Abstract
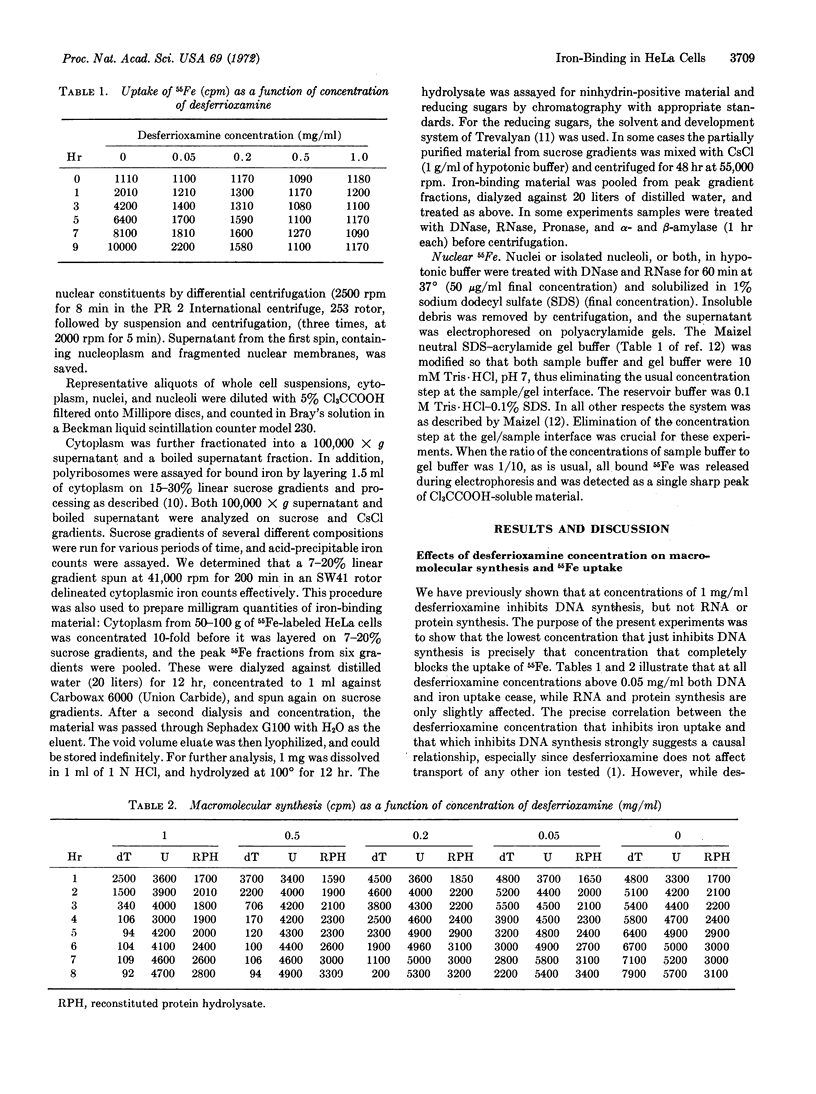
The concentration of the iron-chelating agent, desferrioxamine (Desferal), that just inhibits iron entry into HeLa cells is also the concentration that inhibits DNA synthesis. As a first step in clarification of the mechanism whereby iron may partake in DNA synthesis, we have partially characterized several of the intracellular iron-binding sites. Most cytoplasmic iron appears to be bound to a polysaccharide containing glucose that sediments at about 32 S. Nucleolar iron is bound to a single protein, the mobility of which is independent of the concentration of sodium dodecyl sulfate in an acrylamide gel. In contrast the pattern and mobility of nuclear iron, other than nucleolar, is heterogeneous and markedly affected by the concentration of sodium dodecyl sulfate. The evidence suggests that nuclear iron is bound to protein through one or more intermediate(s).
Keywords: chelation, polysaccharide, protein, DNA synthesis, desferrioxamine
Full text
PDF
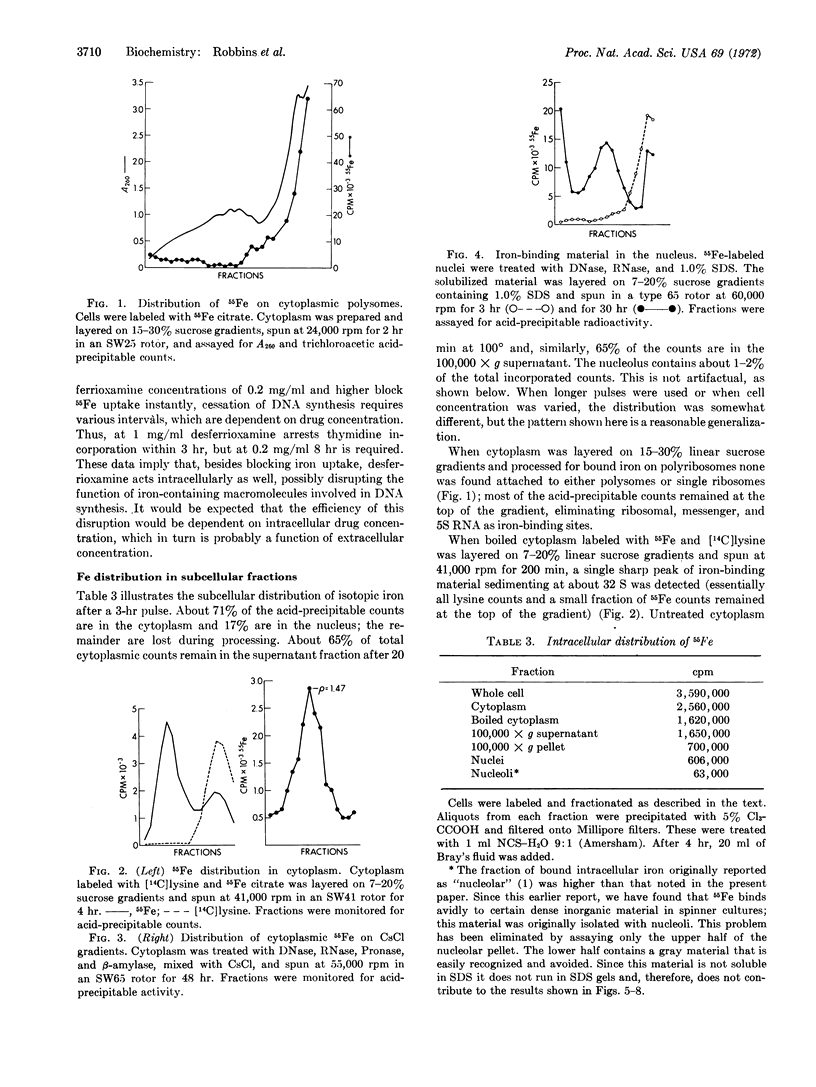

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
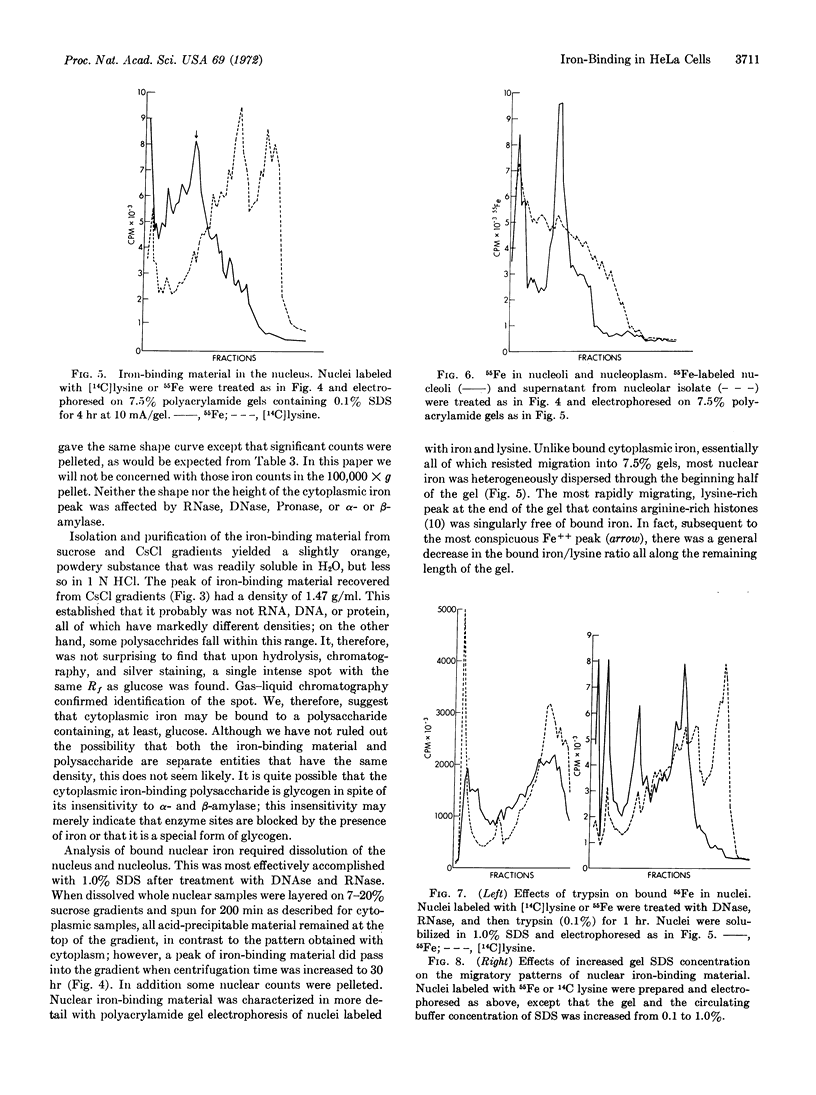
- EICHHORN G. L., CLARK P. INTERACTIONS OF METAL IONS WITH POLYNUCLEOTIDES AND RELATED COMPOUNDS. V. THE UNWINDING AND REWINDING OF DNA STRANDS UNDER THE INFLUENCE OF COPPER (II) IONS. Proc Natl Acad Sci U S A. 1965 Mar;53:586–593. doi: 10.1073/pnas.53.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANICK S. Structure and physiological functions of ferritin. Physiol Rev. 1951 Oct;31(4):489–511. doi: 10.1152/physrev.1951.31.4.489. [DOI] [PubMed] [Google Scholar]
- Robbins E., Borun T. W. The cytoplasmic synthesis of histones in hela cells and its temporal relationship to DNA replication. Proc Natl Acad Sci U S A. 1967 Feb;57(2):409–416. doi: 10.1073/pnas.57.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins E., Pederson T. Iron: its intracellular localization and possible role in cell division. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1244–1251. doi: 10.1073/pnas.66.4.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildkraut C. L., Maio J. J. Studies on the intranuclear distribution and properties of mouse satellite DNA. Biochim Biophys Acta. 1968 Jun 18;161(1):76–93. doi: 10.1016/0005-2787(68)90296-7. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]


