Abstract
T helper type 9 (Th9) cells are a novel identified subset of CD4+ T helper cells, which could partly contribute to allergic inflammation, while the precise contribution of Th9 cells in atopic dermatitis (AD) remains unknown. We aimed to explore the possible role of Th9 cells in AD pathogenesis. The Th9 cell percentage, transcription factor PU.1 and cytokine interleukin (IL)-9 mRNA levels, as well as IL-9 serum concentration in peripheral circulation, were measured in AD patients, psoriasis patients and healthy controls. The Th9 cell percentage, PU.1 and IL-9 expression levels of AD patients were all increased significantly compared with the other two control groups (P < 0·01), and correlated positively with SCORing Atopic Dermatitis index, serum immunoglobulin (Ig)E and thymus- and activation-regulated chemokine (TARC) levels (P < 0·05). In simple AD patients and AD patients complicated by allergic rhinitis or asthma, there were no significant differences in the Th9 cell percentage, PU.1 and IL-9 expression levels between them. At the same time, IL-9 and vascular endothelial growth factor (VEGF) mRNA levels were detected in AD lesions and normal skin samples, which were both distinctly elevated in AD lesions, and there was a positive association between them (P < 0·01). Keratinocytes were cultured with IL-9 stimulation and the secretion of VEGF was detected. IL-9 can promote the secretion of VEGF by keratinocytes in a time- and dose-dependent manner. In conclusion, the expansion of the Th9 cell subset, up-regulation of the PU.1 transcription factor and increased secretion of the IL-9 cytokine may contribute to the pathogenesis of AD, which may be supported by the increased release of VEGF by keratinocyes after IL-9 stimulation.
Keywords: atopic dermatitis, IL-9, Th9 cells, vascular endothelial growth factor
Introduction
Atopic dermatitis (AD) is an inflammatory skin disease characterized by pruritus and chronic or relapsing eczematous lesions that usually occurs in early infancy, but can also begin or persist in adulthood. It is well known that AD is attributed to the interaction of multiple genetic, immunological and environmental factors 1–2. Evidence from human and animal studies have demonstrated that CD4+ T helper (Th) cells play an important role in the pathogenesis of AD. Originally, CD4+ Th cells were thought to differentiate into Th1 and Th2 subsets based on their cytokine expression profiles and immune regulatory function. Interleukin (IL)-17-expressing T cells (termed Th17) and forkhead box protein 3 (FoxP3+) regulatory T cells (Treg) are newly identified subsets of CD4+ Th cells, and developing studies have reported that both of them have a pathogenic role in AD 3,4. More recently, a novel independent Th-cell subset, characterized by expression of high amounts of IL-9, which do not express T-bet, GATA-3, retinoic acid-related orphan receptor gamma T (RORγt) or FoxP3, subset-determining transcription factors associated with Th1, Th2, Th17 and Treg cells, had been recognized as ‘Th9’ cells 6–9. Although the mechanism for the differentiation and proliferation of Th9 cells has not been elucidated fully, the transcription factor PU.1 is required for the differentiation and functional activation of Th9 cells; indeed, PU.1 binds directly to the IL-9 promoter to trigger specific chromatin modifications 10,11. It has been reported that Th9 cells are involved in the development of allergic asthma and allergic rhinitis 13–14, while the precise contribution of Th9 cells in AD remains unknown. In the present study, we detected the expression levels of Th9 cells, PU.1 and IL-9 in AD peripheral circulation and skin lesions, and investigated the effect of IL-9 on the production of vascular endothelial growth factor (VEGF) by keratinocytes, to explore the possible role of Th9 cells and IL-9 in the pathogenesis of AD.
Materials and methods
Individuals and samples
Peripheral blood samples were acquired from 36 AD patients (20 males and 16 females, aged 3–16 years), among whom were 22 AD patients complicated by allergic airway diseases such as allergic rhinitis or asthma and increased serum immunoglobulin (Ig)E levels, as well as 18 psoriasis vulgaris patients (11 males and 7 females, aged 7–34 years) as a disease control and 32 healthy control subjects (18 males and 14 females, aged 6–16 years), who had not thus far been reported with atopic disorders or inflammatory skin diseases. AD was diagnosed according to the criteria defined by Rajka and Langeland 15 and clinical severity was evaluated by SCORing Atopic Dermatitis (SCORAD) index 16. Skin samples were skin punch biopsies of eight AD patients and five normal skin samples of healthy donors undertaking aesthetic or reconstructive surgery. None of the patients had been treated with systemic glucocorticoid, other immunosuppressive agents or desensitization therapy within 6 months, as well as local glucocorticoid or calcineurin inhibitor within 1 week. The human ethics committee of Binzhou Medical University Hospital approved the study, and written informed consent was obtained from all subjects.
Flow cytometric analysis of Th9 cell percentage
Peripheral blood mononuclear cells (PBMCs) were collected by Ficoll-Hypaque density gradient centrifugation, suspended in RPMI-1640 supplemented with 10% fetal calf serum (FCS), 100 U/ml penicillin, 100 μg/ml streptomycin and 1% 1 M Hepes buffer (Sigma, St Louis, MO, USA), and stimulated with 25 ng/ml phorbol myristate acetate (PMA) and 1 μg/ml ionomycin (Sigma) in the presence of GolgiStop (2 mmol/ml, BD Biosciences, San Jose, CA, USA) at 37°C under a 5% CO2 environment for 4 h. After harvesting and washing with phosphate-buffered saline (PBS), cells were stained with fluorescein isothiocyanate (FITC)-labelled CD4 antibody (eBioscience, San Diego CA, USA), fixed and permeabilized using fluorescence activated cell sorter (FACS) Perm Solution (eBioscience), according to the manufacturer's instructions. The cells were then incubated with phycoerythrin (PE)-labelled IL-9 antibody, allophycocyanin (APC)-labelled IL-4 antibody, peridinin chlorophyll-cyanin 5·5 (PerCP-Cy5·5)-labelled IL-17A antibody and PE-Cy7-labelled interferon (IFN)-γ antibody (eBioscience) for 30 min at 4°C in the dark. After washing in stain buffer [fetal bovine serum (FBS)] (eBioscience), cell samples were subjected to FACScanto flow cytometry, and data were collected and analysed using CellQuest software (BD Biosciences).
Preparation and culture of human keratinocytes
Skin samples (three AD lesions and three normal skin) were incubated at 4°C overnight in 0·3% dispase solution (Invitrogen, Carlsbad, CA, USA). After peeling off the biopsy tissue, the epidermis was digested and keratinocytes were cultured in RPMI-1640 supplemented with 20% FCS, 100 U/ml penicillin and 100 μg/ml streptomycin at 37°C, 5% CO2. Cultured keratinocytes were identified by immunocytochemistry. The complete medium was gradually replaced by keratinocyte serum-free medium (K-SFM) and the third-passage keratinocytes were used in the following experiment. Keratinocytes (1 × 104 cells/100 μl) were distributed in 96-well plates and cultured for 12 h. After discarding the culture supernatant, cell pellets were resuspended in 100 μl K-SFM with the indicated concentrations of IL-9 (10 ng/ml and 20 ng/ml) for 24 and 48 h, respectively.
Real-time quantitative reverse transcription–polymerase chain reaction (RT–PCR) analysis of PU.1, IL-9 and VEGF
Total RNA was isolated from PBMCs and skin samples with Trizol reagent (Invitrogen), following the manufacturer's instructions. The quality of RNA samples was assessed by inspecting the 28S and 18S bands after 1·5% agarose gel electrophoresis. The 260/280 absorbance ratio was between 1·9 and 2·0 for each RNA sample. A reverse transcriptase kit (PrimeScript™RT reagent kit; TaKaRa, Shiga, Japan) was used for complementary DNA (cDNA) synthesis. The expression of PU.1, IL-9 and VEGF mRNA was analysed with the primers shown in Table 1, and β-actin was used as an endogenous reference. Real-time RT–PCR was performed on a Rotor-Gene 3000 (Corbett Research, Sydney, Australia) and mRNA levels were quantified using SYBR Premix Ex Taq™ II (TaKaRa). Each reaction was performed in triplicate. Data were analysed using the Rotor-Gene real-time analysis software version 6.0.
Table 1.
List of PU.1, interleukin (IL)-9, vascular endothelial growth factor (VEGF) and β-actin primers for real-time reverse transcription–polymerase chain reaction (RT–PCR).
| Primers | Sequence (5′–3′) | Product length |
|---|---|---|
| PU.1 | Forward: 5′-AGAAGAAGATCCGCCTGTACCA-3′ | 116 bp |
| Reverse: 5′-GTGCTTGGACGAGAACTGGAA-3′ | ||
| IL-9 | Forward: 5′-GGGATCCTGGACATCAACTTC-3′ | 132 bp |
| Reverse: 5′-GAAGCATGGTCTGGTGCAGTT-3′ | ||
| VEGF | Forward: 5′-CACCCACCCACATACATACA-3′ | 161 bp |
| Reverse: 5′-TCAAGTCCACAGCAGTCAA-3′ | ||
| β-actin | Forward: 5′-AGTTGCGTTAC ACCCTTTCTTG-3′ | 150 bp |
| Reverse: 5′-TCACCTTCACCGTTCCAGTTT-3′ |
bp: base pairs.
Enzyme-linked immunosorbent assay (ELISA) for IL-9 and thymus- and activation-regulated chemokine (TARC) in serum as well as VEGF in cell-free supernatant
Peripheral venous blood was taken after an overnight fast in the morning between 08:00 and 09:00 h. Serum was centrifuged within 30 min of blood collection and stored at −80°C for further IL-9 and TARC ELISA. VEGF was detected in cell-free supernatant of the above keratinocyte culture with IL-9 stimulation by a commercial ELISA kit. The experimental procedure was conducted strictly according to the manufacturer's instructions (R&D Systems, Minneapolis, MN, USA and eBioscience).
Statistical analysis
Data were expressed as mean ± standard deviation (s.d.) and analysed statistically using spss for Windows (version 17.0; SPSS Inc., Chicago, IL, USA). The one-way analysis of variance (anova), an independent-samples t-test and Pearson's correlation were used for statistical analysis, with the level of significance set at P < 0·05.
Results
Th9 cell percentage in PBMCs
Representative pictures for the Th9 cell percentage (CD4+IL-9+/CD4+ T cells) are shown in Fig. 1a1–a2. In PBMCs, AD patients showed a significant increase of the Th9 cell percentage (1·75 ± 0·54%) compared with psoriasis patients (1·15 ± 0·24%) and healthy controls (0·88 ± 0·21%, P < 0·01, Fig. 1b), while there was no significant difference between psoriasis patients and healthy controls (P > 0·05).
Figure 1.
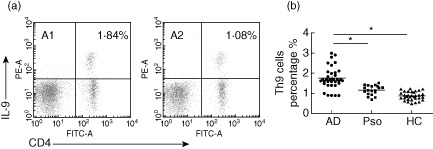
Flow cytometric analysis of T helper type 9 (Th9) cells percentage in atopic dermatitis (AD) patients, psoriasis patients and healthy controls. (a) Peripheral blood mononuclear cells (PBMCs) were isolated from a 16-year-old male AD patient (a1) and a 16-year-old male healthy individual (a2). These cells were stimulated with phorbol myristate acetate (PMA), ionomycin and GolgiStop for 4 h, stained for interleukin (IL)-9, interferon (IFN)-γ, IL-4 and IL-17A and analysed by flow cytometry. The numbers in the upper right quadrant represent the percentage of IL-9+ cells with expression of CD4+ cells in the gated population of CD4+ IFN-γ− IL-4− IL-17A− cells. (b) The percentage of Th9 cells in PBMCs was increased significantly in AD patients compared with psoriasis patients and healthy controls (*P < 0·01). Pso: psoriasis; HC: healthy controls.
PU.1 and IL-9 mRNA levels in PBMCs
The PU.1 mRNA levels in AD patients (6·08 ± 1·48) were increased compared with psoriasis patients (2·12 ± 0·78) and healthy controls (1·55 ± 0·58, P < 0·01, Fig. 2). IL-9 mRNA levels were also significantly higher in AD patients (3·41 ± 1·36) than psoriasis patients (1·68 ± 0·34) and healthy controls (1·22 ± 0·51, P < 0·01, Fig. 2). Although the PU.1 and IL-9 mRNA levels in psoriasis patients were slightly higher than healthy controls, the difference was not statistically significant (both P > 0·05).
Figure 2.
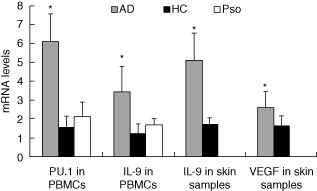
Measurement of PU.1, interleukin (IL)-9 and vascular endothelial growth factor (VEGF) mRNA levels in peripheral blood mononuclear cells (PBMCs) and skin samples by real-time reverse transcription–polymerase chain reaction (RT–PCR). The expression of PU.1 and IL-9 mRNA levels in PBMCs as well as IL-9 and VEGF mRNA levels in skin samples were all elevated significantly in atopic dermatitis (AD) patients (*P < 0·01). Pso: psoriasis; HC: healthy controls.
Serum IL-9 concentrations
In accordance with flow cytometry analysis of the Th9 cell percentage as well as real-time RT–PCR analysis of PU.1 and IL-9 mRNA levels, serum IL-9 concentrations were also up-regulated in AD patients (32·90 ± 6·86 pg/ml) compared with psoriasis patients (19·83 ± 4·37 pg/ml) and healthy controls (15·59 ± 6·12 pg/ml, P < 0·01, Fig. 3), and there was no significant difference between psoriasis patients and healthy controls (P > 0·05).
Figure 3.
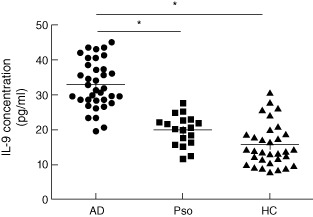
Interleukin (IL)-9 serum concentrations in AD patients, psoriasis patients and healthy controls by enzyme-linked immunosorbent assay (ELISA). The serum IL-9 concentrations were distinctively higher in atopic dermatitis (AD) patients than psoriasis patients and healthy controls (*P < 0·01). Pso: psoriasis; HC: healthy controls.
Correlation analysis of the Th9 cell percentage, PU.1 and IL-9 expression levels with SCORAD index, serum IgE and TARC levels in AD patients
Correlation analysis revealed that the Th9 cell percentage, PU.1 and IL-9 mRNA levels, as well as serum IL-9 concentration, correlated positively with SCORAD index (r = 0·422, 0·387, 0·397 and 0·435, respectively, all P < 0·05, Fig. 4a1–a4), serum IgE (r = 0·412, 0·353, 0·398 and 0·458, respectively, all P < 0·05, Fig. 4b1–b4) and TARC levels (r = 0·398, 0·409, 0·382 and 0·381, respectively, all P < 0·05, Fig. 4c1–c4).
Figure 4.
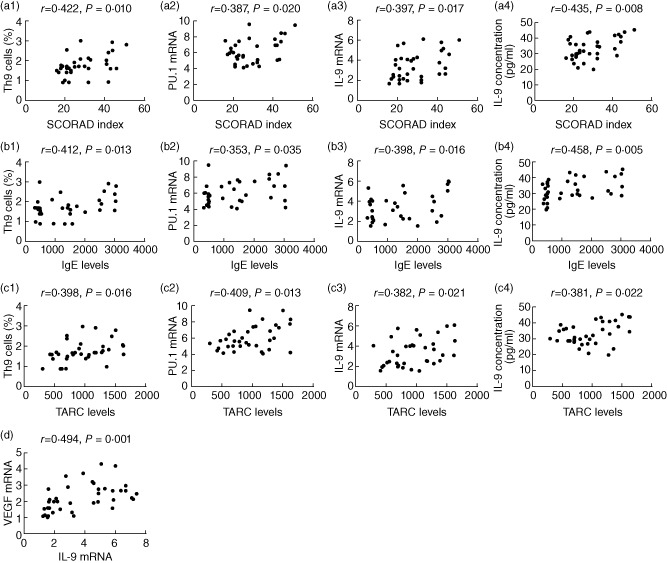
The correlation analysis of T helper type 9 (Th9) cells percentage, PU.1 and interleukin (IL)-9 expression levels with SCORing Atopic Dermatitis (SCORAD) index, immunoglobulin (IgE) and thymus- and activation-regulated chemokine (TARC) levels in atopic dermatitis (AD) peripheral circulation, as well as IL-9 with vascular endothelial growth factor (VEGF) mRNA levels in AD lesions. Th9 cells percentage, PU.1 mRNA level, IL-9 mRNA level in peripheral blood mononuclear cells (PBMCs) and serum IL-9 concentration all correlated positively with SCORAD index (a1–a4), IgE (b1–b4) and TARC (c1–c4) levels (all P < 0·05). In AD lesions, IL-9 mRNA levels showed a positive association with VEGF mRNA levels (d, P < 0·01).
Comparison of the Th9 cell percentage, PU.1 and IL-9 expression levels between simple AD patients and AD patients complicated by allergic rhinitis or asthma
Although expression levels of the Th9 cell subset, PU.1 and IL-9 in AD patients complicated by allergic rhinitis or asthma were higher than those of simple AD patients, there were no significant differences between the two subgroups (Th9 cell percentage, 1·82 ± 0·57 versus 1·64 ± 0·48, t = 0·958; PU.1 mRNA level, 6·40 ± 1·49 versus 5·58 ± 1·37, t = 1·668; IL-9 mRNA level, 3·61 ± 1·47 versus 3·10 ± 1·14, t = 1·113; serum IL-9 concentration, 33·97 ± 6·72 pg/ml versus 31·21 ± 6·99 pg/ml, t = 1·183, all P > 0·05).
IL-9 and VEGF mRNA levels in skin samples
IL-9 mRNA levels were significantly higher in AD lesions than normal skin samples (5·11 ± 1·43 versus 1·72 ± 0·32, t = 11·159, P < 0·01, Fig. 2). VEGF mRNA levels in AD lesions were also increased compared with normal skin samples (2·60 ± 0·82 versus 1·64 ± 0·50, t = 4·051, P < 0·01, Fig. 2). Correlation analysis showed a positive association between IL-9 and VEGF mRNA levels in AD lesions (r = 0·494, P = 0·001, Fig. 4d).
IL-9 stimulates VEGF secretion in human keratinocytes
To examine the effect of IL-9 on VEGF secretion, keratinocytes were treated with IL-9 (10 and 20 ng/ml) for 24 and 48 h, respectively. At the same IL-9 stimulation time, there was a significant difference in the VEGF secretion among the control, 10 ng/ml IL-9 and 20 ng/ml IL-9 groups (24-h groups, F = 53·671, P < 0·01; 48-h groups, F = 67·669, P < 0·01). In addition, at the same IL-9 stimulation concentration, VEGF secretion was significantly different between the two stimulation times (48 h, 10 ng/ml IL-9 group versus 24 h, 10 ng/ml IL-9 group, t = 10·290, P < 0·01; 48 h, 20 ng/ml IL-9 group versus 24 h, 20 ng/ml IL-9 group, t = 5·678, P < 0·01). The results are shown in Fig. 5.
Figure 5.
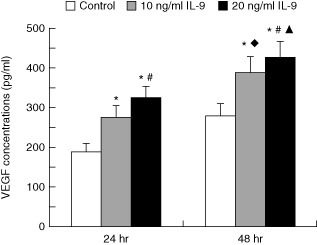
The effect of interleukin (IL)-9 on vascular endothelial growth factor (VEGF) secretion in human keratinocytes. The concentration of VEGF in the control, 10 ng/ml IL-9 and 20 ng/ml IL-9 groups was 189·79 ± 10·94 pg/ml, 275·83 ± 18·58 pg/ml and 322·06 ± 32·34 pg/ml, respectively (24 h); 279·84 ± 14·34 pg/ml, 388·39 ± 19·31 pg/ml, 425·32 ± 30·75 pg/ml, respectively (48 h). The concentrations of VEGF were elevated significantly in 10 ng/ml and 20 ng/ml IL-9 groups compared with the control group at 24 h and 48 h, respectively (*P < 0·01). The concentrations of VEGF were elevated significantly in 20 ng/ml IL-9 groups compared with the 10 ng/ml IL-9 groups at 24 h and 48 h, respectively (#P < 0·01). There were significant differences between 24 h and 48 h in the 10 ng/ml IL-9 groups (♦P < 0·01) as well as at 20 ng/ml IL-9 groups (▴P < 0·01).
Discussion
Th9 cells are a newly identified CD4+ T cell subset, which predominantly produce the effective IL-9 cytokine. Several recent publications have identified the possible relationship of Th9 cells with the two other common allergic respiratory diseases, asthma and rhinitis 13–14. Our results show for the first time that the Th9 cell percentage was elevated significantly in AD patients compared with psoriasis patients and healthy controls, which indicate that Th9 cells may participate in the pathogenesis of AD. It is well known that AD is characterized by a Th2 inflammatory response and IL-4 is one of the important Th2-specific cytokines. Th2 cells can also produce a certain amount of IL-9, which is significantly less than that of Th9 cells 8–17. Previous studies have demonstrated that transforming growth factor (TGF)-β can reprogram Th2 cells to lose their characteristic profile and switch to IL-9 secretion, and drive the differentiation of Th9 cells directly. In addition, IL-4 can enhance TGF-β-mediated IL-9 production by neutralizing the inhibitory effect of IFN-γ and by directly supporting IL-9 production 8–18. Therefore, we hypothesize that in the Th2-cytokine-rich microenvironment of AD, the reprogramming process from Th2 cells to Th9 cells may contribute, to some extent, to the increased Th9 cell number and amplified IL-9 function.
PU.1 plays a critical role in generating the Th9 phenotype and is the Th9 cell transcription factor. PU.1 binds to the IL-9 gene and is required for IL-9 production in human T cells. Decreasing PU.1 expression, either by conditional deletion in murine T cells or siRNA in human T cells, impaired IL-9 production, while ectopic PU.1 expression promoted IL-9 production 10–19. In agreement with the higher frequency of Th9 cells, we first found that PU.1 and IL-9 mRNA levels in PBMCs were increased significantly in AD patients. It has been shown that there is an autocrine-positive feedback in IL-9 secretion: the expression of IL-9 mRNA and protein is concordant and IL-9 mRNA will be up-regulated further in the presence of recombinant IL-9 8. In this study, we also found significantly higher serum IL-9 levels in AD patients, in accordance with a previous study 20. More importantly, we found that the Th9 cell percentage, PU.1 and IL-9 expression levels all correlated positively with SCORAD index, further indicating the important role exerted by Th9 cells in AD.
The IL-9 gene maps to the long arm of chromosome 5 (5q31-35), which also contains genes encoding IL-4, IL-5 and IL-13 21. In allergic asthma, polymorphisms in this region have significant associations with the development of atopy, bronchial hyper-responsiveness and elevated total IgE levels 22,23. As far as AD is concerned, the IL-9 gene rs31563 single nucleotide polymorphism (SNP) has been reported to be associated with an increased susceptibility to AD 25. TARC is an important molecule in the development and maintenance of allergic diseases, which has highly selective chemotic ability for Th2 cells, participates in the inflammation reaction of Th2 cells and associates with AD disease severity 26. In this study, we also assessed a positive association of the Th9 cell percentage, PU.1 and IL-9 expression levels with serum IgE and TARC levels, which support the possible function of Th9 cells in AD in another way. In comparison of the Th9 cell percentage, PU.1 and IL-9 expression levels between the two AD subgroups, there were no significant differences, although the subgroup of AD patients complicated by allergic rhinitis or asthma had slightly higher expression levels than those of simple AD patients. Therefore, we may consider that the involvement of Th9 cells in the pathogenesis of AD is relatively specific.
The effect of IL-9 on airway epithelial cells has been addressed in some studies. In particular, IL-9 can trigger the production of proinflammatory chemokines 27. However, there are few reports on the interaction between IL-9 and skin keratinocytes. VEGF is a major pro-angiogenic factor involved in many inflammatory diseases, which not only promotes angiogenesis but also enhances vascular permeability. In AD, significantly increased expression of biologically active VEGF has been found in the stratum corneum, which indicates that VEGF may participate in AD histological change of capillary dilatation and dermal oedema, as well as associating with the AD clinical manifestation of erythema and inflammatory oedema 28–29. In this study, increased IL-9 and VEGF mRNA levels were both detected in AD lesions, in agreement with previous studies 29–30; in addition, a positive association between IL-9 and VEGF mRNA levels was measured. Mast cells are resident dermis cells, and it has been demonstrated that IL-9 can promote the proliferation and differentiation of mast cells as well as the secretion of VEGF from mast cells 30–31. Keratinocytes are the major epidermis cells, and keratinocyte-derived VEGF has been shown as a potent mitogen for dermal microvascular endothelial cells 32. In our further in vitro study, we found the first indication that IL-9 can promote the secretion of VEGF by keratinocytes in a time- and dose-dependent manner, which adds new evidence that IL-9 can also participate in AD pathogenesis through interaction with VEGF.
In conclusion, our results suggest that expansion of the Th9 cell subset, up-regulation of the PU.1 transcription factor and increased secretion of the IL-9 cytokine may contribute to the pathogenesis of AD, and the increased release of VEGF by IL-9-stimulated keratinocyes may further support this point.
Acknowledgments
This work was funded by Ministry of Education and Education Committee, Shandong Province, China (no.J10LF90), Shandong Provincial Natural Science Foundation of China (no.ZR2010HQ013), Medical Science and Technology Development Project of Shandong Province, China (no. 2011QZ003), Science and Technology Planning Project of Shandong Province, China (no.2011YD18069) and Program of Science and Technology Development of Binzhou city, China (no.2011ZC0914).
Disclosure
The authors have no conflicts of interest to declare.
References
- 1.Ma L, Gao XH, Zhao LP, et al. Brain-derived neurotrophic factor gene polymorphisms and serum levels in Chinese atopic dermatitis patients. J Eur Acad Dermatol Venereol. 2009;23:1277–1281. doi: 10.1111/j.1468-3083.2009.03308.x. [DOI] [PubMed] [Google Scholar]
- 2.Ma L, Zhang L, Di ZH, et al. Association analysis of filaggrin gene mutations and atopic dermatitis in Northern China. Br J Dermatol. 2010;162:225–227. doi: 10.1111/j.1365-2133.2009.09539.x. [DOI] [PubMed] [Google Scholar]
- 3.Fukuyama T, Kosaka T, Miyashita L, et al. Role of regulatory T cells in the induction of atopic dermatitis by immunosuppressive chemicals. Toxicol Lett. 2012;213:392–401. doi: 10.1016/j.toxlet.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 4.Koga C, Kabashima K, Shiraishi N, et al. Possible pathogenic role of Th17 cells for atopic dermatitis. J Invest Dermatol. 2008;128:2625–2630. doi: 10.1038/jid.2008.111. [DOI] [PubMed] [Google Scholar]
- 5.Ma L, Xue HB, Guan XH, et al. Possible role of Th17 cells and IL-17 in the pathogenesis of atopic dermatitis in northern China. J Dermatol Sci. 2012;68:66–68. doi: 10.1016/j.jdermsci.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Fan Y, Weifeng W, Yuluan Y, et al. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of coxsackievirus b3-induced viral myocarditis reduces myocardium inflammation. Virol J. 2011;8:17. doi: 10.1186/1743-422X-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan J, Yu M, Lin QW, et al. Th17 cells contribute to viral replication in coxsackievirus B3-induced acute viral myocarditis. J Immunol. 2010;185:4004–4010. doi: 10.4049/jimmunol.1001718. [DOI] [PubMed] [Google Scholar]
- 8.Veldhoen M, Uyttenhove C, van Snick J, et al. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an IL-9-producing subset. Nat Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 9.Dardalhon V, Awasthi A, Kwon H, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(–) effector T cells. Nat Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perumal NB, Kaplan MH. Regulating IL9 transcription in T helper cells. Trends Immunol. 2011;32:146–150. doi: 10.1016/j.it.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang HC, Sehra S, Goswami R, et al. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat Immunol. 2010;11:527–534. doi: 10.1038/ni.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staudt V, Bothur E, Klein M, et al. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity. 2010;33:192–202. doi: 10.1016/j.immuni.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 13.Ciprandi G. Serum interleukin 9 in allergic rhinitis. Ann Allergy Asthma Immunol. 2010;104:180–181. doi: 10.1016/j.anai.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 14.Xing J, Wu Y, Ni B. Th9: a new player in asthma pathogenesis? J Asthma. 2011;48:115–125. doi: 10.3109/02770903.2011.554944. [DOI] [PubMed] [Google Scholar]
- 15.Rajka G, Langeland T. Grading of severity of atopic dermatitis. Acta Derm Venereol Suppl (Stockh) 1989;144:13–14. doi: 10.2340/000155551441314. [DOI] [PubMed] [Google Scholar]
- 16.Stadler JF, Taïeb A. Severity scoring of atopic dermatitis: the SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology. 1993;186:23–31. doi: 10.1159/000247298. [DOI] [PubMed] [Google Scholar]
- 17.Noelle RJ, Nowak EC. Cellular sources and immune functions of interleukin-9. Nat Rev Immunol. 2010;10:683–687. doi: 10.1038/nri2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmitt E, Germann T, Goedert S, et al. IL-9 production of naive CD4+ T cells depends on IL-2, is synergistically enhanced by a combination of TGF-beta and IL-4, and is inhibited by IFN-gamma. J Immunol. 1994;153:3989–3996. [PubMed] [Google Scholar]
- 19.Goswami R, Kaplan MH. Gcn5 is required for PU.1-dependent IL-9 induction in Th9 cells. J Immunol. 2012;189:3026–3033. doi: 10.4049/jimmunol.1201496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ciprandi G, De Amici M, Giunta V, et al. Serum interleukin-9 levels are associated with clinical severity in children with atopic dermatitis. Pediatr Dermatol. 2013;30:222–225. doi: 10.1111/j.1525-1470.2012.01766.x. [DOI] [PubMed] [Google Scholar]
- 21.van Leeuwen BH, Martinson ME, Webb GC, et al. Molecular organization of the cytokine gene cluster, involving the human IL-3, IL-4, IL-5, and GM-CSF genes, on human chromosome 5. Blood. 1989;73:1142–1148. [PubMed] [Google Scholar]
- 22.Postma DS, Bleecker ER, Amelung PJ, et al. Genetic susceptibility to asthma – bronchial hyperresponsiveness coinherited with a major gene for atopy. N Engl J Med. 1995;333:894–900. doi: 10.1056/NEJM199510053331402. [DOI] [PubMed] [Google Scholar]
- 23.Noguchi E, Shibasaki M, Arinami T, et al. Evidence for linkage between asthma/atopy in childhood and chromosome 5q31-q33 in a Japanese population. Am J Respir Crit Care Med. 1997;156:1390–1393. doi: 10.1164/ajrccm.156.5.9702084. [DOI] [PubMed] [Google Scholar]
- 24.Doull IJ, Lawrence S, Watson M, et al. Allelic association of gene markers on chromosomes 5q and 11q with atopy and bronchial hyperresponsiveness. Am J Respir Crit Care Med. 1996;153:1280–1284. doi: 10.1164/ajrccm.153.4.8616554. [DOI] [PubMed] [Google Scholar]
- 25.Namkung JH, Lee JE, Kim E, et al. An association between IL-9 and IL-9 receptor gene polymorphisms and atopic dermatitis in a Korean population. J Dermatol Sci. 2011;62:16–21. doi: 10.1016/j.jdermsci.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Kakinuma T, Nakamura K, Wakugawa M, et al. Thymus and activation-regulated chemokine in atopic dermatitis: serum thymus and activation-regulated chemokine level is closely related with disease activity. J Allergy Clin Immunol. 2001;107:535–541. doi: 10.1067/mai.2001.113237. [DOI] [PubMed] [Google Scholar]
- 27.Soroosh P, Doherty TA. Th9 and allergic disease. Immunology. 2009;127:450–458. doi: 10.1111/j.1365-2567.2009.03114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen L, Marble DJ, Agha R, et al. The progression of inflammation parallels the dermal angiogenesis in a keratin 14 IL-4-transgenic model of atopic dermatitis. Microcirculation. 2008;15:49–64. doi: 10.1080/10739680701418416. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Matsuo H, Morita E. Increased production of vascular endothelial growth factor in the lesions of atopic dermatitis. Arch Dermatol Res. 2006;297:425–429. doi: 10.1007/s00403-006-0641-9. [DOI] [PubMed] [Google Scholar]
- 30.Sismanopoulos N, Delivanis DA, Alysandratos KD, et al. IL-9 induces VEGF secretion from human mast cells and IL-9/IL-9 receptor genes are overexpressed in atopic dermatitis. PLOS One. 2012;7:e33271. doi: 10.1371/journal.pone.0033271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demoulin JB, Renauld JC. Interleukin 9 and its receptor: an overview of structure and function. Int Rev Immunol. 1998;16:345–364. doi: 10.3109/08830189809043001. [DOI] [PubMed] [Google Scholar]
- 32.Detmar M, Yeo KT, Nagy JA, et al. Keratinocyte-derived vascular permeability factor (vascular endothelial growth factor) is a potent mitogen for dermal microvascular endothelial cells. J Invest Dermatol. 1995;105:44–50. doi: 10.1111/1523-1747.ep12312542. [DOI] [PubMed] [Google Scholar]


