support research with potentially cost-saving outcomes, the Australian and New Zealand Society of Nephrology (ANZSN) and Kidney Health Australia identified the need for an Australasian-based Collaborative Research Group (CRG) to drive investigator-initiated clinical trials in the region. A call for expressions of interest was circulated via the ANZSN, inviting clinical and academic nephrologists and public health researchers to become involved in establishing the Network. Utilizing seed funding provided by these groups, and advice and assistance from the Australian and New Zealand Intensive Care Society, the George Institute for Global Heath, and the National Health and Medical Research Council Clinical Trials Center, the AKTN (the ‘Network') was established in 2005 to address questions of clinical importance to renal patients and nephrologists. Key opinion leaders from across Australia and New Zealand were invited through their ANZSN membership and engaged to form the inaugural Scientific Committee and Operations Secretariat, whose roles were to identify clinical research priorities of the renal community, secure funding for the AKTN, and engage renal clinicians in trial participation. Engagement was to occur primarily via distribution of expressions of interest to the ANZSN membership. In 2005, a National Health and Medical Research Council enabling grant was able to be successfully leveraged from initial seed funding, allowing the first AKTN clinical trial protocols to be developed and the AKTN website to be published to provide the community access to AKTN information and resources. This grant also enabled core AKTN staff to be engaged, specifically an Operations Manager to run daily financial, contractual, and personnel matters, a Project Manager to advance protocol development, a Biostatistician, and an Executive Support Officer. Additional trial-specific staff (Clinical Research Associates, Biostatisticians and Data Managers) were engaged using trial-specific funding. Important milestones for the AKTN are depicted in the timeline shown in Figure 2.
Figure 2.
Important AKTN milestones. AKTN, Australasian Kidney Trials Network; ANZSN, Australian and New Zealand Society of Nephrology; CRG, Collaborative Research Group; KHA, Kidney Health Australia; NHMRC, National Health and Medical Research Council.
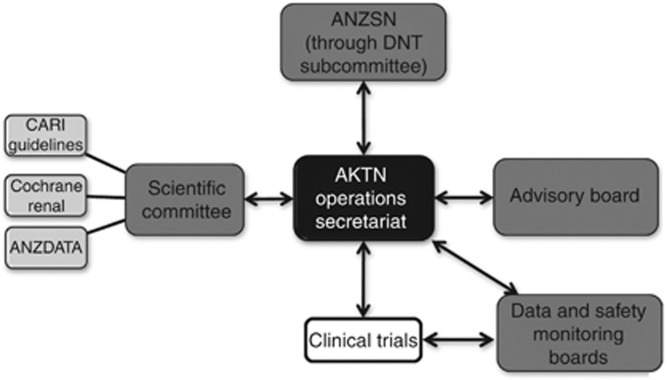
The AKTN governance structure is depicted in Figure 3. The Operations Secretariat manages the daily activities of the Network. Supporting the Operations Secretariat is a Scientific Committee comprising nephrologists, renal nurses, renal dietitians, biostatisticians, a public health economist, and a patient with kidney disease. The Scientific Committee's primary responsibility is to ensure that Network trials maintain a high level of scientific and clinical merit and are representative of the priorities of the nephrological and kidney patient community. Further supporting the Operations Secretariat is an independent Advisory Board that provides high-level advice on the strategic direction of the Network, including funding, success measures, education, and overall Network progress.
Figure 3.
AKTN governance and infrastructure. AKTN, Australasian Kidney Trials Network; ANZDATA, Australia and New Zealand Dialysis and Transplant Registry; ANZSN, Australian and New Zealand Society of Nephrology; CARI, Caring for Australasians with Renal Impairment; DNT, Dialysis Nephrology Transplantation.
AKTN TRIAL DEVELOPMENT: FROM PROPOSAL TO PUBLICATION
The AKTN provides support to clinician researchers with a clinical trial concept but without the experience or resources to implement it. Without the AKTN, early career or experienced researchers may not be in a position to instigate clinical trials in nephrology; by providing a central hub from which trials can be coordinated, the Network offers a more streamlined approach to renal trials. The services offered to researchers are given in Table 1.
Table 1. Services offered by the AKTN.
| Before funding procurement | For funded trials |
|---|---|
| Scientific peer review of trial proposal | Protocol development and amendments |
| Trial design | Trial coordination |
| Protocol development | Financial management |
| Identification of funding sources | Contract negotiations |
| Assistance with funding applications | Site management |
| Access to AKTN Scientific Committee | Ethics and governance documentation and correspondence |
| Assistance with statistical analysis plans | Investigational product procurement and management |
| Development of trial resources | |
| Regulatory reporting | |
| Safety reporting | |
| Trial promotion | |
| Data management | |
| Statistical analysis | |
| Data and safety monitoring | |
| Manuscript preparation and submission |
Abbreviation: AKTN, Australasian Kidney Trials Network.
ACHIEVEMENTS OF THE AKTN
The formation of the AKTN has successfully addressed previously unmet research needs of the renal community by appreciably improving clinical trial support, education, quality, funding, and output (Table 2).
Table 2. Achievements of the AKTN.
| Category | Benefit |
|---|---|
| Support | Delivered infrastructure and governance required to conduct investigator-initiated trials |
| Promoted research collaborations | |
| Enabled the effective organization of research priorities | |
| Quality | Improved the quality of investigator-initiated research in the region |
| Funding | Increased funding opportunities |
| Education | Promoted renal research in the region |
| Provided educational and career opportunities in renal clinical trials |
Abbreviation: AKTN, Australasian Kidney Trials Network.
Augmented infrastructure to support research
The Network has accrued a critical mass of trials expertise. Access to these resources has provided investigators with the tools to develop, conduct, monitor, analyze, and report on a clinical trial. The Network has also facilitated the formation of Trial Management Committees and Data and Safety Monitoring Boards.
Increased collaboration
It has been proposed that a number of the barriers to successful completion of clinical trials can be overcome by multicenter collaboration.10 The AKTN has successfully brought together renal units from Australia and New Zealand, and has had recent success in partnering with centers in Malaysia and the United Kingdom, owing to similar research priorities with collaborators in these countries. In mid-2012, 60 renal units were participating in at least one AKTN trial, including 45 in Australia and New Zealand. Furthermore, the AKTN has been invited to engage Australian and New Zealand sites in four large, multinational clinical trials by local or overseas-based CRGs (see Table 3). International research groups collaborating on large-scale clinical trials have expanded the patient pool available, thereby increasing the likelihood of achieving recruitment success and trial completion. This has the additional advantage of enhancing the heterogeneity of the trial population, which can increase the generalizability and external validity of trial results.15 The use of AKTN links to international CRGs and to national and international experts in other specialties has also provided access to international experts in the field of nephrology, cardiology, statistics, pharmacology, dietetics, nursing, health economics, and clinical trial conduct for membership on trial Data and Safety Monitoring Boards and Trial Management Committees. Collaboration with local academic groups, The George Institute for Global Health and the National Health and Medical Research Council Clinical Trials Center, has contributed substantially to the growth of the Network in its early years. Increased academic collaboration will enhance the AKTN's future ability to conduct high-quality, high-impact clinical trials.
Table 3. AKTN clinical trials: coordinated, facilitated, and endorsed.
| Trial acronym | ANZCTR number | Support type | Trial status |
|---|---|---|---|
| FAVOURED: Fish oil and Aspirin in Vascular access OUtcomes in REnal Disease | 12607000569404 | Coordinated | Recruiting |
| HONEYPOT: HOney versus Nasal Eradication of staphYlococci for the Prevention Of Tenckhoff infections in PD | 12607000537459 | Coordinated | Completed |
| HERO: Handling Erythropoietin-Resistance with Oxpentifylline | 12608000199314 | Coordinated | Completed |
| ACCORD: Assessment of Carnitine for Clinical Outcomes in Renal Disease | 12609000024246 | Endorsed | Completed |
| BLOCADE: Beta-blocker to LOwer CArdiovascular Dialysis Events | 12609000174280 | Coordinated | Ongoing |
| PEXIVAS: Plasma exchange and glucocorticoids in anti-neutrophil cytoplasm antibody associated systemic vasculitis | Clinicaltrials.gov no.: NCT00987389 | Facilitated | Recruiting |
| ACTIVE: A Clinical Trial of IntensiVE Dialysis | Clinicaltrials.gov no.: NCT00649298 | Endorsed | Ongoing |
| IMPROVE-CKD: IMpact of Phosphate Reduction On Vascular End points in Chronic Kidney Disease | 12610000650099 | Coordinated | Recruiting |
| ACEi: Ace inhibition for the preservation of renal function and patient survival in kidney transplantation | Controlled-trials.com no.: MCT-78844 | Facilitated | Ongoing |
| SOLID: The SOdium Lowering In Dialysate Trial | 12611000975998 | Endorsed | Recruiting |
| TESTING: Therapeutic Evaluation of Steroids in IgA Nephropathy Global Study | Clinicaltrials.gov no.: NCT01560052 | Endorsed | Recruiting |
| CKD-FIX: a randomized Controlled trial of slowing of Kidney Disease progression From the Inhibition of Xanthine oxidase | 12611000791932 | Coordinated | Recruiting |
| TransDiab: A randomized controlled trial of metformin versus gliclizide in overt diabetes and metformin versus placebo in glucose intolerance in the treatment of NODAT: Feasibility study | TBA | Coordinated | In development |
| Blockade of uremic toxins in prevention of Heart Failure and CKD | TBA | Endorsed | In development |
Abbreviations: AKTN, Australasian Kidney Trials Network; ANZCTR, Australian New Zealand Clinical Trials Registry; PD, peritoneal dialysis.
Establishment of clinical research priorities
Clinical research priorities for the AKTN have been determined via a combination of (a) evaluation of surveys of all AKTN clinician and health consumer members; (b) development of special interest groups in renal transplantation, hemodialysis, peritoneal dialysis, and chronic kidney disease; (c) recommendations of the Caring for Australasians with Renal Impairment (CARI) Guidelines Writing Groups; and (d) dialog with the Cochrane Renal Group to identify important gaps in evidence. In conjunction with the broader renal community, the AKTN Scientific Committee has established key research priorities to be addressed by investigator-initiated trials.
Expanded educational and career opportunities for renal clinician scientists
As a medical specialty, nephrology is experiencing a workforce shortage in Australasia.16 A survey conducted across Australian physician trainees in 2009 identified barriers to medical trainees pursuing nephrology as a career choice, and suggested that engaging trainees' research interests may demystify renal medicine and increase uptake.17 The AKTN has provided significant educational opportunities to the nephrology community, particularly targeting Early Career Researchers with an interest in clinical trial design, conduct, and the tenets of good clinical practice. Furthermore, opportunities for participation in clinical trials have been provided by the AKTN through encouragement of Early Career Researchers as trial principal investigators and Trial Management Committee members, and the provision of scholarships for PhD and Master degree candidates.
Enhanced clinical trial quality
Samuels and Molony18 outlined the importance of maintaining high standards in several features of clinical trial design and conduct. The AKTN has provided clinician investigators access to biostatisticians with proven expertise and specialist training in clinical trial design and analysis. In addition, all AKTN trials are conducted with full International Conference on Harmonization Good Clinical Practice (ICHGCP) guideline compliance, AKTN staff are accredited with the Association of Clinical Research Professionals, and each trial has been assigned an independent Data and Safety Monitoring Board to ensure ongoing data quality and participant safety. The provision of educational opportunities to clinician investigators, combined with AKTN's rigorous standards and operating procedures, ensures that investigator-initiated renal trials in the region are conducted ethically and deliver high-quality data.
Increased funding opportunities
Lack of funding availability is the main reason research studies do not proceed to commencement.19 Furthermore, research studies that attract external, competitive grants are more likely to achieve publication of their primary results.20, 21 By providing resources necessary for competitive grant application submission to major funding bodies, the AKTN increases the likelihood of a trial's commencement, completion, and subsequent publication, thereby contributing to the body of clinical trial evidence in the renal specialty and increasing the opportunity to impact on patient care. Through its national and global cooperative associations, the AKTN provides opportunities for linking international and local researchers on trial management teams, thereby strengthening funding proposals and boosting the track record of collaborators.
A National Health and Medical Research Council enabling grant was successfully leveraged by the AKTN in 2005 following initial seed funding provided by Kidney Health Australia and ANZSN. Demonstrated network success in key performance areas, as well as established in-kind support from the University of Queensland, Queensland Health, and the George Institute for Global Health, led to a second National Health and Medical Research Council enabling grant being awarded in 2010, indicating the importance placed on the renal clinical trial network by the federal government funding agency.
Increased renal clinical trial output
Since 2005, 23 clinical trial proposals have been presented to the AKTN for consideration of coordination, facilitation, or endorsement, and have undergone review for scientific quality, clinical merit, and statistical rigor. Of those, seven have been approved for coordination, two for facilitation (the AKTN runs the regional arm of an international study), and five for endorsement (the AKTN provides support/advice but does not coordinate the trial) (Table 3). All nine coordinated or facilitated trials are multicenter in nature, and would likely not have been initiated without the proposer's access to network facilities. Completion of the first two AKTN trials occurred in 2012.
It has been a priority of the AKTN to publish manuscripts in the areas of clinical trial research results, statistical analysis plans, trial protocols, systematic reviews, meta-analyses, and research methodologies. Three trial protocols,22, 23, 24 three systematic reviews,25, 26, 27 two research methodology papers,28, 29 and one letter to the editor30 have been published based on AKTN research activities; a further four manuscripts have been submitted, and several more are in preparation. Once current AKTN trials are completed and outcomes are made available, the publication rate will further increase.
LESSONS LEARNT BY THE AKTN
The 6 years of AKTN operations have provided insights into the main challenges experienced in clinical trials, particularly investigator-initiated trials, as well as the opportunity to devise solutions to these challenges.
A slower than anticipated recruitment rate is often the primary challenge in conducting a clinical trial. The AKTN has identified an effective method to counteract recruitment difficulties: inclusion of international collaborators and sites early in the life of the trial. This proactive strategy is used to prevent slow recruitment rather than wait for international groups to ‘rescue' failing trials. Complex trial protocols can also contribute to recruitment difficulties. A simple trial design that closely mirrors standard clinical practice and has minimal restriction by inclusion and exclusion criteria reduces trial management costs, facilitates participation by a large number of centers, enables an easier recruitment process, and has the added advantage of maximizing the trial generalizability.
The central coordination processes require careful management to maximize trial success. Realistic budget projections ensure that trials are not underfunded on commencement, and close monitoring of expenditure and remodeling of financial requirements as necessary provides the opportunity to identify the need for additional funding procurement to facilitate trial completion. The longevity of Network operations is contingent on diversification of revenue streams, including financing from government, industry, research foundations and societies, and the provision of commercial functions such as education and contract research or management services. In addition to the administration of trial research grants, close financial management of routine operational expenditure is essential.
The AKTN promotes a culture of inclusivity, with all research units encouraged to participate in clinical research and contribute according to the availability of resources, thereby fostering engagement within the community and a sense of ‘ownership' of the Network. However, this policy is tempered with the intermittent exclusion of underperforming sites to ensure that resources are used appropriately and efficiently. Responsible fiscal management in clinical trial conduct is also demonstrated by the undertaking of small feasibility or rigorous proof-of-concept trials devised to inform large-scale trial design and feasibility in a clinical setting before major financial outlay.
Access to a research legal team with expertise in legal issues specific to clinical trials is vital for CRGs co-ordinating multicenter trials. The application of the legal team's expertise in contractual negotiations with external stakeholders ensures the protection of trial participants and safeguards the intellectual property and interests of investigators.
The daily operational activities of the AKTN provide a unique insight into the culture of health-care facilities across Australia and New Zealand. The barriers and hurdles encountered in conducting clinical trials at these facilities suggest a widespread perception that clinical research, and investigator-initiated clinical trials in particular, are a drain on hospital resources. The recognition of clinical trials as an integral part of patient care, and the promotion of a tripartite model of clinical service, research, and teaching, is an urgent necessity requiring a widespread paradigm shift in how research is approached within hospitals.
FUTURE PRIORITIES OF THE AKTN
The AKTN will continue to progress renal research in the region by facilitating investigator-initiated clinical trials across Australia and New Zealand. Priorities in the immediate future will be to publish the first AKTN clinical trials, continue educational and promotional activities, strengthen national and international collaborations, and engage Australian/New Zealand Nephrologists in renal research.
Trials
With seven coordinated or facilitated trials currently active, a primary priority of the Network is completing the first of these and publishing clinical trial results to inform clinical practice. Research priorities for future trials will continue to be focused on trials that deliver patient-centered outcomes, have hard-clinical end points with the potential to have an impact on and improve clinical care, and include quality-of-life assessments. Incorporating cost-effectiveness assessments and health economic evaluations will remain critical to inform policy designed to mitigate continuing economic challenges surrounding health care.
Refining operational processes
The importance of simplified protocols that closely mirror standard practice of clinical care for renal patients has been identified through the AKTN experience. Simplified protocols help manage cost by reducing the number of assessments required and, where possible, removing investigations extraneous to usual care, and therefore requiring additional funding through the Network. Protocols using schedules of assessments in line with standard clinical care allow trial assessments to be conducted at usual clinic visits instead of imposing extra appointments on staff and participants. Simplified protocols that are uncomplicated for clinical staff and participants also improve site and participant compliance, which aids the generalizability of trial results, and thus will continue to be a priority for AKTN trials. Responsible financial management will also remain a focus, as the Network continues to make clinical trials financially viable in the region to offer the best possible care for renal patients, as well as exposure to the latest evidence for Renal Physicians. This will include making trials cost-neutral to participating sites, assisting research units to negotiate costs with hospital-based services such as pathology and pharmacy, and lobbying for adequate funding for trial-specific costs and infrastructural support for units participating in investigator-initiated clinical trials. More emphasis will also be placed on the conduct of rigorous feasibility surveys and feasibility trials at units before commencing larger trials with hard-clinical end points and substantial recruitment targets. This will serve to save resources in the long term by only moving forward with feasible trials that can realistically expect to be completed in a suitable time frame and by using appropriate resources.
Energizing the renal research community
Several strategies to engage and energize the nephrology community in the region have been identified and adopted by the AKTN. It will be imperative to maintain these approaches, as the success of the AKTN relies on an engaged membership base and enthusiastic support of, and participation in, its research activities. Strategic engagement of site personnel includes the following: (1) ensuring a geographical balance of Trial Management Committee members to drive trials in their local state or district; (2) inclusion of Early Career Researchers on Trial Management Committees to perpetuate clinical trials interest and skills in the region; (3) placement of renal nurses on Trial Management Committees to encourage their input into recruitment and trial conduct at sites; (4) encouraging site staff to engage members of the hospital-based workforce outside the core trials group, including Dialysis Nurses, Vascular Access Coordinators, and Pharmacists, to encourage cooperation and promote efficiencies on site; (5) conducting regular Steering Committee meetings to provide a voice to local Investigators and Study Coordinators on trial progress and issues; and (6) developing an inclusive publication policy that acknowledges the input of all trial site staff, including the Investigator and Research Nurse/Study Coordinator.
The AKTN has a responsibility to the nephrology community to educate its stakeholders in clinical trial design, conduct, and good clinical practice. Formal educational opportunities, including scholarships for the higher research degrees of PhD and Masters in Public Health, will continue to be offered, as well as regular workshops targeting specific aspects of trial design and conduct. Informal educational opportunities, such as involvement on Trial Management Committees and assistance through AKTN trial endorsement, will also be available to researchers. The AKTN will continue to work with other trial networks to promote clinical trials as a career stream for Early Career Researchers.
Engaging kidney patients
Patient-centered outcomes have always been a focus of AKTN trials. As the Network has progressed, representatives of the kidney patient community have been included on core AKTN committees to ensure that Network activities are representative of the community's needs. Quality-of-life outcomes that are meaningful for kidney patients will continue to feature in AKTN trials.
Sustainable funding
The largest threat to the future of clinical trial CRGs is ongoing funding for core operations. The UK's National Institute for Health Research implemented a Clinical Research Network in 2008 to provide funding and network support to investigator-initiated trials in the United Kingdom (http://www.crncc.nihr.ac.uk/). Unlike the Australian model where research funding is sourced via a time-consuming, annual competitive federal government grant process for individual investigators or ad hoc funding made available from participation in pharmaceutical company sponsored research, research units in the UK are encouraged to participate in research through the provision of infrastructural support directly from the department of health in the form of clinical research personnel to facilitate investigator-initiated trial design and conduct. Although it is yet to be formally evaluated whether this resource allocation has been successful in increasing clinical trial quality and output, the UK Clinical Research Network has been identified as world-class infrastructure that has improved clinical trial uptake by participants through raising public awareness of the importance of clinical trials.31 The AKTN provides personnel and educational support to renal trials in Australia and New Zealand, but in the absence of a funding model similar to the UK Clinical Research Network, its sustainability is in jeopardy. The AKTN aims to join with other clinical trial networks to lobby government funding bodies to recognize clinical trials as part of routine clinical care to allow provision of world-class, evidence-based clinical care to Australian and New Zealand patients. This campaigning is to be at both state and federal health department levels, via the National Health and Medical Research Council, in conjunction with a working group established at a Medical Journal of Australia symposium, and directly to the relevant ministers.
National and International collaborations
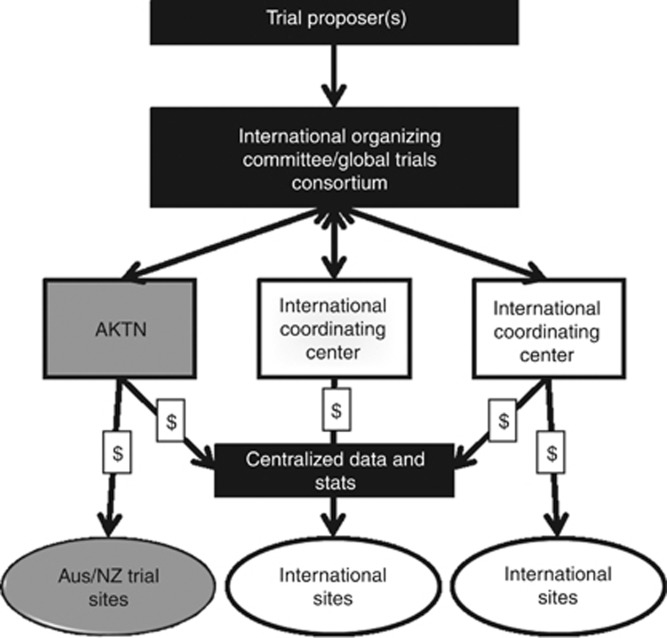
To increase recruitment success, international collaboration will continue to be a priority for individual trials, as will partnering with other national academic research groups to improve research efficiencies and output. An important goal of the AKTN is membership of a global clinical trials consortium consisting of multiple clinical trial networks that connect in order to (1) identify international research and clinical priorities, (2) provide a larger pool of renal patients available for trials, and (3) avoid duplication of research trials and development of methodologies. The AKTN has already partnered with international researchers to facilitate an ANZ arm of existing trials (see Table 3—PEXIVAS and ACEi), and to expand AKTN-led trials to overseas sites to improve recruitment rates and generalizability of outcomes (Table 3—FAVOURED, ACTIVE, and IMPROVE-CKD). A formalized model of international collaboration would streamline this process for the future, saving time in setting up contractual and logistical arrangements. A proposed model of a global trials consortium would include a joint funding model, and is outlined in Figure 4.
Figure 4.
Proposed model for international collaboration in a global clinical trials consortium.
SUMMARY
The aim of the AKTN is to conduct investigator-driven, patient-focused clinical research directed to improving patient outcomes. As a CRG established to facilitate and promote renal clinical trials in the region, the AKTN strives to ensure that Australian and New Zealand Investigator-initiated research is conducted in compliance with ICH GCP, and is held to a high standard of quality. The Network has a role in educating renal physicians, nurses, and allied health professionals in trial design and conduct. The objective of the AKTN is to increase nephrology research output from the region, reduce the dearth of clinical evidence in the specialty, and be an important link for international collaboration, with the ultimate aim of improving health outcomes for patients with kidney disease.
All the authors declared no competing interests.
References
- Levey AS, Atkins R, Coresh J, et al. Chronic kidney disease as a global public health problem: approaches and initiatives—a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72:247–259. doi: 10.1038/sj.ki.5002343. [DOI] [PubMed] [Google Scholar]
- White SL, Polkinghorne KR, Atkins RC, et al. Comparison of the prevalence and mortality risk of CKD in Australia using the CKD Epidemiology Collaboration (CKD-EPI) and Modification of Diet in Renal Disease (MDRD) Study GFR estimating equations: the AusDiab (Australian Diabetes, Obesity and Lifestyle) Study. Am J Kidney Dis. 2010;55:660–670. doi: 10.1053/j.ajkd.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Chadban SJ, Briganti EM, Kerr PG, et al. Prevalence of kidney damage in Australian adults: The AusDiab kidney study. J Am Soc Nephrol. 2003;14 (Suppl 2:S131–S138. doi: 10.1097/01.asn.0000070152.11927.4a. [DOI] [PubMed] [Google Scholar]
- Coresh J, Astor BC, Greene T, et al. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- Australian Government Budget—Budget Paper No. 1. Statement 6: Expenses and Net Capital Investment. Parkes, ACT: The Commonwealth of Australia201121–25.
- Annual Data Report, Chapter 11. United States Renal Data System, 2011. Available at http://www.usrds.org/2011/pdf/v2_ch011_11.pdf (29 May 2012).
- Annual Data Report, Chapter 12. United States Renal Data System. Available at http://www.usrds.org/2011/pdf/v2_ch012_11.pdf (29 May 2012).
- National Health Insurance Annual Statistical Report 1995–2009. Taiwan: Bureau of National Health Insurance, 2011. Available at http://www.nhi.gov.tw/webdata/webdata.aspx?menu=17&menu_id=661&WD_ID=689&webdata_id=3351 (29 May 2012).
- Couser WG, Remuzzi G, Mendis S, et al. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011;80:1258–1270. doi: 10.1038/ki.2011.368. [DOI] [PubMed] [Google Scholar]
- Strippoli GF, Craig JC, Schena FP. The number, quality, and coverage of randomized controlled trials in nephrology. J Am Soc Nephrol. 2004;15:411–419. doi: 10.1097/01.asn.0000100125.21491.46. [DOI] [PubMed] [Google Scholar]
- Palmer SC, Sciancalepore M, Strippoli GF. Trial quality in nephrology: how are we measuring up. Am J Kidney Dis. 2011;58:335–337. doi: 10.1053/j.ajkd.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Deo A, Schmid CH, Earley A, et al. Loss to analysis in randomized controlled trials in CKD. Am J Kidney Dis. 2011;58:349–355. doi: 10.1053/j.ajkd.2011.04.023. [DOI] [PubMed] [Google Scholar]
- Califf RM, Zarin DA, Kramer JM, et al. Characteristics of clinical trials registered in ClinicalTrials.gov, 2007–2010. JAMA. 2012;307:1838–1847. doi: 10.1001/jama.2012.3424. [DOI] [PubMed] [Google Scholar]
- Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363:609–619. doi: 10.1056/NEJMoa1000552. [DOI] [PubMed] [Google Scholar]
- Vincent JL. Logistics of large international trials: the good, the bad, and the ugly. Crit Care Med. 2009;37 (Suppl:S75–S79. doi: 10.1097/CCM.0b013e3181922c2d. [DOI] [PubMed] [Google Scholar]
- Ad Hoc Committee on Nephrology Manpower Needs Estimating workforce and training requirements for nephrologists through the year 2010. J Am Soc Nephrol. 1997;8 (Suppl 5:S9–S13. doi: 10.1681/ASN.V85s9. [DOI] [PubMed] [Google Scholar]
- Lane CA, Brown MA. Nephrology: a specialty in need of resuscitation. Kidney Int. 2009;76:594–596. doi: 10.1038/ki.2008.685. [DOI] [PubMed] [Google Scholar]
- Samuels JA, Molony DA. Randomized controlled trials in nephrology: state of the evidence and critiquing the evidence. Adv Chronic Kidney Dis. 2012;19:40–46. doi: 10.1053/j.ackd.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Easterbrook PJ, Matthews DR. Fate of research studies. Journal of the Royal Society of Medicine. 1992;85:71–76. doi: 10.1177/014107689208500206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decullier E, Chapuis F. Impact of funding on biomedical research: a retrospective cohort study. BMC Public Health. 2006;6:165. doi: 10.1186/1471-2458-6-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickersin K, Min YI.Publication bias: the problem that won't go away Ann NY Acad Sci 1993703135–146.discussion 46–48.. [DOI] [PubMed] [Google Scholar]
- Irish A, Dogra G, Mori T, et al. Preventing AVF thrombosis: the rationale and design of the Omega-3 fatty acids (Fish Oils) and Aspirin in Vascular access OUtcomes in REnal Disease (FAVOURED) study. BMC Nephrol. 2009;10:1. doi: 10.1186/1471-2369-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DW, Clark C, Isbel NM, et al. The honeypot study protocol: a randomized controlled trial of exit-site application of medihoney antibacterial wound gel for the prevention of catheter-associated infections in peritoneal dialysis patients. Periton Dial Int. 2009;29:303–309. [PubMed] [Google Scholar]
- Johnson DW, Hawley CM, Rosser B, et al. Oxpentifylline versus placebo in the treatment of erythropoietin-resistant anaemia: a randomized controlled trial. BMC Nephrol. 2008;9:8. doi: 10.1186/1471-2369-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badve SV, Hawley CM, Johnson DW. Is the problem with the vehicle or the destination? Does high-dose ESA or high haemoglobin contribute to poor outcomes in CKD. Nephrology. 2011;16:144–153. doi: 10.1111/j.1440-1797.2010.01407.x. [DOI] [PubMed] [Google Scholar]
- Badve SV, Brown F, Hawley CM, et al. Challenges of conducting a trial of uric-acid-lowering therapy in CKD. Nat Rev Nephrol. 2011;7:295–300. doi: 10.1038/nrneph.2010.186. [DOI] [PubMed] [Google Scholar]
- Badve SV, Roberts MA, Hawley CM, et al. Effects of beta-adrenergic antagonists in patients with chronic kidney disease: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58:1152–1161. doi: 10.1016/j.jacc.2011.04.041. [DOI] [PubMed] [Google Scholar]
- Navaneethan SD, Palmer SC, Smith A, et al. How to design a randomized controlled trial. Nephrology. 2010;15:732–739. doi: 10.1111/j.1440-1797.2010.01428.x. [DOI] [PubMed] [Google Scholar]
- Smith A, Palmer S, Johnson DW, et al. How to conduct a randomized trial. Nephrology. 2010;15:740–746. doi: 10.1111/j.1440-1797.2010.01433.x. [DOI] [PubMed] [Google Scholar]
- Badve SV, Smith A, Hawley CM, et al. Adherence to guideline recommendations for infection prophylaxis in peritoneal dialysis patients. Letter: NDT Plus. 2011;2:508. doi: 10.1093/ndtplus/sfp095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KS, Koller K, Foster K, et al. The UK clinical research network—has it been a success for dermatology clinical trials. Trials. 2011;12:153. doi: 10.1186/1745-6215-12-153. [DOI] [PMC free article] [PubMed] [Google Scholar]