Abstract
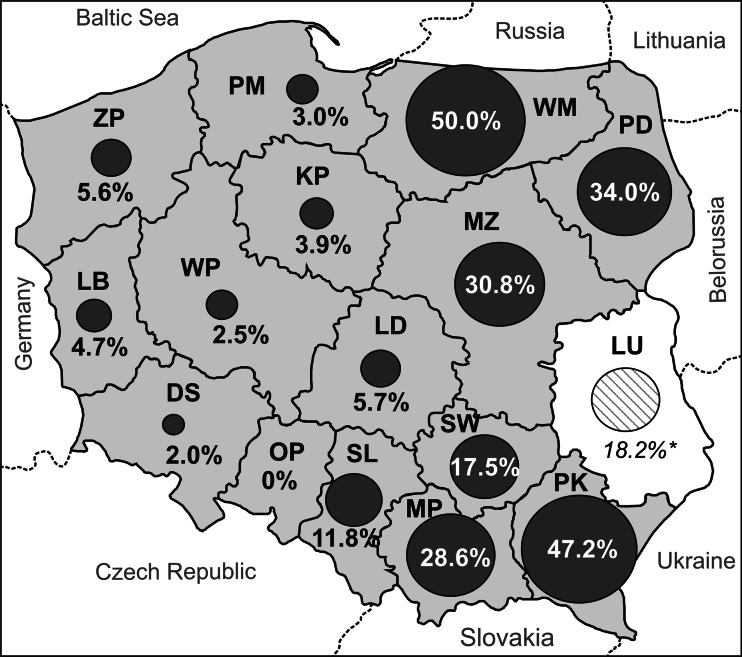
The aim of the study was to determine the prevalence of Echinococcus multilocularis in red foxes (Vulpes vulpes) in Poland. Overall, 1,546 intestinal samples from 15 of the 16 provinces in Poland were examined by the sedimentation and counting technique (SCT). The mean prevalence of E. multilocularis in Poland was 16.5 % and was found in 14 of the 15 examined provinces. The mean intensity of infection was 2,807 tapeworms per intestine. Distinct differences in prevalence were observed between regions. In some provinces of eastern and southern Poland, the level of prevalence was 50.0 % (Warmińsko-Mazurskie), 47.2 % (Podkarpackie), 30.4 % (Podlaskie) and 28.6 % (Małopolskie), while in other provinces (west and south-west), only a few percent was found: 2.0 % (Dolnośląskie), 2.5 % (Wielkopolskie) and 0.0 % (in Opolskie). The border between areas with higher and lower prevalence seems to coincide with a north–south line running through the middle of Poland, with prevalence from 17.5 to 50.0 % in the eastern half and from 0.0 to 11.8 % in the western half. The dynamic situation observed in the prevalence of this tapeworm indicated the necessity of continuing to monitor the situation concerning E. multilocularis in red foxes in Poland.
Introduction
Alveolar echinococcosis is one of the most dangerous of parasitic zoonoses. The causative agent of this disease in humans is the larval form of the Echinococcus multilocularis tapeworm (man plays the role of nonspecific intermediate host in the life cycle of this parasite). Cases of this disease are being noted increasingly often in people (Davidson et al. 2012; Schweiger et al. 2007). A total of 121 cases of alveolar echinococcosis have been confirmed in Poland so far (Nahorski et al. 2013). People were infected by accidental ingestion of E. multilocularis invasive eggs passed to the environment in the faeces of definitive hosts. The final hosts in the life cycle of E. multilocularis are some species of carnivores, the most typical being the red fox (Vulpes vulpes). Therefore, it is evident that the red fox is the most important species for environmental contamination with eggs of this tapeworm (Kapel et al. 2006). For this reason, red foxes are most often taken into consideration in investigations concerning the distribution of this infection in animals in the context of the potential risk for public health. There are some different types of diagnostic techniques used in such investigations. Among them, the most sensitive is a postmortem examination by the sedimentation and counting technique (SCT) (Davidson et al. 2009; Dinkel et al. 2011; Duscher et al. 2005; Hofer et al. 2000; Machnicka et al. 2003).
In the past few years (decades), a significant increase in the prevalence of E. multilocularis in red fox populations has been observed in many endemic regions in Europe (Combes et al. 2012; Denzin et al. 2012). Moreover, a dynamic spread and expansion in its geographical range in Europe has been observed. For example, in the Netherlands, the parasite population has increased in number and spread northward at a speed of 2.7 km per year (Takumi et al. 2008). Furthermore, just 2 years ago (2011), the first case of E. multilocularis infection in red foxes in Sweden was recorded (Osterman et al. 2011). Therefore, from now on, only four countries in the European Union are regarded as free from E. multilocularis: three island countries, where the movement of wild animals is limited by the sea (Great Britain, Ireland and Malta), and Finland (EFSA 2006). In Poland, these tapeworms were detected in foxes for the first time in 1994 (Malczewski et al. 1995). Afterwards, some prevalence studies were carried out in different regions of the country, and in selected areas, a distinct increase in the percentage of infected foxes has been observed (Borecka et al. 2008; Malczewski et al. 2008a, b; Karamon et al. 2011).
The aim of the study was to obtain current data concerning the prevalence of E. multilocularis in foxes in Poland.
Materials and methods
The red foxes (V. vulpes) used in the study were obtained from 15 (out of a total of 16) provinces in Poland. They were shot by hunters in the years 2009–2013 (during an official survey concerning the efficacy of an anti-rabies vaccination). Overall, 1,546 red foxes were used in the investigation (90–120 foxes from each province) (Table 1). The small intestines were the material for investigation, and these were frozen for at least 7 days at −80 °C before examination for safety reasons. All samples of intestines were examined with the use of the SCT (OIE Office International des Epizooties 2008).
Table 1.
Prevalence and intensity of E. multilocularis in red foxes in Poland
| Provinces | No. of examined foxes | % of positive foxes (95 % CI) | Mean no. of worms per intestine (CV) |
|---|---|---|---|
| Dolnośląskie (DS) | 102 | 2.0 (0.3–7.6) | 410 (117 %) |
| Kujawsko-Pomorskie (KP) | 103 | 3.9 (1.5–9.6) | 1,685 (197 %) |
| Łódzkie (LD) | 104 | 5.8 (2.7–12.0) | 3,187 (233 %) |
| Małopolskie (MP) | 98 | 28.6 (20.6–38.2) | 216 (168 %) |
| Mazowieckie (MZ) | 120 | 30.8 (23.3–39.6) | 2,253 (367 %) |
| Lubuskie (LB) | 107 | 4.7 (2.0–10.5) | 1,610 (160 %) |
| Opolskie (OP) | 100 | 0.0 (0.0–3.7) | 0 |
| Podkarpackie (PK) | 106 | 47.2 (37.9–56.6) | 8,704 (459 %) |
| Podlaskie (PD) | 100 | 34.0 (25.5–43.7) | 2,219 (285 %) |
| Pomorskie (PM) | 100 | 3.0 (1.0–8.5) | 359 (155 %) |
| Śląskie (SL) | 102 | 11.8 (6.9–19.4) | 513 (172 %) |
| Świętokrzyskie (SW) | 97 | 17.5 (11.2–26.3) | 736 (197 %) |
| Warmińsko-Mazurskie (WM) | 98 | 50.0 (40.3–59.7) | 1,222 (234 %) |
| Wielkopolskie (WP) | 119 | 2.5 (0.9–7.6) | 15 (83 %) |
| Zachodniopomorskie (ZP) | 90 | 5.6 (2.4–12.4) | 256 (180 %) |
| Total | 1,546 | 16.5 (14.7–18.4) | 2,807 (656 %) |
Statistical analysis
Confidence intervals concerning the percentages of infected foxes in Poland and separately for each province were calculated according to the method described by Newcombe (1998). Differences concerning the intensity of infection between provinces were analysed with the use of the Kruskal–Wallis test (P < 0.05). Correlations between E. multilocularis prevalence and density of fox population, forest cover and agricultural land were calculated with the use of the Spearman coefficient of correlation. Statistical analyses were performed using STATISTICA 7.0. (StatSoft, Poland).
Results and discussion
E. multilocularis tapeworms were found in the intestines of 255 out of the 1,546 red foxes (16.5 %). Distinct differences in prevalence were observed between regions. There was some observable regularity in the distribution of the infection in Poland, namely that those areas with a higher prevalence were located in the eastern half of the country while those areas with a lower prevalence were in the western half. The border between these zones seems to lie on a north–south line running through the middle of Poland.
The highest level of prevalence was noted in Warmińsko-Mazurskie Province, where every other examined fox was infected. Moreover, a very high prevalence was also observed in two neighbouring provinces (Podlaskie—34.0 % and Mazowieckie—30.8 %), as well as provinces in southern Poland (Małopolskie—30 % and Podkarpackie—47.2 %), while being relatively high in Świętokrzyskie (17.4 %). This picture of the eastern half of Poland as being an area characterized by a high percentage of infected foxes became more distinct when the results obtained earlier in Lubelskie Province were taken into account (Fig. 1). The comparison of our results obtained from the eastern half of Poland with the results of investigations conducted in the same area in the past (Table 2) demonstrated a very distinct and dynamic rise in E. multilocularis prevalence in red foxes during the past 15–20 years. Moreover, it can be observed that the rapid increase in the percentage of infected foxes began in the late 1990s and early 2000s. However, one exception to this rule seems to be Świętokrzyskie Province, where the very significant increase in E. multilocularis prevalence (from 3.6 to 17.5 %) took place a few years later (2008–2012) (Karamon et al. 2011).
Fig. 1.
Prevalence of E. multilocularis in Poland. Gray-coloured provinces were included in the investigation described in this paper. DS Dolnośląskie, KP Kujawsko-Pomorskie, LD Łódzkie, MP Małopolskie, LB Lubuskie, LU Lubelskie, MZ Mazowieckie, OP Opolskie, PK Podkarpackie, PD Podlaskie, PM Pomorskie, SL Śląskie, SW Świętokrzyskie, WM Warmińsko-Mazurskie, WP Wielkopolskie, ZP Zachodniopomorskie. Asterisk indicates results from 2007 to 2008 (Karamon et al. 2011)
Table 2.
Comparison of previous and current prevalence of E. multilocularis in red foxes in selected provinces of Poland
| Provinces | % of E. multilocularis positive foxes (95 % CI) | |||
|---|---|---|---|---|
| 1995–2000a | 2001–2006b | 2007–2008c | 2009–2013 | |
| Małopolskie (MP) | 0.6 (0.1–3.5) | 20.1 (15.3–26.0) | – | 28.6 (20.6–38.2) |
| Mazowieckie (MZ) | – | 13.5 (10.6–17.6) | – | 30.8 (23.3–39.6) |
| Podkarpackie (PK) | 7.2 (4.0–12.8) | 36.8 (32.2–41.5) | – | 47.2 (37.9–56.6) |
| Podlaskie (PD) | 6.5 (3.0–13.5) | – | – | 34.0 (25.5–43.7) |
| Świętokrzyskie (SW) | – | – | 3.6 (1.4–8.9) | 17.5 (11.2–26.3) |
| Warmińsko-Mazurskie (WM) | 18.8 (13.6–25.4) | 39.6 (34.9–44.8) | – | 50.0 (40.3–59.7) |
| Lubelskie (LU) | 1.0 (0.2–5.5) | – | 18.2 (13.8–23.5) | – |
| Dolnośląskie (DS) | 0.0 (0.0–1.2) | 1.0 (0.2–5.5) | – | 2.0 (0.3–7.6) |
| Kujawsko-Pomorskie (KP) | 0.6 (0.1–3.3) | – | – | 3.9 (1.5–9.6) |
| Lubuskie (LB) | 1.2 (0.8–1.8) | – | – | 4.7 (2.0–10.5) |
| Pomorskie (PM) | 7.3 (4.9–10.2) | 7.9 (6.2–10.1) | – | 3.0 (1.0–8.5) |
| Zachodniopomorskie (ZP) | 1.2 (0.6–2.4) | – | – | 5.6 (2.4–12.4) |
The situation concerning the prevalence of E. multilocularis in foxes was different for the western half of Poland. The percentage share of infected foxes in the western, north-western, south-western and partially central provinces was relatively low. Moreover, in one of these provinces (Opolskie), no E. multilocularis tapeworms were detected at all. Of course, this is not the same as saying it is an E. multilocularis-free region because the sampling rate for possible prevalence has a confidence interval of 0.0–3.7 %. However, it is interesting to note that, in contrast to the eastern regions described above, the western half of Poland lacked the significant increase in the number of infected foxes—the low prevalence in these areas has remained at the same level (or increased only minimally) for about 15 years (Table 2).
E. multilocularis has been detected in bordering countries. Over the western border (Germany), the epidemiological situation is well known—with continuous monitoring in different regions showing a significant increase in the prevalence of this parasite in foxes. For example, in Thuringia, the prevalence of E. multilocularis increased from 11.9 % in 1990 to 42.0 % in 2005 (Staubach et al. 2011), while in Saxony-Anhalt, it increased from 13.6 % (1998–2005) to 23.4 % (2006–2010) (Denzin et al. 2012). Over the southern border of Poland, the tapeworms have also been detected in foxes: in Slovakia (30.3 %) (Miterpáková and Dubinský 2011) and in the Czech Republic (from 2.5 to 12.0 %) (Pavlasek 1998). The presence of alveolar echinococcosis in foxes near the eastern border of Poland confirmed the data obtained from Western Ukraine, with 36.0 % of infected foxes (Kharchenko et al. 2008) and from Lithuania (57.3 %) (Bružinskaitė et al. 2007).
It is difficult to arrive at a definite conclusion at this stage of the investigation concerning what is the main factor influencing such distinct differentiation in the distribution of E. multilocularis in foxes in Poland.
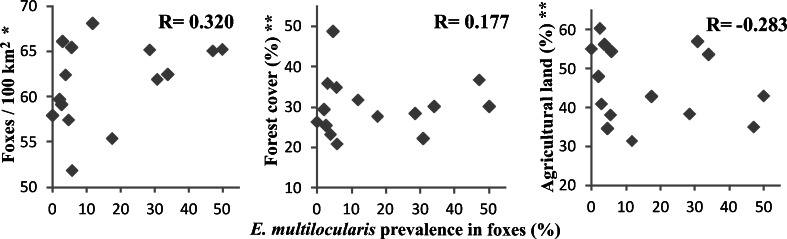
The increase in the prevalence of E. multilocularis in Poland was explained largely by the rise in the fox population, strongly correlated to the anti-rabies campaigns (Borecka et al. 2008). During the past 10–15 years the red fox population in Poland increased by about four times while simultaneously, the E. multilocularis prevalence increased significantly from 5 % (Rocki et al. 1999) to 16.5 %—according to our estimates. However, the size of the fox population and the E. multilocularis prevalence in foxes increased together only in the eastern half of Poland, which might indicate that the increase in the red fox population is not an absolute factor determining the high prevalence of the tapeworm. Moreover, the analysis of the relationship between the density of fox population and the prevalence of E. multilocularis in foxes in Poland revealed only a weak positive correlation (R = 0.320) (Fig. 2). An additional comparison of E. multilocularis prevalence in foxes with data concerning agricultural areas and forests in Poland showed only a very weak correlation, negative with agricultural land (R = −0.283) or positive with forest cover (R = 0.177) (Fig. 2).
Fig. 2.
Comparison of E. multilocularis prevalence in red foxes with density of fox population, forest cover and agricultural land. Single asterisk indicates data from the Polish Hunting Association Report (2011/2012). Double asterisks indicate data from the Concise Statistical Yearbook of Poland 2013
Some researchers have shown a relationship between percentage of infected foxes and mean annual temperature or rainfall, or other geomorphological factors in the region. This relates to the conditions necessary for the survival of tapeworm eggs in the environment, as well as for the biology of the definitive and the intermediate hosts. For example, an investigation conducted in Slovakia confirmed that a low mean annual air temperature, high mean annual rainfall and high soil humidity were important for E. multilocularis distribution (Miterpáková et al. 2006). Such a strict connection between the higher annual rainfalls and the higher occurrence of E. multilocularis in foxes does not appear to be confirmed by our study. Some of the Polish provinces having a low prevalence of this parasite (Opolskie, Zachodniopomorskie, Dolnośląskie and Pomorskie) were characterized by higher annual mean rainfalls than provinces where the percentage of infected foxes was very high (Warmińsko-Mazurskie), Podlaskie, Lubelskie and Mazowieckie). Climatic factors certainly play a crucial role in limiting the distribution of E. multilocularis throughout the world (limitation for north hemisphere) or the continent—in Europe, the southern limit in the prevalence of this tapeworm occurs in northern Italy (Casulli et al. 2005), central Hungary (Casulli et al. 2010) and northern Romania (Sikó et al. 2011). Therefore, on the scale of one region or country (like Poland), located fully within the zone of E. multilocularis occurrence, climatic factors seem to be one of the many elements that affect the prevalence of this parasite in foxes. Most likely, the most favourable conditions for the spread of this infection in the final hosts consist of a specific compilation of many factors, both biological and physical.
The mean intensity of infection was 2,807 tapeworms per intestine (ranging from 1 to 260,000). The mean intensity varied distinctly depending on the province, from 15 in Wielkopolskie to 8,704 in Podkarpackie, although there were no statistically significant differences between foxes from different provinces.
This investigation presents the current data concerning E. multilocularis occurrence in red foxes in Poland. It shows the risk for the human health connected with these parasites in Poland, especially in those regions with a very high prevalence. It must be stressed that most cases of human alveococcosis in Poland have been observed in Warmińsko-Mazurskie Province (Nahorski et al. 2013) where, according to our investigations, about 50 % of the foxes were infected. Moreover, the danger of infection with E. multilocularis for people in Poland was additionally emphasized by the recent detection of the larval form of this parasite in the livers of pigs (Karamon et al. 2012), an infection risk for humans connected with environments closer to people.
The dynamic situation observed in the prevalence of this tapeworm underlined the necessity of continuing to monitor the prevalence of E. multilocularis in red foxes in Poland.
The results were obtained during a project covering the whole of Poland concerning the epidemiology of E. multilocularis—a project realized as part of the multi-year program “Protection of animal and public health”.
References
- Borecka A, Gawor J, Malczewska M, Malczewski A. Prevalence of Echinococcus multilocularis tapeworm in red foxes in central Poland. Med Weter. 2007;63:1333–1335. [Google Scholar]
- Borecka A, Gawor J, Malczewska M, Malczewski A. Occurence of Echinococcus multilocularis in red foxes (Vulpes vulpes) in southern Poland. Helminthologia. 2008;45:24–27. doi: 10.2478/s11687-008-0004-5. [DOI] [Google Scholar]
- Bružinskaitė R, Marcinkutė A, Strupas K, Sokolovas V, Deplazes P, Mathis A, Eddi C, Sarkūnas M. Alveolar echinococcosis, Lithuania. Emerg Infect Dis. 2007;13:1618–1619. doi: 10.3201/eid1310.061161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casulli A, Manfredi MT, La Rosa G, Di Cerbo AR, Dinkel A, Romig T, Deplazes P, Genchi C, Pozio E. Echinococcus multilocularis in red foxes (Vulpes vulpes) of the Italian Alpine region: is there a focus of autochthonous transmission? Int J Parasitol. 2005;35:1079–1083. doi: 10.1016/j.ijpara.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Casulli A, Széll Z, Pozio E, Sréter T. Spatial distribution and genetic diversity of Echinococcus multilocularis in Hungary. Vet Parasitol. 2010;174:241–246. doi: 10.1016/j.vetpar.2010.08.023. [DOI] [PubMed] [Google Scholar]
- Combes B, Comte S, Raton V, Raoul F, Boué F, Umhang G, Favier S, Dunoyer C, Woronoff N, Giraudoux P. Westward spread of Echinococcus multilocularis in foxes, France, 2005–2010. Emerg Infect Dis. 2012;18:2059–2062. doi: 10.3201/eid1812.120219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RK, Øines Ø, Madslien K, Mathis A. Echinococcus multilocularis—adaptation of a worm egg isolation procedure coupled with a multiplex PCR assay to carry out large-scale screening of red foxes (Vulpes vulpes) in Norway. Parasitol Res. 2009;104:509–514. doi: 10.1007/s00436-008-1222-y. [DOI] [PubMed] [Google Scholar]
- Davidson RK, Romig T, Jenkins E, Tryland M, Robertson LJ. The impact of globalisation on the distribution of Echinococcus multilocularis. Trends Parasitol. 2012;28:239–247. doi: 10.1016/j.pt.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Denzin N, Schliephake A, Fröhlich A, Ziller M, Conraths FJ. On the move? Echinococcus multilocularis in red foxes of Saxony-Anhalt (Germany) Transbound Emerg Dis. 2012 doi: 10.1111/tbed.12026. [DOI] [PubMed] [Google Scholar]
- Dinkel A, Kern S, Brinker A, Oehme R, Vaniscotte A, Giraudoux P, Mackenstedt U, Romig T. A real-time multiplex-nested PCR system for coprological diagnosis of Echinococcus multilocularis and host species. Parasitol Res. 2011;109:493–498. doi: 10.1007/s00436-011-2272-0. [DOI] [PubMed] [Google Scholar]
- Duscher G, Prosl H, Joachim A. Scraping or shaking—a comparison of methods for the quantitative determination of Echinococcus multilocularis in fox intestines. Parasitol Res. 2005;95:40–42. doi: 10.1007/s00436-004-1260-z. [DOI] [PubMed] [Google Scholar]
- EFSA Assessment of the risk of echinococcosis introduction into the UK, Ireland, Sweden, Malta and Finland as a consequence of abandoning national rules. The EFSA Journal. 2006;441:1–54. [Google Scholar]
- Hofer S, Gloor S, Muller U, Mathis A, Hegglin D, Deplazes P. High prevalence of Echinococcus multilocularis in urban red foxes (Vulpes vulpes) and voles (Arvicola terrestris) in the city of Zurich, Switzerland. Parasitology. 2000;120:135–142. doi: 10.1017/S0031182099005351. [DOI] [PubMed] [Google Scholar]
- Kapel CMO, Torgerson PR, Thompson RCA, Deplazes P. Reproductive potential of Echinococcus multilocularis in experimentally infected foxes, dogs, raccoon dogs and cats. Int J Parasitol. 2006;36:79–86. doi: 10.1016/j.ijpara.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Karamon J, Sroka J, Cencek T, Michalski MM, Zięba P, Karwacki J. Prevalence of Echinococcus multilocularis in red foxes in two eastern provinces of Poland. Bull Vet Inst Pulawy. 2011;55:429–433. [Google Scholar]
- Karamon J, Sroka J, Cencek T. The first detection of Echinococcus multilocularis in slaughtered pigs in Poland. Vet Parasitol. 2012;185:327–329. doi: 10.1016/j.vetpar.2011.09.022. [DOI] [PubMed] [Google Scholar]
- Kharchenko VA, Kornyushin VV, Varodi EI, Malega OM. Occurrence of Echinococcus multilocularis (Cestoda, Taeniidae) in red foxes (Vulpes vulpes) from Western Ukraine. Acta Parasitologica. 2008;53:36–40. doi: 10.2478/s11686-008-0008-9. [DOI] [Google Scholar]
- Machnicka B, Dziemian E, Rocki B, Kołodziej-Sobocińska M. Detection of Echinococcus multilocularis antigens in faeces by ELISA. Parasitol Res. 2003;91:491–496. doi: 10.1007/s00436-003-0994-3. [DOI] [PubMed] [Google Scholar]
- Machnicka-Rowińska B, Rocki B, Dziemian E, Kołodziej-Sobocińska M. Racoon dog (Nyctereutes procyonoides)—the new host of Echinococcus multilocularis in Poland. Wiad Parazytol. 2002;48:65–68. [PubMed] [Google Scholar]
- Malczewski A, Rocki B, Ramisz A, Eckert J. Echinococcus multilocularis (Cestoda), the causative agent of alveolar echinococcosis in humans: first record in Poland. J Parasitol. 1995;81:318–321. doi: 10.2307/3283945. [DOI] [PubMed] [Google Scholar]
- Malczewski A, Borecka A, Malczewska M, Gawor J. An attempt to determine intermediate hosts of the tapeworm Echinococcus multilocularis in Poland. Wiad Parazytol. 2008;54:137–141. [PubMed] [Google Scholar]
- Malczewski A, Gawor J, Malczewska M. Infection of red foxes (Vulpes vulpes) with Echinococcus multilocularis during the years 2001–2004 in Poland. Parasitol Res. 2008;103:501–505. doi: 10.1007/s00436-008-0990-8. [DOI] [PubMed] [Google Scholar]
- Miterpáková M, Dubinský P. Fox tapeworm (Echinococcus multilocularis) in Slovakia—summarizing the long-term monitoring. Helminthologia. 2011;48:155–161. doi: 10.2478/s11687-011-0023-5. [DOI] [Google Scholar]
- Miterpáková M, Dubinský P, Reiterová K, Stanko M. Climate and environmental factors influencing Echinococcus multilocularis occurrence in the Slovak Republic. Ann Agric Environ Med. 2006;13:235–242. [PubMed] [Google Scholar]
- Nahorski WL, Knap JP, Pawłowski ZS, Krawczyk M, Polański J, Stefaniak J, Patkowski W, Szostakowska B, Pietkiewicz H, Grzeszczuk A, Felczak-Korzybska I, Gołąb E, Wnukowska N, Paul M, Kacprzak E, Sokolewicz-Bobrowska E, Niścigorska-Olsen J, Czyrznikowska A, Chomicz L, Cielecka D, Myjak P. Human alveolar echinococcosis in Poland: 1990–2011. PLoS Negl Trop Dis. 2013;7(1):e1986. doi: 10.1371/journal.pntd.0001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17:857–872. doi: 10.1002/(SICI)1097-0258(19980430)17:8<857::AID-SIM777>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- OIE (Office International des Epizooties) Manual of diagnostic tests and vaccines for terrestrial animals. 6. Paris: World Organisation for Animal Health; 2008. pp. 175–189. [Google Scholar]
- Osterman E, Juremalm M, Christensson D, Widgren S, Hallgren G, Ågren EO. Uhlhorn H, Lindberg A, Cedersmyg M, Wahlström H (2011) First detection of Echinococcus multilocularis in Sweden, February to March 2011. Euro Surveill. 16(14):pii = 19836 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19836 [PubMed]
- Pacoń J, Sołtysiak Z, Nicpoń J, Janczak M. Prevalence of internal helminths in red foxes (Vulpes vulpes) in selected regions of Lower Silesia. Med Weter. 2006;62:67–69. [Google Scholar]
- Pavlasek I. Current situation in Echinococcus multilocularis occurrence in red foxes in Europe and Czech Republic, respectively. Klin Mikrobiol. 1998;2:224–233. [Google Scholar]
- Ramisz A, Eckert J, Balicka-Ramisz A, Grupiński T, Pilarczyk B, Król-Pośpieszny, Słowikowski P. Prevalence of Echinococcus multilocularis in foxes in western Poland. Med Weter. 1997;53:340–342. [Google Scholar]
- Rocki B, Malczewski A, Eckert J. Studies on the incidence of Echinococcus multilocularis in red foxes (Vulpes vulpes) in north-east, central and south of Poland. Wiad Parazytol. 1999;45:391–393. [PubMed] [Google Scholar]
- Schweiger A, Ammann RW, Candinas D, Clavien PA, Eckert J, Gottstein B, Halkic N, Muellhaupt B, Prinz BM, Reichen J, Tarr PE, Torgerson PR, Deplazes P. Human alveolar echinococcosis after fox population increase, Switzerland. Emerg. Infect Dis. 2007;13:878–882. doi: 10.3201/eid1306.061074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikó SB, Deplazes P, Ceica C, Tivadar CS, Bogolin I, Popescu S, Cozma V. Echinococcus multilocularis in south-eastern Europe (Romania) Parasitol Res. 2011;108:1093–1097. doi: 10.1007/s00436-010-2150-1. [DOI] [PubMed] [Google Scholar]
- Staubach C, Hoffmann L, Schmid VJ, Ziller M, Tackmann K, Conraths FJ. Bayesian space-time analysis of Echinococcus multilocularis-infections in foxes. Vet Parasitol. 2011;179:77–83. doi: 10.1016/j.vetpar.2011.01.065. [DOI] [PubMed] [Google Scholar]
- Takumi K, de Vries A, Chu ML, Mulder J, Teunis P, van der Giessen J. Evidence for an increasing presence of Echinococcus multilocularis in foxes in The Netherlands. Int J Parasitol. 2008;38:571–578. doi: 10.1016/j.ijpara.2007.09.014. [DOI] [PubMed] [Google Scholar]