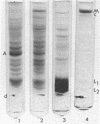
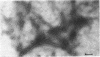
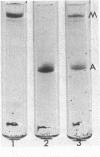
Abstract
Myosin has been isolated from cloned mouse fibroblasts, line L-929. Fibroblast myosin: (i) binds to rabbit muscle actin and is dissociated from it by ATP, (ii) has an ATPase activity that is suppressed by Mg2+ in 0.6 M KCl and is activated by rabbit muscle actin in the presence of Mg2+ in 14 mM KCl, (iii) forms thin bipolar aggregates in 0.1 M KCl when viewed in the electron microscope, (iv) possesses a heavy chain with the same mobility as muscle myosin (molecular weight 200,000) in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. In these respects, fibroblast myosin appears to be similar to muscle myosin in structure and function.
Keywords: muscle, SDS-acrylamide gel electrophoresis, actin binding, agarose filtration
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Pollard T. D., Kuehl W. M. Isolation and characterization of myosin and two myosin fragments from human blood platelets. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2703–2707. doi: 10.1073/pnas.68.11.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bárány M. ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol. 1967 Jul;50(6 Suppl):197–218. doi: 10.1085/jgp.50.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsie A. W., Puck T. T. Morphological transformation of Chinese hamster cells by dibutyryl adenosine cyclic 3':5'-monophosphate and testosterone. Proc Natl Acad Sci U S A. 1971 Feb;68(2):358–361. doi: 10.1073/pnas.68.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H., Bischoff R., Holtzer H. Formation of arrowhead complexes with heavy meromyosin in a variety of cell types. J Cell Biol. 1969 Nov;43(2):312–328. [PMC free article] [PubMed] [Google Scholar]
- Johnson G. S., Friedman R. M., Pastan I. Restoration of several morphological characteristics of normal fibroblasts in sarcoma cells treated with adenosine-3':5'-cyclic monphosphate and its derivatives. Proc Natl Acad Sci U S A. 1971 Feb;68(2):425–429. doi: 10.1073/pnas.68.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. S., Morgan W. D., Pastan I. Regulation of cell motility by cyclic AMP. Nature. 1972 Jan 7;235(5332):54–56. doi: 10.1038/235054a0. [DOI] [PubMed] [Google Scholar]
- Johnson G. S., Pastan I. Role of 3',5'-adenosine monophosphate in regulation of morphology and growth of transformed and normal fibroblasts. J Natl Cancer Inst. 1972 May;48(5):1377–1387. [PubMed] [Google Scholar]
- KIELLEY W. W., BRADLEY L. B. The relationship between sulfhydryl groups and the activation of myosin adenosinetriphosphatase. J Biol Chem. 1956 Feb;218(2):653–659. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nachmias V. Filament formation by purified Physarum myosin. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2011–2014. doi: 10.1073/pnas.69.8.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrie W. T., Smillie L. B., Perry S. V. A phosphorylated light-chain component of myosin. Biochem J. 1972 Jul;128(3):105P–106P. doi: 10.1042/bj1280105p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard J. R. Restoration of contact-inhibited growth to transformed cells by dibutyryl adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1316–1320. doi: 10.1073/pnas.68.6.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Stull J. T., Brostrom C. O., Krebs E. G. Phosphorylation of the inhibitor component of troponin by phosphorylase kinase. J Biol Chem. 1972 Aug 25;247(16):5272–5274. [PubMed] [Google Scholar]
- Yang Y. Z., Perdue J. F. Contractile proteins of cultured cells. I. The isolation and characterization of an actin-like protein from cultured chick embryo fibroblasts. J Biol Chem. 1972 Jul 25;247(14):4503–4509. [PubMed] [Google Scholar]