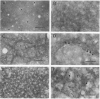
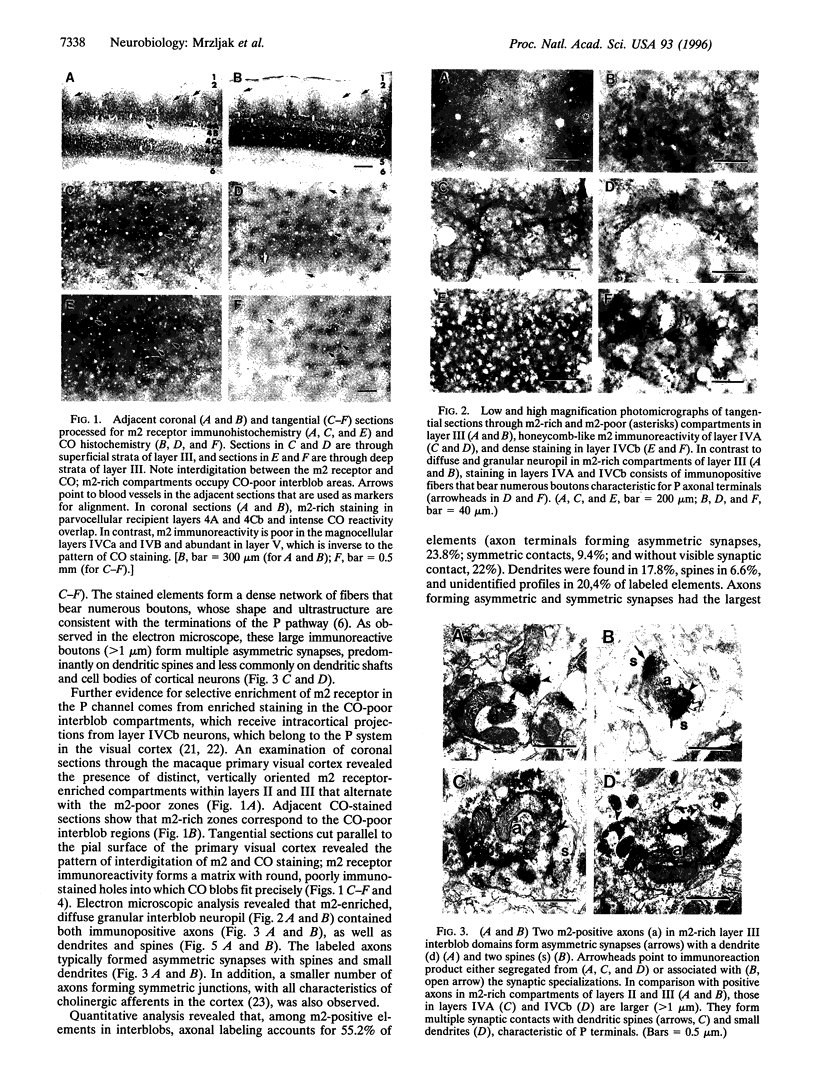
Abstract
Visual information in primates is relayed from the dorsal lateral geniculate nucleus to the cerebral cortex by three parallel neuronal channels designated the parvocellular, magnocellular, and interlaminar pathways. Here we report that m2 muscarinic acetylcholine receptor in the macaque monkey visual cortex is selectively associated with synaptic circuits subserving the function of only one of these channels. The m2 receptor protein is enriched both in layer IV axons originating from parvocellular layers of the dorsal lateral geniculate nucleus and in cytochrome oxidase poor interblob compartments in layers II and III, which are linked with the parvocellular pathway. In these compartments, m2 receptors appear to be heteroreceptors, i.e., they are associated predominantly with asymmetric, noncholinergic synapses, suggesting a selective role in the modulation of excitatory neurotransmission through the parvocellular visual channel.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agnati L. F., Zoli M., Strömberg I., Fuxe K. Intercellular communication in the brain: wiring versus volume transmission. Neuroscience. 1995 Dec;69(3):711–726. doi: 10.1016/0306-4522(95)00308-6. [DOI] [PubMed] [Google Scholar]
- Aoki C., Kabak S. Cholinergic terminals in the cat visual cortex: ultrastructural basis for interaction with glutamate-immunoreactive neurons and other cells. Vis Neurosci. 1992 Mar;8(3):177–191. doi: 10.1017/s0952523800002832. [DOI] [PubMed] [Google Scholar]
- Baughman R. W., Gilbert C. D. Aspartate and glutamate as possible neurotransmitters in the visual cortex. J Neurosci. 1981 Apr;1(4):427–439. doi: 10.1523/JNEUROSCI.01-04-00427.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasdel G. G., Lund J. S., Fitzpatrick D. Intrinsic connections of macaque striate cortex: axonal projections of cells outside lamina 4C. J Neurosci. 1985 Dec;5(12):3350–3369. doi: 10.1523/JNEUROSCI.05-12-03350.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner T. I., Buckley N. J., Young A. C., Brann M. R. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987 Jul 31;237(4814):527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- Carder R. K., Hendry S. H. Neuronal characterization, compartmental distribution, and activity-dependent regulation of glutamate immunoreactivity in adult monkey striate cortex. J Neurosci. 1994 Jan;14(1):242–262. doi: 10.1523/JNEUROSCI.14-01-00242.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casagrande V. A. A third parallel visual pathway to primate area V1. Trends Neurosci. 1994 Jul;17(7):305–310. doi: 10.1016/0166-2236(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Celio M. R., Schärer L., Morrison J. H., Norman A. W., Bloom F. E. Calbindin immunoreactivity alternates with cytochrome c-oxidase-rich zones in some layers of the primate visual cortex. Nature. 1986 Oct 23;323(6090):715–717. doi: 10.1038/323715a0. [DOI] [PubMed] [Google Scholar]
- Conti F., DeFelipe J., Farinas I., Manzoni T. Glutamate-positive neurons and axon terminals in cat sensory cortex: a correlative light and electron microscopic study. J Comp Neurol. 1989 Dec 1;290(1):141–153. doi: 10.1002/cne.902900109. [DOI] [PubMed] [Google Scholar]
- De Lima A. D., Singer W. Cholinergic innervation of the cat striate cortex: a choline acetyltransferase immunocytochemical analysis. J Comp Neurol. 1986 Aug 15;250(3):324–338. doi: 10.1002/cne.902500306. [DOI] [PubMed] [Google Scholar]
- Doty R. W. Nongeniculate afferents to striate cortex in macaques. J Comp Neurol. 1983 Aug 1;218(2):159–173. doi: 10.1002/cne.902180204. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick D., Itoh K., Diamond I. T. The laminar organization of the lateral geniculate body and the striate cortex in the squirrel monkey (Saimiri sciureus). J Neurosci. 1983 Apr;3(4):673–702. doi: 10.1523/JNEUROSCI.03-04-00673.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund T. F., Martin K. A., Soltesz I., Somogyi P., Whitteridge D. Arborisation pattern and postsynaptic targets of physiologically identified thalamocortical afferents in striate cortex of the macaque monkey. J Comp Neurol. 1989 Nov 8;289(2):315–336. doi: 10.1002/cne.902890211. [DOI] [PubMed] [Google Scholar]
- Freund T. F., Meskenaite V. gamma-Aminobutyric acid-containing basal forebrain neurons innervate inhibitory interneurons in the neocortex. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):738–742. doi: 10.1073/pnas.89.2.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson A. E., Hunt S. P., Wu J. Y. Immunocytochemical localization of glutamic acid decarboxylase in monkey striate cortex. Nature. 1981 Aug 13;292(5824):605–607. doi: 10.1038/292605a0. [DOI] [PubMed] [Google Scholar]
- Hendrickson A. E., Wilson J. R., Ogren M. P. The neuroanatomical organization of pathways between the dorsal lateral geniculate nucleus and visual cortex in Old World and New World primates. J Comp Neurol. 1978 Nov 1;182(1):123–136. doi: 10.1002/cne.901820108. [DOI] [PubMed] [Google Scholar]
- Hendry S. H., Huntsman M. M., Viñuela A., Möhler H., de Blas A. L., Jones E. G. GABAA receptor subunit immunoreactivity in primate visual cortex: distribution in macaques and humans and regulation by visual input in adulthood. J Neurosci. 1994 Apr;14(4):2383–2401. doi: 10.1523/JNEUROSCI.14-04-02383.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry S. H., Schwark H. D., Jones E. G., Yan J. Numbers and proportions of GABA-immunoreactive neurons in different areas of monkey cerebral cortex. J Neurosci. 1987 May;7(5):1503–1519. doi: 10.1523/JNEUROSCI.07-05-01503.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry S. H., Yoshioka T. A neurochemically distinct third channel in the macaque dorsal lateral geniculate nucleus. Science. 1994 Apr 22;264(5158):575–577. doi: 10.1126/science.8160015. [DOI] [PubMed] [Google Scholar]
- Kisvarday Z. F., Cowey A., Smith A. D., Somogyi P. Interlaminar and lateral excitatory amino acid connections in the striate cortex of monkey. J Neurosci. 1989 Feb;9(2):667–682. doi: 10.1523/JNEUROSCI.09-02-00667.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachica E. A., Beck P. D., Casagrande V. A. Parallel pathways in macaque monkey striate cortex: anatomically defined columns in layer III. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3566–3570. doi: 10.1073/pnas.89.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey A. I., Edmunds S. M., Hersch S. M., Wiley R. G., Heilman C. J. Light and electron microscopic study of m2 muscarinic acetylcholine receptor in the basal forebrain of the rat. J Comp Neurol. 1995 Jan 16;351(3):339–356. doi: 10.1002/cne.903510303. [DOI] [PubMed] [Google Scholar]
- Levey A. I., Kitt C. A., Simonds W. F., Price D. L., Brann M. R. Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J Neurosci. 1991 Oct;11(10):3218–3226. doi: 10.1523/JNEUROSCI.11-10-03218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone M., Hubel D. Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science. 1988 May 6;240(4853):740–749. doi: 10.1126/science.3283936. [DOI] [PubMed] [Google Scholar]
- McCormick D. A. Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog Neurobiol. 1992 Oct;39(4):337–388. doi: 10.1016/0301-0082(92)90012-4. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Prince D. A. Two types of muscarinic response to acetylcholine in mammalian cortical neurons. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6344–6348. doi: 10.1073/pnas.82.18.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M. M., Hersh L. B., Mash D. C., Geula C. Differential cholinergic innervation within functional subdivisions of the human cerebral cortex: a choline acetyltransferase study. J Comp Neurol. 1992 Apr 15;318(3):316–328. doi: 10.1002/cne.903180308. [DOI] [PubMed] [Google Scholar]
- Mrzljak L., Goldman-Rakic P. S. Low-affinity nerve growth factor receptor (p75NGFR)- and choline acetyltransferase (ChAT)-immunoreactive axons in the cerebral cortex and hippocampus of adult macaque monkeys and humans. Cereb Cortex. 1993 Mar-Apr;3(2):133–147. doi: 10.1093/cercor/3.2.133. [DOI] [PubMed] [Google Scholar]
- Mrzljak L., Levey A. I., Goldman-Rakic P. S. Association of m1 and m2 muscarinic receptor proteins with asymmetric synapses in the primate cerebral cortex: morphological evidence for cholinergic modulation of excitatory neurotransmission. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5194–5198. doi: 10.1073/pnas.90.11.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrzljak L., Pappy M., Leranth C., Goldman-Rakic P. S. Cholinergic synaptic circuitry in the macaque prefrontal cortex. J Comp Neurol. 1995 Jul 10;357(4):603–617. doi: 10.1002/cne.903570409. [DOI] [PubMed] [Google Scholar]
- Murakoshi T. Cholinergic modulation of synaptic transmission in the rat visual cortex in vitro. Vision Res. 1995 Jan;35(1):25–35. doi: 10.1016/0042-6989(94)e0056-q. [DOI] [PubMed] [Google Scholar]
- Peters A., Sethares C. Organization of pyramidal neurons in area 17 of monkey visual cortex. J Comp Neurol. 1991 Apr 1;306(1):1–23. doi: 10.1002/cne.903060102. [DOI] [PubMed] [Google Scholar]
- Pohorecki R., Head R., Domino E. F. Effects of selected muscarinic cholinergic antagonists on [3H]acetylcholine release from rat hippocampal slices. J Pharmacol Exp Ther. 1988 Jan;244(1):213–217. [PubMed] [Google Scholar]
- Schwartz M. L., Dekker J. J., Goldman-Rakic P. S. Dual mode of corticothalamic synaptic termination in the mediodorsal nucleus of the rhesus monkey. J Comp Neurol. 1991 Jul 15;309(3):289–304. doi: 10.1002/cne.903090302. [DOI] [PubMed] [Google Scholar]
- Sillito A. M. GABA mediated inhibitory processes in the function of the geniculo-striate system. Prog Brain Res. 1992;90:349–384. doi: 10.1016/s0079-6123(08)63622-5. [DOI] [PubMed] [Google Scholar]
- Sillito A. M., Kemp J. A. Cholinergic modulation of the functional organization of the cat visual cortex. Brain Res. 1983 Dec 19;289(1-2):143–155. doi: 10.1016/0006-8993(83)90015-x. [DOI] [PubMed] [Google Scholar]
- Sillito A. M. The cholinergic neuromodulatory system: an evaluation of its functional roles. Prog Brain Res. 1993;98:371–378. doi: 10.1016/s0079-6123(08)62421-8. [DOI] [PubMed] [Google Scholar]
- Umbriaco D., Watkins K. C., Descarries L., Cozzari C., Hartman B. K. Ultrastructural and morphometric features of the acetylcholine innervation in adult rat parietal cortex: an electron microscopic study in serial sections. J Comp Neurol. 1994 Oct 15;348(3):351–373. doi: 10.1002/cne.903480304. [DOI] [PubMed] [Google Scholar]
- Van Essen D. C., Gallant J. L. Neural mechanisms of form and motion processing in the primate visual system. Neuron. 1994 Jul;13(1):1–10. doi: 10.1016/0896-6273(94)90455-3. [DOI] [PubMed] [Google Scholar]
- Vidal C., Changeux J. P. Nicotinic and muscarinic modulations of excitatory synaptic transmission in the rat prefrontal cortex in vitro. Neuroscience. 1993 Sep;56(1):23–32. doi: 10.1016/0306-4522(93)90558-w. [DOI] [PubMed] [Google Scholar]
- Winfield D. A., Rivera-Dominguez M., Powell T. P. The termination of geniculocortical fibres in area 17 of the visual cortex in the macaque monkey. Brain Res. 1982 Jan 7;231(1):19–32. doi: 10.1016/0006-8993(82)90004-x. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979 Jul 27;171(1):11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- Yoshioka T., Levitt J. B., Lund J. S. Independence and merger of thalamocortical channels within macaque monkey primary visual cortex: anatomy of interlaminar projections. Vis Neurosci. 1994 May-Jun;11(3):467–489. doi: 10.1017/s0952523800002406. [DOI] [PubMed] [Google Scholar]
- de Lima A. D., Bloom F. E., Morrison J. H. Synaptic organization of serotonin-immunoreactive fibers in primary visual cortex of the macaque monkey. J Comp Neurol. 1988 Aug 8;274(2):280–294. doi: 10.1002/cne.902740211. [DOI] [PubMed] [Google Scholar]