Abstract
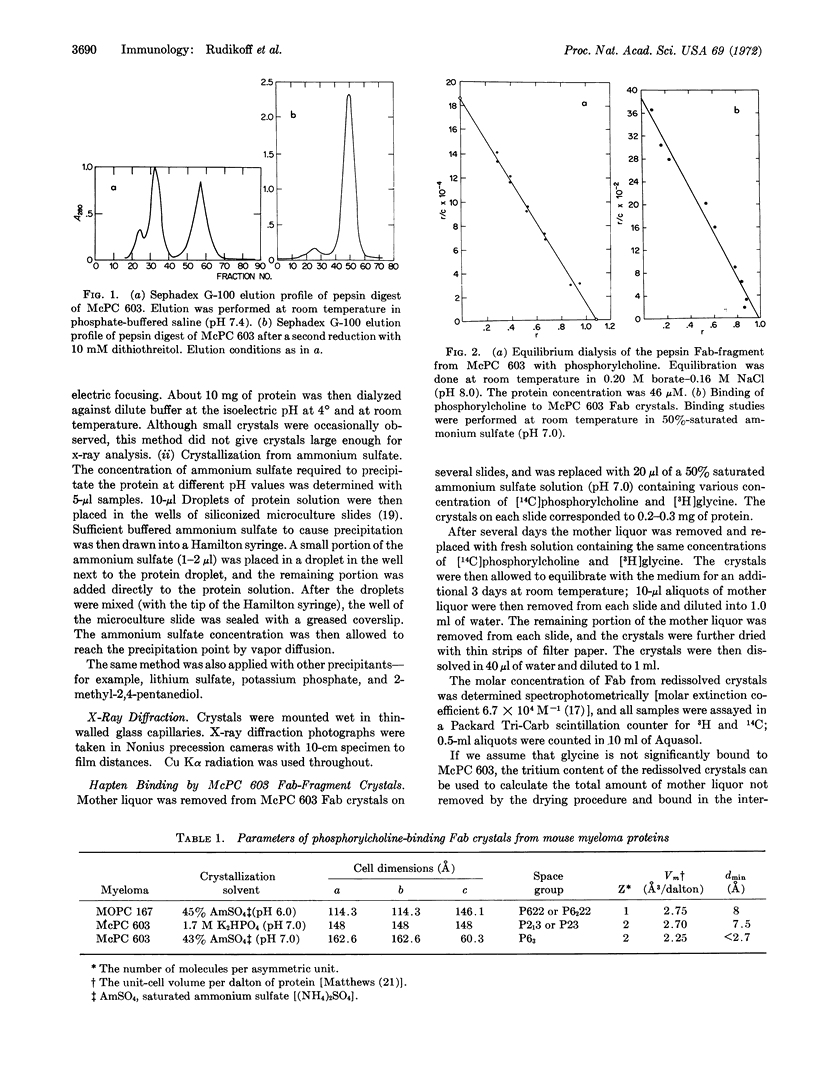
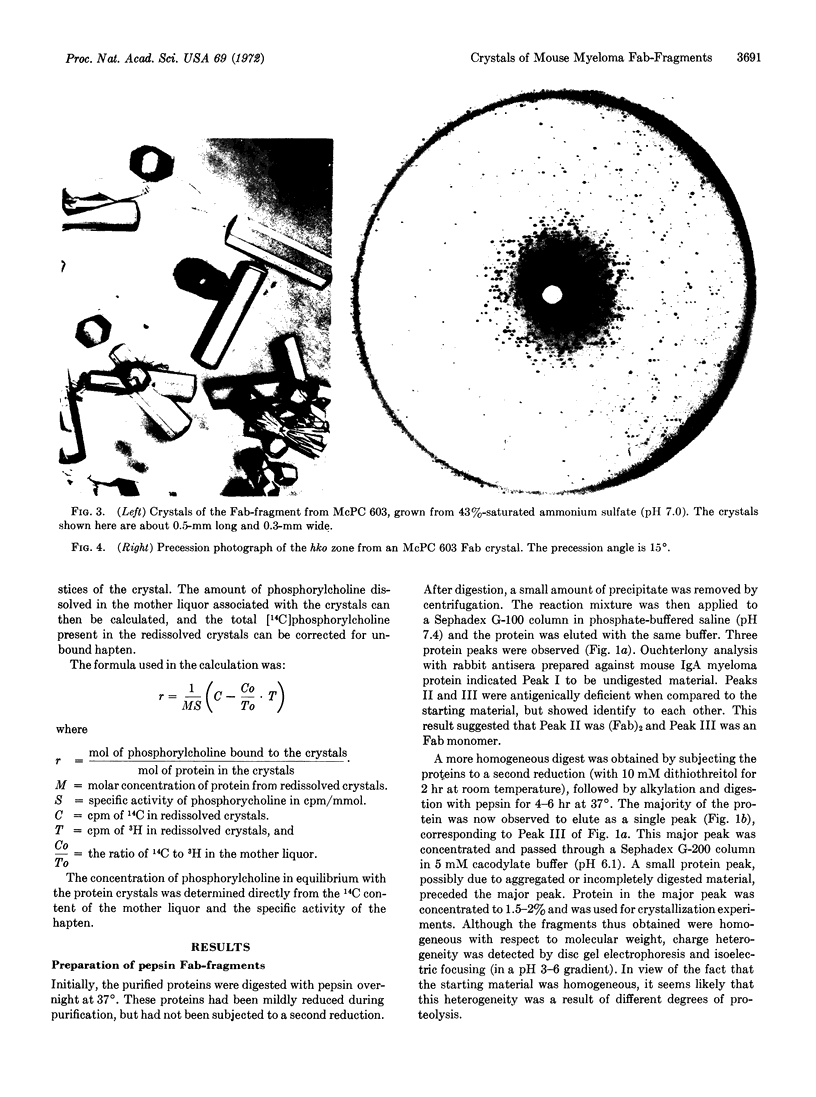
Fab-fragments of several phosphorylcholine-binding mouse-myeloma proteins have been prepared by pepsin digestion; two of these, MOPC 167 and McPC 603, gave large crystals from ammonium sulfate solutions. The Fab-fragment from MOPC 167 crystallizes in a hexagonal space group, but does not diffract to a resolution greater than about 8 Å. In contrast, McPC 603 crystals (space group P63) diffract to about 2.7 Å. An isotopic double-labeling technique was developed that demonstrated that the 603 crystals bind 1 mol of hapten per mol of Fab-fragment, but with a binding constant significantly lower than that observed in solution. The findings indicate that a three-dimensional model of this homogeneous antigen-binding immunoglobulin can be constructed. Accordingly, a search for heavy-atom derivatives and determination of the primary structure are in progress.
Keywords: IgA, pepsin fragments, hapten binding, x-ray crystallography
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bridges S. H., Little J. R. Recovery of binding activity in reconstituted mouse myeloma proteins. Biochemistry. 1971 Jun 22;10(13):2525–2530. doi: 10.1021/bi00789a016. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Metzger H. Affinity labeling of a phosphorylcholine binding mouse myeloma protein. Biochemistry. 1972 Feb 29;11(5):766–771. doi: 10.1021/bi00755a014. [DOI] [PubMed] [Google Scholar]
- Edmundson A. B., Wood M. K., Schiffer M., Hardman K. D., Ainsworth C. F., Ely K. R. A crystallographic investigation of a human IgG immunoglobulin. J Biol Chem. 1970 May 25;245(10):2763–2764. [PubMed] [Google Scholar]
- Eisen H. N., Simms E. S., Potter M. Mouse myeloma proteins with antihapten antibody acitivity. The protein produced by plasma cell tumor MOPC-315. Biochemistry. 1968 Nov;7(11):4126–4134. doi: 10.1021/bi00851a048. [DOI] [PubMed] [Google Scholar]
- Inbar D., Rotman M., Givol D. Crystallization with hapten of the Fab' fragment from a mouse IgA myeloma protein with antidinitrophenyl activity. J Biol Chem. 1971 Oct 25;246(20):6272–6275. [PubMed] [Google Scholar]
- Leon M. A., Young N. M., McIntire K. R. Immunochemical studies of the reaction between a mouse myeloma macroglobulin and dextrans. Biochemistry. 1970 Feb 17;9(4):1023–1030. doi: 10.1021/bi00806a043. [DOI] [PubMed] [Google Scholar]
- Matthews B. W. Solvent content of protein crystals. J Mol Biol. 1968 Apr 28;33(2):491–497. doi: 10.1016/0022-2836(68)90205-2. [DOI] [PubMed] [Google Scholar]
- Poljak R. J., Amzel L. M., Avey H. P., Becka L. N. Structure of Fab' New at 6 A resolution. Nat New Biol. 1972 Feb 2;235(57):137–140. doi: 10.1038/newbio235137a0. [DOI] [PubMed] [Google Scholar]
- Potter M. Antigen-binding myeloma proteins in mice. Ann N Y Acad Sci. 1971 Dec 31;190:306–321. doi: 10.1111/j.1749-6632.1971.tb13543.x. [DOI] [PubMed] [Google Scholar]
- Potter M., Leon M. A. Three IgA myeloma immunoglobulins from the BALB/ mouse: precipitation with pneumococcal C polysaccharide. Science. 1968 Oct 18;162(3851):369–371. doi: 10.1126/science.162.3851.369. [DOI] [PubMed] [Google Scholar]
- Potter M., Lieberman R. Common individual antigenic determinants in five of eight BALB-c IgA myeloma proteins that bind phosphoryl choline. J Exp Med. 1970 Oct 1;132(4):737–751. doi: 10.1084/jem.132.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter M., Mushinski E. B., Glaudemans C. P. Antigen-binding IgA myeloma proteins in mice: specificities to antigens containing -D 1 leads to 6 linked galactose side chains and a protein antigen in wheat. J Immunol. 1972 Feb;108(2):295–300. [PubMed] [Google Scholar]
- Rossi G., Nisonoff A. Crystallization of fragment Fab of human IgG myeloma proteins. Biochem Biophys Res Commun. 1968 Jun 28;31(6):914–918. doi: 10.1016/0006-291x(68)90539-1. [DOI] [PubMed] [Google Scholar]
- Schubert D., Jobe A., Cohn M. Mouse myelomas producing precipitating antibody to nucleic acid bases and-or nitrophenyl derivatives. Nature. 1968 Nov 30;220(5170):882–885. doi: 10.1038/220882a0. [DOI] [PubMed] [Google Scholar]
- Terry W. D., Boyd M. M., Rea J. S., Stein R. Human M-proteins with antibody activity for nitrophenyl ligands. J Immunol. 1970 Jan;104(1):256–259. [PubMed] [Google Scholar]
- Vicari G., Sher A., Cohn M., Kabat E. A. Immunochemical studies on a mouse myeloma protein with specificity for certain beta-linked terminal residues of N-acetyl-D-glucosamine. Immunochemistry. 1970 Oct;7(10):829–838. doi: 10.1016/0019-2791(70)90059-5. [DOI] [PubMed] [Google Scholar]




