Abstract
Timely detection and follow-up of abnormal cellular changes can aid in early diagnosis of breast cancer, thus leading to better treatment outcomes. However, despite substantial breast cancer screening initiatives, the proportion of female breast cancer cases diagnosed at late stages remains high. Distance to screening clinics may affect access to care, particularly for women living in impoverished areas with limited means of reliable transportation. Utilizing breast cancer screening data collected by the Illinois Breast and Cervical Cancer Program between 1996 and 2010, we examined the effect of travel distance to the clinic from which women received breast cancer screening tests on stage of diagnosis.
The proportion of abnormal mammograms in White women (1.6%) was higher than in Black women (1.1%) or Hispanic women (0.5%). The average distance traveled to a clinic was also farthest among White women (6.7 mi) than for Hispanic (5.3 mi) or Black women (4.4 mi). Distance to a clinic was significantly associated with increased odds of having abnormal results. When distance to clinic was controlled for, the observed disparity in odds of having an abnormal mammogram between White and Black women was no longer statistically significant. Individual and neighborhood sociodemographic characteristics were significantly associated with distance to clinic, but were not associated with increased odds of having an abnormal mammogram, controlling for distance to the clinic.
Findings showed that individual and neighborhood sociodemographic characteristics are directly and indirectly associated with abnormal mammogram results, and that distance to a clinic may mediate, in part, the effects of individual characteristics and neighborhood disadvantage on the probability of having an abnormal mammogram.
Keywords: Breast cancer screening, travel distance, abnormal mammogram
INTRODUCTION
Breast Cancer Disparities
Breast cancer is the second-leading cause of cancer-related death in women (Edwards et al., 2010; National Cancer Institute, 2011). Although during the 2000s the breast cancer death rate has dropped steadily, in 2011, an estimated 230,480 new cases of invasive breast cancer were expected to be diagnosed in women in the U.S., along with 57,650 new cases of non-invasive (in situ) breast cancer (Breastcancer.org, 2011).
The incidence rate of breast cancer has been consistently higher for white women, and in 2008, the incidence was 130 per 100,000 white women, compared with 126 per 100,000 black women (National Cancer Institute, 2010b). However, survival rates are lower among black women. Between 2003 and 2007, the breast cancer mortality rate was 155/100,000 for white women and 181/100,000 for black women. Similarly, five-year survival was also higher among white women compared with black women (91% vs. 78%, respectively) (National Cancer Institute, 2010b). This pattern holds for the state of Illinois, where the mortality rate was 30 per 100,000 for white women and 39 per 100,000 for black women (Illinois Department of Public Health, 2009).
Breast cancer survival rates can be significantly improved when it is diagnosed at an earlier stage: the 5-year survival rate for women who were diagnosed at the localized breast cancer stage is 98%, compared with 23% for those who were diagnosed at the distant stage (National Cancer Institute, 2009). Despite the availability of relatively simple and inexpensive screening tools, racial/ethnic minority women of low socioeconomic status (SES) are more often diagnosed at a later stage of breast cancer and, subsequently, the mortality rates are higher for racial/ethnic minority women compared with the rates among white women (Cross, Harris, & Recht, 2002; Eley et al., 1994; Grisby, Hall-Daniels, Baker, & Perez, 2000; Mandelblatt, Andrews, J., Zauber, & Burnett, 1991; Mundt, Connell, & Campbell, 1998; Roetzheim et al., 1999; Wang, McLafferty, Escamilla, & Luo, 2008).
Disparities in Cancer Screening
One of the reasons for the late diagnosis of cancer may be lack of routine screening (Brookfield, Cheung, Lucci, Fleming, & Koniaris, 2009; Ferrante, Gonzalez, Roetzheim, Pal, & Woodard, 2000; Liu, Wang, Waterbor, Weiss, & Soong, 1998). Routine screening such as mammography can improve early detection of abnormal changes and early stage diagnosis, which can greatly reduce the cancer mortality rates (Berry et al., 2005; Bradley, Given, & Roberts, 2001; Brown, Riley, Schussler, & Etzioni, 2002; Clegg et al., 2009; Ferrante, et al., 2000; Lundin, Christopherson, Mendez, & Parker, 1965; National Cancer Institute, 2010a; Nelson et al., 2009; Sassi, Luft, & Guadagnoli, 2006). The Centers for Disease Control and Prevention (CDC) reported no difference in mammogram uptake between white and black women among 40 years and older (both being 68%) (CDC, 2011). On the other hand, a meta-analysis using 33 studies between 1980 and 2006 concluded that African American women (OR 0.87, 95% CI 0.75, 1.00) and Hispanic women (OR 0.65, 95% CI 0.50, 0.85) were less likely to obtain breast cancer screening than white women (Purc-Stephenson & Gorey, 2008). Similar findings have been reported based on the 2000 National Health Interview Survey where the rate of mammogram uptake was lower for African American and Hispanic women compared with the rate for white women (Swan, Breen, Coates, Rimer, & Lee, 2003).
A myriad of psychosocial and economic factors may affect breast cancer screening behavior (Consedine, Magai, Krivoshekova, Ryzewicz, & Neugut, 2004; Magai, Consedine, Conway, Neugut, & Culver, 2004). Studies have argued that one of the reasons for the late diagnosis of cancer may be due to the lack of routine screening (Brookfield, et al., 2009; Ferrante, et al., 2000; Liu, et al., 1998). Education was known to be associated with having a recent mammogram: 54% of women with no high school or GED diploma had a mammogram in the past two years, but 65% of women with a high school degree and 73% of women with at least some college had a mammogram in the past two years (American Cancer Society, 2007). Insurance status was also found to be associated with late stage cancer diagnosis. Halpern and others documented that in the US National Cancer Registry database, uninsured individuals or individuals on Medicaid were more likely to be diagnosed with cancer at a late stage compared with privately insured patients (Halpern et al., 2008). Using the 2000 and 2001 Medical Expenditure Panel Survey, national-level survey data, Kirby and colleagues also documented that differences in individual health insurance coverage and SES, as well as neighborhood-level racial composition, explained some of the racial differences in health outcomes (Kirby, Taliaferro, & Zuvekas, 2006).
Access to mammography sites seems to influence cancer screening and follow-up behavior (Celaya et al., 2010). Particularly, distance to the nearest mammography facility (Engelman et al., 2002) and/or to nearest hospital (Silverstein, Nietert, Ye, & Lackland, 2002) was associated with the rate of cancer screening uptake. For example, a study conducted in Australia found that among women living in disadvantaged areas, those who lived closer to clinics were more likely to have a mammogram (Hyndman, Holman, & Dawes, 2000). Travel time and distance to screening facilities was also shown to be inversely associated with neighborhood poverty level (Zenk, Tarlov, & Sun, 2006). Predominantly black neighborhoods in Atlanta had the longest travel times to the facilities (Peipins et al., 2011). Similarly in Chicago, individuals living in predominantly African American neighborhoods had longer travel distance to clinics (Zenk, et al., 2006).
The effect of travel distance to a clinic on women’s screening participation and follow-up behavior has not been thoroughly examined (Hyndman, et al., 2000). While people in disadvantaged neighborhoods often lack access to primary care clinics, individuals who have limited access to reliable means of transportation could particularly be affected by the distance they have to travel to receive preventive screening tests. In this study we examined racial/ethnic differences in distance to screening facilities among women living in poverty.
Conceptual model
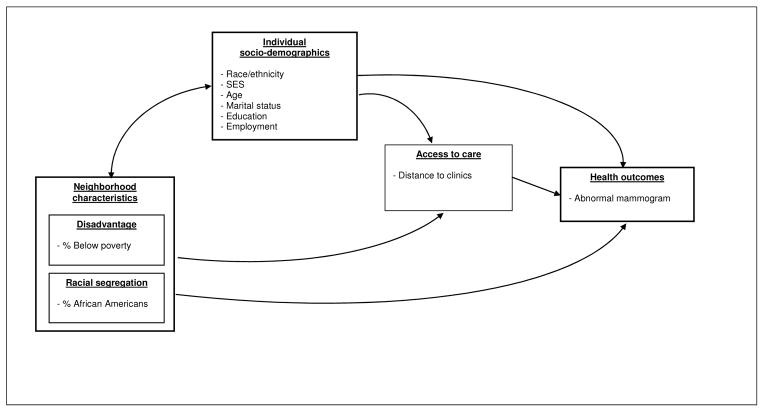
This study draws from multi-level ecological approaches to health disparities (Campbell et al., 2009; Cho et al., 2011; Warnecke, Oh, Breen, & et al, 2008). Ecological approaches explore distal factors, fundamental causes of health disparities, and examine interactions among multilevel factors that affect health. Individual and neighborhood socioeconomic characteristics affect access to care, quality, and timeliness of care, which consequently affect health outcomes. In addition, we specifically focus on potential factors that can introduce differential breast cancer outcomes in the overall process from routine screening, follow-up of abnormal findings, early diagnosis, timely treatment, and quality care (Figure 1). One of the ways in which distal factors such as race/ethnicity and socioeconomic status may influence an individual’s health outcome is through impacting access to care. In this analysis, we treat the distance traveled to the clinic as a barrier of access to routine screening and timely follow-up of abnormal changes.
Figure 1.
Associations between explanatory variables, distance to the clinic, and abnormal screening result
We hypothesize that 1) the average distance to the clinic may differ by race/ethnicity, controlling for other relevant variables; 2) distance to screening facilities has an independent effect on the probability of having an abnormal mammogram, controlling for other factors. Finally, we expect that 3) although the crude rate of abnormal mammograms is higher for white women compared with black women, the racial difference in the likelihood of having an abnormal mammogram may be reduced when distance to clinic is taken into account.
METHODS
Datasets
We utilized the breast cancer screening data collected by the Illinois Breast and Cervical Cancer Program (IBCCP), between November 20, 1996 and May 10, 2010. The IBCCP aims to provide breast and cervical cancer screening and diagnostic tests to low-income, uninsured, and underinsured women. IBCCP services are available to women living below 250% of federal poverty level. Since the program was launched in Illinois in 1995, more than 154,000 women have been screened for breast and cervical cancers (Illinois Department of Public Health, 2011). Overall, 48,368 screening encounters throughout the state of Illinois were recorded in the dataset, which included 32,772 unique individuals who received one or more mammogram. Of those, we restricted our analysis to the 98% of individuals (n=32,175) who resided in Cook County, IL.
Individual residential addresses were geocoded using ArcGIS 10. The 10.0 North America Geocode Service was used as the Address Locator. Census tract-level socio-demographic data from the US Census Bureau were added to the dataset. Census data from 2000 were used to match as a midpoint for the time period the IBCCP data were collected (1996–2010). Using the Hawth’s Analysis Tools extension (ArcGIS 9), we calculated the distance in miles between the residential location to the IBCCP clinic where women received mammograms.
A total of 17 IBCCP agencies provided care. Of those, five agencies were excluded from the analysis. Because these agencies had more than one clinic site, we were not able to calculate the actual distance to the clinic where women obtained a mammogram. Mammogram results from the 12 remaining agencies included 21,085 unique individuals. Home addresses were used to calculate distance to clinic. Additionally, individual sociodemographic information and census tract-level socioeconomic variables were included in this study.
Variables
Dependent variables
The primary variable of interest in this analysis was the distance to breast cancer screening clinics. We used the distance as a dependent variable examining the effect of race/ethnicity and other relevant variables. We also used the distance variable as an independent variable to explain abnormal mammogram results, controlling for all other variables.
The residential address and the location of the IBCCP clinic from which each individual received her screening service were geocoded. The distance traveled to a clinic was calculated by measuring the distance in miles between longitude and latitude of residential location and the clinic from which the women received the screening service. Census tract numbers were assigned to the cases based on residential addresses.
A dichotomous variable indicating mammogram results (normal vs. abnormal findings) was created. Abnormal findings included: Lobular in situ (LCIS), Ductal in situ (DCIS), mild dysplasia (CIN 1), moderate dysplasia (CIN 2), severe dysplasia, carcinoma in situ, or adenocarcinoma in situ (AIS) (CIN 3).
Independent variables
Individual sociodemographic variables included race/ethnicity, age, income, marital status, education, and employment status. Race/ethnicity included four categories (Black, Hispanic, White, and other race/ethnic group). Age was categorized into four groups: less than 40 years (omitted category), 40 to 49 years, 50 to 64 years, and 65 and older. Income was grouped into quartiles: less than $7,200, $7,200–$12,000, $12,000–$16,320, and more than $16,320. Marital status was categorized in to the following: never married, married, widowed, and divorced/separated. Education level was dichotomized: less than high school education vs. high school graduate or more. Employment status was divided into full-time employment, part-time employment, or unemployed.
Census tract-level demographic information included percent of residents living below poverty line and percent of African American residents. We calculated a factor score indicating the level of disadvantage using: % poverty, % African Americans, % female headed households with children, % less than high school education, median income, and % unemployed. The factor analysis generated two components. We compared the model using the factor score and the model with % poverty and % African Americans as separate variables (results not shown). We found no difference between the two approaches and therefore used the raw variables.
Analysis
Descriptive statistics were used to characterize the sample (Tables 1 & 2). First we examined the differences in the percent of abnormal screening results and the average distance to clinic by race/ethnicity, age, education, employment status, marital status, and income (Table 1). Second, we examined racial/ethnic differences in the independent and explanatory variables at the individual level as well as at the residential census tract level (Table 2). We used Chi-square tests to examine proportional differences and ANOVA to test mean differences.
Table 1.
Abnormal changes by screening type (N=21,085)
| % sample distribution | % abnormal at initial visit | Sig. for % abnormal | |
|---|---|---|---|
| Mammogram results | |||
| Normal | 99.2 | - | - |
| Abnormal | 0.8 | ||
| Race/Ethnicity | |||
| White | 9.3 | 1.6 | |
| Black | 36.8 | 1.1 | <.01 |
| Hispanic | 44.5 | 0.5 | |
| Other | 8.3 | 0.7 | |
| Age | |||
| < 40 | 3.9 | 1.0 | |
| 40–49 | 32.7 | 0.6 | <.05 |
| 50–64 | 60.8 | 0.9 | |
| > 65 | 2.6 | 1.8 | |
| Education | |||
| Less than high school | 34.0 | 0.6 | <.01 |
| High school or more | 66.0 | 1.1 | |
| Employment | |||
| Unemployed | 56.2 | 0.9 | <.01 |
| Part time | 19.2 | 1.1 | |
| Full time | 24.6 | 0.5 | |
| Household income | |||
| < $7,200 | 24.4 | 0.9 | |
| $7,200 – $12,000 | 25.4 | 0.8 | n.s. |
| $12,000 – $16,320 | 25.2 | 0.7 | |
| > $16,320 | 25.0 | 0.8 | |
| 250% Poverty | |||
| Yes | 98.5 | 0.8 | n.s. |
| No | 1.5 | 1.8 | |
| Marital Status | |||
| Never married | 22.9 | 1.0 | |
| Married | 39.6 | 0.5 | <.01 |
| Widowed | 10.2 | 1.2 | |
| Divorced/Separated | 27.3 | 0.9 | |
| Distance to clinic | |||
| Below average (<5.19) | 58.9 | 0.7 | n.s. |
| Above average (>=5.19) | 41.1 | 1.0 | |
Table 2.
Descriptive summary of characteristics of racial/ethnic group differences among abnormal screening results
| Race/ethnicity
|
|||||
|---|---|---|---|---|---|
| White | Black | Hispanics | Other | p | |
| Individual level characteristics | |||||
|
| |||||
| % Abnormal | 1.6 | 1.1 | 0.7 | 0.5 | <.01 |
| Mean miles traveled to clinic | 6.7 | 4.4 | 5.3 | 6.3 | <.01 |
| Mean Age | 52.7 | 53.6 | 51.4 | 52.5 | <.01 |
| HS or More Education | 72.2 | 75.1 | 59.8 | 76.8 | <.01 |
| Mean Household Income | $14,339 | $10,518 | $13,835 | $15,552 | <.01 |
| Mean Household Size | 1.6 | 1.7 | 2.6 | 2.2 | <.01 |
| Employment | |||||
| % Unemployed | 53.4 | 59.8 | 54.8 | 50.4 | <.01 |
| % Part Time Employed | 29.0 | 21.0 | 14.5 | 25.5 | |
| % Full Time Employed | 17.6 | 19.2 | 30.7 | 24.2 | |
| Marital Status | |||||
| % Never married | 20.5 | 33.2 | 18.0 | 7.4 | |
| % Married | 36.6 | 18.4 | 51.7 | 69.5 | <.01 |
| % Widowed | 10.9 | 12.6 | 8.2 | 9.6 | |
| % Divorced/Separated | 32.1 | 35.8 | 22.0 | 13.5 | |
|
| |||||
| Census tract characteristics | |||||
|
| |||||
| % Poverty | 11.1 | 22.2 | 17.3 | 15.5 | <.01 |
| % Female headed households | 9.8 | 23.0 | 12.1 | 9.9 | <.01 |
| % Unemployed | 6.7 | 26.9 | 9.4 | 7.5 | <.01 |
| % Less than HS educated | 24.1 | 26.9 | 41.1 | 27.0 | <.01 |
| % White | 68.2 | 17.2 | 50.9 | 56.4 | <.01 |
| % Black | 12.3 | 75.9 | 12.0 | 11.1 | <.01 |
| % Hispanic | 22.9 | 7.4 | 54.6 | 20.2 | <.01 |
Third, to test Hypothesis 1, the association between race/ethnicity and the distance to the clinic, controlling for individual and census tract level variables, we performed two -level hierarchical linear models (Table 3). The multilevel model accounts for clustering of individual outcome measures within census tracts: this allows for the correlation of error terms within each community (Goldstein, 1995). For the continuous outcome variable (distance in miles), we modeled two-level linear regressions (Bryk & Raudenbush, 1992). In all models, only the intercept was treated as random to explore differences in average measures between communities along with individual variance.
Table 3.
Hierarchical linear regression models explaining distance to the clinic
| Models | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| I | II | III | ||||
|
| ||||||
| Coeff | SE | Coeff | SE | Coeff | SE | |
| Race/Ethnicity | ||||||
| White | - | - | - | - | - | - |
| Black | −0.67** | 0.17 | −0.84** | 0.19 | −0.68** | 0.19 |
| Hispanic | 0.15** | 0.09 | 0.02 | 0.09 | 0.05 | 0.09 |
| Other | −0.25 | 0.20 | −0.03 | 0.21 | −0.01 | 0.21 |
| Age | ||||||
| < 40 | 0.42* | 0.17 | 0.43* | 0.17 | ||
| 40–49 | - | - | - | - | ||
| 50–64 | −0.22 | 0.14 | −0.24 | 0.14 | ||
| > 65 | −0.05 | 0.15 | −0.07 | 0.15 | ||
| High school or more | 0.02 | 0.06 | 0.01 | 0.06 | ||
| Household income | ||||||
| < $7,200 | −0.31** | 0.07 | −0.30** | 0.07 | ||
| $7,200 – $12,000 | −0.30** | 0.07 | −0.29** | 0.07 | ||
| $12,000 – $16,320 | −0.22** | 0.07 | −0.22** | 0.07 | ||
| > $16,320 | - | - | - | - | ||
| Marital Status | ||||||
| Never married | 0.08 | 0.07 | 0.08 | 0.07 | ||
| Married | - | - | - | - | ||
| Widowed | 0.08 | 0.08 | 0.08 | 0.08 | ||
| Divorced/Separated | −0.05 | 0.06 | −0.06 | 0.06 | ||
| Employment | ||||||
| Unemployed | −0.03 | 0.06 | −0.03 | 0.06 | ||
| Part time | - | - | - | - | ||
| Full time | −0.29** | 0.07 | −0.28** | 0.07 | ||
| % Poverty | −0.19** | 0.01 | ||||
| % African Americans | 0.002 | 0.004 | ||||
|
| ||||||
| Intercept | 7.70** | 0.19 | 7.68** | 0.22 | 10.72** | 0.31 |
|
| ||||||
| Variance component | 31.25** | 27.02** | 20.29** | |||
p < .05;
p < .01
Note: Unstandardized coefficients are presented. Model I included only race/ethnicity. Model II included individual level variables. Model III included census tract level variables.
First, we examined the effect of race/ethnicity only (Model 1). We then introduced other sociodemographic variables (Model 2). Finally, we included census tract level variables (Model 3). To test Hypothesis 2, the association between distance to clinic and abnormal mammogram results, we specified nonlinear Bernoulli models since the outcome variable was a dichotomous variable (abnormal vs. normal), accounting for census tract level clustering and controlling for individual-level characteristics (Table 4). To explore changes in the size of coefficients of independent variables, we introduced explanatory variables step-wise: first, race/ethnicity and other individual level socioeconomic measures (Model I) were included. We added the distance measure to the model (Model II). Alternatively, we included census tract level measures (Model III). The final model included both census tract level measures and the distance (Model IV). To test Hypothesis 3, we compared the effect of race/ethnicity variables in Model I with the rest of the models. We compared odds ratios of each variable in relation to the dependent variable to determine the size and the direction of the effect on the likelihood of having abnormal mammogram results.
Table 4.
Logistic regressions explaining having abnormal mammogram results
| Models
|
||||
|---|---|---|---|---|
| I | II | III | IV | |
| Race/Ethnicity | ||||
| White | - | - | - | - |
| Black | 0.58* | 0.68 | 0.66 | 0.73 |
| Hispanic | 0.33** | 0.36** | 0.34** | 0.36** |
| Other | 0.35* | 0.35* | 0.36* | 0.36* |
| Age | ||||
| < 40 | 1.82 | 1.73 | 1.80 | 1.73 |
| 40–49 | - | - | - | - |
| 50–64 | 0.43 | 0.43 | 0.42* | 0.43* |
| > 65 | 3.30* | 3.32* | 3.36* | 3.35** |
| High school or more | 1.39 | 1.35 | 1.36 | 1.34 |
| Household income | ||||
| < $7,200 | 0.74 | 0.78 | 0.75 | 0.78 |
| $7,200 – $12,000 | 0.76 | 0.81 | 0.78 | 0.81 |
| $12,000 – $16,320 | 0.74 | 0.77 | 0.75 | 0.77 |
| > $16,320 | - | - | - | - |
| Marital Status | ||||
| Never married | 1.55 | 1.62 | 1.57 | 1.62 |
| Married | - | - | - | - |
| Widowed | 1.52 | 1.56 | 1.52 | 1.56 |
| Divorced/Separated | 1.11 | 1.14 | 1.11 | 1.13 |
| Employment | ||||
| Unemployed | 0.80 | 0.82 | 0.80 | 0.81 |
| Part time | - | - | - | - |
| Full time | 0.53* | 0.55* | 0.55* | 0.56* |
| Distance in miles | 1.06** | 1.06** | ||
| % Poverty | 0.99 | 0.99 | ||
| % African Americans | 1.00 | 1.00 | ||
|
| ||||
| Intercept | 0.01** | 0.01** | 0.01** | 0.01** |
| Variance component | 0.30n.s. | 0.30n.s. | 0.28n.s. | 0.30n.s. |
p < .05;
p < .01
Note: Odds ratios are presented; Models differ based on variables included in the analysis.
RESULTS
Sample characteristics
Overall, more than 72% of women received one service and 95% utilized the IBCCP services three times or less (ranged 1 to 11 visits). Table 1 describes characteristics of women in the analysis. About 45% of women who received mammogram from the IBCCP were Hispanic and about 37% were black. Women who were White or other ethnicities each accounted for less than 10% of the sample. More than 60% of women were between 50 years and 64 years old, and an additional 33% were between 40 and 49 years old. The majority of women had more than a HS education, but more than 56% were unemployed. The mean household income was $12,790 (median=$12,000), and women in the top 25% quartile had an annual household income over $16,320. Adjusting for the number of people living in household, only 1.5% did not meet the IBCCP’s 250% poverty line criteria. Nearly 40% were married and 23% had never been married/single.
The mean distance from women’s residential location to the clinic was 5.2 miles; with the median of 4.4 miles. Just over 41% traveled further than the average. The majority of follow-up test results were normal; a total of 173 women (0.8%) were found to have abnormal breast cancer screening results. Overall, bivariate comparison results showed that women who are white, older than 65 years of age, with a high school or more education, employed part-time, and the widowed were more likely to have abnormal results (Table 1).
Racial/Ethnic differences
All explanatory variables included in the study were significantly different between racial/ethnic groups (Table 2). White women had the highest % of abnormal results, and traveled the farthest distance to clinic. Hispanic women traveled the shortest distance to clinic. On average, black women were older (54 years) than other racial/ethnic women; and Hispanic women were younger (51 years) than other racial/ethnic women. Only 60% of Hispanic women had a high school education; while 77% of other racial/ethnic women, 75% of black women, and 72% of white women had completed high school or more education. The average income was also the highest among other racial/ethnic women, followed by white and Hispanic women. Black women had the lowest average household income. More than 30% of Hispanic women were employed full-time, while 18% of white and 19% of black women were employed full-time.
Census tract level neighborhood characteristics were significantly different by racial/ethnic group. The average percent of residents living below the poverty line was highest for black women (22%), followed by Hispanic women (17%), other women (16%), and white women (11%). A similar pattern was showed for % female headed households and % unemployed. The average proportion of residents with less than high school education was significantly higher for Hispanic women (41%), while it was 27% for black and other racial/ethnic women, and 24% for white women. Overall, white women were living in predominantly white communities (68% whites), compared with black women (17% of residents were whites); and black women were living in predominantly black communities (76% black residents), compared with other racial groups (between 11% and 12% of residents being black).
Distance to the clinics
Table 3 describes the results of hierarchical linear regression models for distance traveled to the clinics in miles. Regression coefficients and standard errors are reported in the table. When only the race/ethnicity dummy variables were in the model, black women traveled a shorter distance but Hispanic women traveled a longer distance to a clinic compared with white women (Model I).
However, controlling for relevant individual and census tract level sociodemographic variables (Models II and III), the difference between Hispanic and white women disappeared; but black women traveled a shorter distance compared with white women. This result supported Hypothesis I: the average distance to the clinic may differ by race/ethnicity. In addition, women younger than 40 years of age traveled longer distances compared with women 40–49 years old. Women in the highest income quartile traveled longer distances than women in the lower income quartiles. Women employed full-time traveled shorter distances compared with those employed part-time. Women living in neighborhoods with a greater proportion of poverty traveled shorter distances, controlling for other variables. However, the census tract level proportion of African American residents was not associated with travel distance to clinics.
Abnormal mammogram results
Table 4 summarizes the results from logistic regression models for having abnormal mammogram results. First, controlling for other variables, white women were more likely than all other race/ethnic women to have an abnormal mammogram (Model I).
When the distance measure was introduced, the difference between black and white women was no longer statistically significant, controlling for other variables (Models II). However, Hispanic and other race/ethnic women were less likely than white women to have an abnormal mammogram. In addition, Model II shows the significant effect of distance on abnormal mammogram: for each one mile increase in the distance to clinic, the likelihood of having abnormal mammogram results increased by six percent. This result supported our Hypothesis II: distance to screening facilities has an independent effect on the likelihood of having an abnormal mammogram.
Finally, introducing two census tract level variables, women 50–64 years old were 57% less likely but women older than 65 were 3.5 times more likely than women 40–49 years of age to have abnormal results (Model III). Women employed full-time were 44% less likely than women employed part-time to have an abnormal mammogram. Census tract level variables, % living below poverty and % African Americans were not associated with the likelihood of having an abnormal mammogram, however, distance continued to be significantly associated with the likelihood of having abnormal mammogram results (Model III), controlling for all other variables. This result confirms Hypothesis III where the difference in the likelihood of having abnormal mammogram between white and black women may be in part accounted for by distance to clinic.
DISCUSSION
Our findings suggest that the distance women traveled to a clinic was a significant predictor for having an abnormal mammogram, controlling for relevant individual and community-level sociodemographic characteristics. IBCCP provides free breast cancer screening services to women without health insurance, so by definition, women in our analysis were predominantly minority women living in poverty. For such women with limited resources who do not have access to reliable transportation, access to and proximity to health care facilities is an important factor that could affect routine screening and timely follow-up.
Racial/ethnic differences were observed in the odds of having abnormal results. White women were more likely to have abnormal mammogram results, compared with black, Hispanic, and other ethnic women, but after controlling for distance to clinic, the black-white difference was no longer significant, while Hispanic and other ethnicities continued to be less likely to have abnormal results. Interestingly, white women traveled the farthest compared to all other racial/ethnic women, although white women were more likely to have abnormal results. This finding posits a significant policy implication in terms of allocating resources, such as determining locations of IBCCP clinics. Clearly, IBCCP clinics are strategically located in poor neighborhoods.
In addition, our finding indicated that women living in poorer census tracts traveled a shorter distance to a clinic, controlling other factors. This finding suggests that IBCCP clinics are primarily located in impoverished neighborhoods and that underserved women utilize these services. However, poor white women may not be living in impoverished neighborhoods, which are often predominantly ethnic minority neighborhoods. This pattern is an example of racial residential segregation, even among those who are living in poverty. White women who live in relatively less poor neighborhoods may have less access to health care and/or social services. Safety net clinics, such as IBCCP, are designed to provide health care to underserved, uninsured individuals, and are strategically located in poor neighborhoods. And, those who are poor and uninsured, but reside in relatively less poor neighborhoods, may have a harder time accessing safety net clinics. This finding illustrates the difficulty in policy decision-making in attempting to meet the needs of all underserved women. It is a challenge to develop effective ways to provide necessary care to those living in areas where social services and health care are scarce, due to the fact that the neighborhoods as a whole are not necessarily considered to be in need. In segregated urban cities, such as Chicago, a high correlation between neighborhood-level poverty and the composition of racial/ethnic minorities has been observed. Poor white women, on the other hand, may not necessarily reside in high poverty neighborhoods where social services agencies and clinics are often located. Clear, this finding illustrates that neighborhood characteristics may influence the level of access to care and resources (Pornet, Dejardin, Morlais, Bouvier, & Launoy, 2010). For illnesses where incidence rates are higher for white women in particular, such as breast cancer, designing and allocating services and resources need more careful consideration. Policies that simply target poor neighborhoods or predominantly minority areas inevitably exclude women whose population-level incidence rates are higher than other race/ethnic groups.
Women employed full-time were 44% less likely to have an abnormal mammogram, compared with women employed part-time. Furthermore, the average travel distance was shorter for women employed full-time than for women employed part-time. This finding might reflect the fact that Hispanic women have relatively lower rates of abnormal mammograms, shorter average travel distances to clinics, and are more likely to be employed full-time (31%) compared with any other women (18%–24%). However, it is not clear whether the association between employment status and access to care among the poor underserved women might mean some other latent characteristics of the individuals and the neighborhoods (such as collective efficacy and social capital) strengthens the capacity for individuals and neighborhoods to advocate themselves and to obtain necessary resources, such as IBCCP clinics or other community health clinics. Further research is warranted to investigate the effect of employment on collective efficacy and access to care.
Non-linear associations are shown between age group and abnormal mammogram results, as well as the distance to the clinic. Women in the oldest quartile were more likely to have an abnormal mammogram. This was expected considering that breast cancer incidence increases with age (DeSantis, Siegel, Bandi, & Jemal, 2011). This observation suggests the need to improve follow-up behavior among older women (Young & Severson, 2005). Although our findings suggested that younger women tended to travel further to clinics, other potential barriers to the follow-up of abnormal test results among older aged women should be considered, including lack of knowledge and information on cancer screening, and fear or fatalistic beliefs (Glanz et al., 1992; Hughes, Lerman, & Lustbader, 1996; Lerman et al., 1993; Rimer, Schildkraut, Lerman, Lin, & Audrain, 1996). Other issues might be lack of social support networks, as older individuals may be socially isolated (Klinenberg, 2003). To develop interventions to improve follow-up of abnormal test results among older women, additional qualitative exploration of behavioral barriers specific to older women may help underlying mechanisms for the differences.
Overall, the findings suggested that how far women live from breast cancer screening facilities was associated with a greater likelihood of having abnormal mammogram results. In addition, race/ethnicity, age, education, income, marital status, employment status, and neighborhood poverty and racial composition were associated with distance to screening facilities. These findings confirm our conceptual model with which we hypothesized that broader social factors influence health outcomes, in this case, abnormal mammogram results. While distance to screening facilities was a significant factor affecting the likelihood of having an abnormal mammogram among disadvantaged women, such access to care and the proximity to care was a function of other individual and neighborhood level sociodemographic factors.
LIMITATIONS
We used data from the Illinois Breast and Cervical Cancer Program, which is designed to provide free cancer screening services: Consequently, women included in our study were low-income, uninsured, and living below 250% of federal poverty level. Our finding showed a significant association between distance to clinics and abnormal mammogram results, which indicates that having cancer screening clinics close to home is an important aspect of access to care, but the finding was limited to underserved women who may not have reliable transportation to and fro clinics. For those who have resources to have a car or someone to provide transportation, distance to clinics may not be an important factor in determining access to care. The effect of distance to health facilities on health outcomes may interact with one’s socioeconomic status. The dataset we used for this analysis did not allow us to explore such individual-level associations. Our study was not designed to explain mechanisms of mediating individual-level factors between sociodemographic factors and cancer screening behavior. For example, social support, knowledge, and attitudes toward health care system may affect health care utilization. Such factors may interact with physical distance to clinics and access to care, which may further mitigate the negative effect of distance to clinics.
Researchers have argued that using neighborhood level characteristics in explaining individual level outcomes introduces significant methodological problems, particularly the spatial uncertainty problem (Kwan, 2012). Research that uses only residential locations to determine distance to health clinics, ignores how individuals spend their time in other neighborhoods where they may spend a great deal of time working, shopping, attending church, or visiting relatives. Our study did not take into account other locations than one’s residential address, which may have overestimated the effect of distance.
Furthermore, the way geographic areas obtain clinics providing free screening programs, such as community health centers (CHCs) and federally qualified health centers (FQHCs), is not only based on how underserved these areas are but also whether they have the political and social capital to organize their constituents and organizations to apply and receive such clinics. In general CHCs and FQHCs are located in areas that are identified as underserved with the Medically Underserved Areas (MUAs). The Health Resources and Services Administration (HRSA) established criteria for MUAs based on the Index of Medical Underservice (IMU); areas found to have less than 62 on this score are eligible for the MUA designation. However, it is not automatic that all neighborhoods with an IMU score less than 62 get MUA status. Thus poor neighborhoods eligible to be MUAs may not be designated when they lack necessary political and social capital to mobilize residents and to acquire such status. Further research is warranted to understand the effects of individual and collective level social, economic, and political resources on access to care and consequently health outcomes.
CONCLUSION
Our study showed that distance to cancer screening clinics is associated with the likelihood of having an abnormal mammogram. Access to quality care helps women obtain routine screening and timely follow-up of abnormal changes, which in turn results in earlier diagnosis of breast cancer. Having cancer screening facilities close to home may be even more important to disadvantaged women who may not have access to reliable transportation. There is a need for developing effective health policy and planning that can help overcome barriers to access to necessary routine cancer screening. In recent years, Chicago and Cook County, IL where this analysis was based, has experienced significant sociodemographic changes. The gentrification process may have contributed to reducing concentrated poverty in certain areas, which could have had a positive effect on the health of residents living in such areas. But such changes in the county triggered sociodemographic changes, and as a consequence, inner city poor minorities may have been relocated/dispersed to suburban areas. This introduces potential challenges as individuals with limited resources may have become dispersed to wider suburban areas, which would require a new way of designing health care and other social services for the underserved.
Other fundamental social factors may determine, at least in part, the level of access to care in the first place. Future research needs to explore other mediating factors, in addition to the distance to the clinic, which may affect cancer outcomes. Neighborhood social characteristics, such as collective efficacy, social capital, and social network, are less researched factors that might affect not only health care utilization and health behavior, but also the level of neighborhood capacity which may increase their access to care and resources (Link & Phelan, 2002; Link & Phelan, 2005; Phelan & Link, 2005).
Health disparities can take different forms depending on cancer types that have reliable screening tools such as breast and cervical compared with cancer types with no screening tools, such as ovarian cancer. Specific characteristics and potential factors in each step of the disease process need to be thoroughly explored, including routine screening, follow-up of abnormal test results, early diagnosis, adequate treatment, and survival.
Acknowledgments
This study is funded in part by the NIMHD Comprehensive Centers of Excellence (P60- MD003424).
Contributor Information
Seijeoung Kim, University of Illinois at Chicago, School of Public Health.
Beverly Chukwudozie, University of Illinois at Chicago, Institute for Health Research and Policy.
Elizabeth Calhoun, University of Illinois at Chicago, School of Public Health.
References
- American Cancer Society. Cancer Facts and Figures, 2007. Atlanta, GA: American Cancer Society; 2007. [Google Scholar]
- Berry D, Cronin K, Plevritis S, Fryback D, Clarke L, Zelen M, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- Bradley C, Given C, Roberts C. Disparities in cancer diagnosis and survival. Cancer. 2001;91:178–188. doi: 10.1002/1097-0142(20010101)91:1<178::aid-cncr23>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Breastcancer.org. US breast cancer statistics. 2011 from http://www.breastcancer.org/symptoms/understand_bc/statistics.jsp.
- Brookfield K, Cheung M, Lucci J, Fleming L, Koniaris L. Disparities in survival among women with invasive cervical cancer: a problem of access to care. Cancer. 2009;115(1):166–178. doi: 10.1002/cncr.24007. [DOI] [PubMed] [Google Scholar]
- Brown M, Riley G, Schussler N, Etzioni R. Estimating health care costs related to cancer treatment from SEER-Medicare data. Med Care. 2002;40(8 Suppl):104–117. doi: 10.1097/00005650-200208001-00014. [DOI] [PubMed] [Google Scholar]
- Campbell RT, Li X, Dolecek TA, Barrett RE, Weaver KE, Warnecke RB. Economic, racial, and ethnic disparities in breast cancer in the US: Towards a more comprehensive model. Health & Place. 2009;15(3):870–879. doi: 10.1016/j.healthplace.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Breast cancer screening rates. 2011 from http://www.cdc.gov/cancer/breast/statistics/screening.htm.
- Celaya MO, Berke EM, Onega TL, Gui J, Riddle BL, Cherala SS, et al. Breast cancer stage at diagnosis and geographic access to mammography screening (New Hampshire, 1998–2004) Rural and Remote Health. 2010;10:1361. [PMC free article] [PubMed] [Google Scholar]
- Cho YI, Johnson TP, Barrett RE, Campbell RT, Dolecek T, Warnecke RB. Neighborhood changes in concentrated immigration and late stage breast cancer diagnosis. Journal of Immigrant and Minority Health. 2011;13(1):9–14. doi: 10.1007/s10903-010-9339-3. [DOI] [PubMed] [Google Scholar]
- Clegg L, Reichman M, Miller B, Hankey B, Singh G, Lin Y, et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes Control. 2009;20(4):417–435. doi: 10.1007/s10552-008-9256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consedine N, Magai C, Krivoshekova Y, Ryzewicz L, Neugut A. Fear, anxiety, worry, and breast cancer screening behavior: A critical review. Cancer Epidemiology, Biomarkers & Prevention. 2004;13:501–510. [PubMed] [Google Scholar]
- Cross CK, Harris J, Recht A. Race, socioeconomic status, and breast carcinoma in the U.S: what have we learned from clinical studies. Cancer. 2002;95(9):1988–1999. doi: 10.1002/cncr.10830. [DOI] [PubMed] [Google Scholar]
- DeSantis C, Siegel R, Bandi P, Jemal A. Breast Cancer Statistics, 2011. CA A Cancer Journal for Clinicians. 2011;61(6):409–418. doi: 10.3322/caac.20134. [DOI] [PubMed] [Google Scholar]
- Edwards B, Ward E, Kohler B, Eheman C, Zauber A, Anderson R, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eley JW, Hill HA, Chen VW, Austin DF, Wesley MN, Muss HB, et al. Racial differences in survival from breast cancer. Results of the National Cancer Institute Black/White Cancer Survival Study. JAMA. 1994;272(12):947–954. doi: 10.1001/jama.272.12.947. [DOI] [PubMed] [Google Scholar]
- Engelman K, Hawley D, Gazaway R, Mosier M, Ahluwalia J, Ellerbeck E. Impact of geographic barriers on the utilization of mammograms by older rural women. Journal of the American Geriatrics Society. 2002;50(1):62–68. doi: 10.1046/j.1532-5415.2002.50009.x. [DOI] [PubMed] [Google Scholar]
- Ferrante J, Gonzalez E, Roetzheim R, Pal N, Woodard L. Clinical and Demographic Predictors of Late-Stage Cervical Cancer. Arch Fam Med. 2000;9(5):439–445. doi: 10.1001/archfami.9.5.439. [DOI] [PubMed] [Google Scholar]
- Glanz K, Resch N, Lerman C, Blake A, Gorchov P, Rimer B. Factors associated with adherence to breast cancer screening among working women. JOM. 1992;34(11):1071–1078. doi: 10.1097/00043764-199211000-00008. [DOI] [PubMed] [Google Scholar]
- Grisby P, Hall-Daniels L, Baker S, Perez C. Comparison of clinical outcomes in black and white women treated with radiotherapy for cervical carcinoma. Gynecol Oncol. 2000;79:357–361. doi: 10.1006/gyno.2000.5974. [DOI] [PubMed] [Google Scholar]
- Halpern MT, Ward EM, Pavluck AL, Schrag NM, Bian J, Chen AY. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: A retrospective analysis. The Lancer Oncology. 2008;9(3):222–231. doi: 10.1016/S1470-2045(08)70032-9. [DOI] [PubMed] [Google Scholar]
- Hughes C, Lerman C, Lustbader E. Ethnic differences in risk perception among women at increased risk for breast cancer. Breast Cancer Res Treatment. 1996;40:25–35. doi: 10.1007/BF01806000. [DOI] [PubMed] [Google Scholar]
- Hyndman J, Holman C, Dawes V. Effect of distance and social disadvantage on the response to invitations to attend mammography screening. J Med Screen. 2000;7:141–145. doi: 10.1136/jms.7.3.141. [DOI] [PubMed] [Google Scholar]
- Illinois Department of Public Health. Cancer Mortality by Race, Illinois, 1986–2006. 2009. [Google Scholar]
- Illinois Department of Public Health. About the Illinois Breast and Cervical Cancer Program (IBCCP) 2011 from http://cancerscreening.illinois.gov/
- Kirby J, Taliaferro G, Zuvekas S. Explaining racial and ethnic disparities in health care. Medical Care. 2006;44(5 Suppl):I-64–I-72. doi: 10.1097/01.mlr.0000208195.83749.c3. [DOI] [PubMed] [Google Scholar]
- Klinenberg E. Heat Wave: A Social Autopsy of Disaster in Chicago (Illinois) Chicago, IL: University of Chicago Press; 2003. [DOI] [PubMed] [Google Scholar]
- Kwan M. The uncertain geographic context problem. Annals of the Association of American Geographers. 2012;102(5):958–968. [Google Scholar]
- Lerman C, Daly M, Sands C, Balshem A, Lustbader E, Heggan T, et al. Psychological distress interferes with mammography adherence among women at risk for breast cancer. J Nat Cancer Inst. 1993;85:1074–1080. doi: 10.1093/jnci/85.13.1074. [DOI] [PubMed] [Google Scholar]
- Link B, Phelan J. McKeown and the idea that social conditions are fundamental causes of disease. Am J Public Health. 2002;92(5):730–732. doi: 10.2105/ajph.92.5.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link B, Phelan J. Fundamental Sources of Health Inequalities. In: Mechanic D, Rogut L, Colby D, Knickman J, editors. Policy challenges in modern health care. New Brunswick, NJ: Rutgers University Press; 2005. [Google Scholar]
- Liu T, Wang X, Waterbor J, Weiss H, Soong S. Relationships between socioeconomic status and race specific cervical cancer incidence in the United States, 1973–1992. J Health Care Poor Underserved. 1998;9:420–432. doi: 10.1353/hpu.2010.0482. [DOI] [PubMed] [Google Scholar]
- Lundin F, Christopherson W, Mendez W, Parker J. Morbidity from cervical cancer: effects of cervical cytology and socioeconomic status. J Natl Cancer Inst. 1965;35:1015–1025. [PubMed] [Google Scholar]
- Magai C, Consedine N, Conway F, Neugut A, Culver C. Diversity Matters: Unique populations of women and breat cancer screening. Cancer. 2004;100(11):2300–2307. doi: 10.1002/cncr.20278. [DOI] [PubMed] [Google Scholar]
- Mandelblatt J, Andrews H, JK, Zauber A, Burnett W. Determinants of late stage diagnosis of breast cancer and cervical cancer: the impact of age, race, social class, and hospital type. Am J Public Health. 1991;81:646–649. doi: 10.2105/ajph.81.5.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelblatt J, Andrews H, Kerner J, Zauber A, Burnett W. Determinants of late stage diagnosis of breast and cervical cancer: the impact of age, race, social class, and hospital type. Am J Public Health. 1991;81(5):646–649. doi: 10.2105/ajph.81.5.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundt A, Connell P, Campbell T. Race and clinical outcomes in patients with carcinoma of the uterine cervix treated with radiation therapy. Gynecol Oncol. 1998;71:151–158. doi: 10.1006/gyno.1998.5203. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. SEER Cancer Statistics Review, 1975–2006. Bethesda, MD: National Cancer Institute; 2009. [Google Scholar]
- National Cancer Institute. Cancer Trends Progress Report, 2009/2010 update. Bethesda, MD: NIH, DHHS; 2010a. [Google Scholar]
- National Cancer Institute. SEER Cancer Statistics Review, 1975–2007. Bethesda, MD: National Cancer Institute; 2010b. [Google Scholar]
- National Cancer Institute. A snapshot of breast cancer. 2011 from http://www.cancer.gov/aboutnci/servingpeople/snapshots/breast.pdf.
- Nelson H, Tyne K, Naik A, Bougatsos C, Chan B, Humphrey L. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151:727–737. doi: 10.1059/0003-4819-151-10-200911170-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peipins L, Graham S, Young R, Lewis B, Foster S, Flanagan B, et al. Time and distance barriers to mammography facilities in the Atlanta metropolitan area. J Community Health. 2011;36(4):675–683. doi: 10.1007/s10900-011-9359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan J, Link B. Controlling disease and creating disparities: A fundamental cause perspective. Journals of Gerontology: Series B. 2005;60B(Special Issue II):27. doi: 10.1093/geronb/60.special_issue_2.s27. [DOI] [PubMed] [Google Scholar]
- Pornet C, Dejardin O, Morlais F, Bouvier V, Launoy G. Socioeconomic and healthcare supply statistical determinants of compliance to mammography screening programs: A multilevel analysis in Calvados, France. Cancer Epidemiology. 2010;34(3):309–315. doi: 10.1016/j.canep.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Purc-Stephenson RJ, Gorey KM. Lower adherence to screening mammography guidelines among ethnic minority women in America: A meta-analytic review. Preventive Medicine. 2008;46(6):479–488. doi: 10.1016/j.ypmed.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimer B, Schildkraut J, Lerman C, Lin T, Audrain J. Participation in a women’s breast cancer risk counseling trial. Cancer. 1996;77:2348–2355. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2348::AID-CNCR25>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Roetzheim R, Pal N, Tennant C, Voti L, Ayanian J, Schwabe A, et al. Effects of health insurance and race on early detection of cancer. J Natl Cancer Inst. 1999;91:1409–1415. doi: 10.1093/jnci/91.16.1409. [DOI] [PubMed] [Google Scholar]
- Sassi F, Luft H, Guadagnoli E. Reducing Racial/Ethnic Disparities in Female Breast Cancer: Screening Rates and Stage at Diagnosis. American Journal of Public Health. 2006;96(12):2165–2172. doi: 10.2105/AJPH.2005.071761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein M, Nietert P, Ye X, Lackland D. Access to care and stage at diagnosis for patients with lung cancer and esophageal cancer: analysis of the Savannah River Region Information System cancer registry data. The Southern Medical Journal. 2002;95(8):900–908. [PubMed] [Google Scholar]
- Swan J, Breen N, Coates R, Rimer B, Lee N. Progress in cancer screening practices in the United States: results from the 2000 National Health Interview Survey. Cancer. 2003;97:1528–1540. doi: 10.1002/cncr.11208. [DOI] [PubMed] [Google Scholar]
- Wang F, McLafferty S, Escamilla V, Luo L. Late-Stage Breast Cancer Diagnosis and Health Care Access in Illinois. Prof Geogr. 2008;60(1):54–69. doi: 10.1080/00330120701724087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnecke R, Oh A, Breen N, et al. Approaching health disparities from a population perspective: The National Institutes of Health Centers for Population Health and Health Disparities. Am J Public Health. 2008;98:1608–1615. doi: 10.2105/AJPH.2006.102525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R, Severson R. Breast cancer screening barriers and mammography completion in older minority women. Breast Cancer Research and Treatment. 2005;89:111–118. doi: 10.1007/s10549-004-1476-8. [DOI] [PubMed] [Google Scholar]
- Zenk SN, Tarlov E, Sun J. Spatial equity in facilities providing low- or no-fee screening mammography in Chicago neighborhoods. Journal of Urban Health: Bulletin of the New York Academy of Medicine. 2006;83(2):195–210. doi: 10.1007/s11524-005-9023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]