Abstract
Primary viral infections induce activation of CD8+ T cells responsible for effective resistance. We sought to characterize the nature of the CD8+ T cell expansion observed after primary viral infection with influenza. Infection of naive mice with different strains of influenza resulted in the rapid expansion of memory CD8+ T cells exhibiting a unique bystander phenotype with significant up-regulation of natural killer group 2D (NKG2D), but not CD25, on the CD44highCD8+ T cells, suggesting an antigen non-specific phenotype. We further confirmed the non-specificity of this phenotype on ovalbumin-specific (OT-I) CD8+ T cells, which are not specific to influenza. These non-specific CD8+ T cells also displayed increased lytic capabilities and were observed primarily in the lung. Thus, influenza infection was shown to induce a rapid, antigen non-specific memory T cell expansion which is restricted to the specific site of inflammation. In contrast, CD8+ T cells of a similar phenotype could be observed in other organs following administration of systemic agonistic anti-CD40 and interleukin-2 immunotherapy, demonstrating that bystander expansion in multiple sites is possible depending on whether the nature of activation is either acute or systemic. Finally, intranasal blockade of NKG2D resulted in a significant increase in viral replication early during the course of infection, suggesting that NKG2D is a critical mediator of anti-influenza responses prior to the initiation of adaptive immunity. These results characterize further the local bystander expansion of tissue-resident, memory CD8+ T cells which, due to their early induction, may play an important NKG2D-mediated, antigen non-specific role during the early stages of viral infection.
Keywords: antigen non-specific, bystander activation, memory CD8, NKG2D, tissue-resident
Introduction
Acute infection with influenza, among other viruses, leads to the activation and proliferation of CD8+ T cells, which play a critical role in cell-mediated immunity and subsequent viral clearance. The resolution of primary viral infections results in accelerated protection against subsequent infection 1–5. While acute infection has been shown to elicit proliferation of virus-specific CD8+ T cells, this expansion is suggested to be only partly antigen-driven, as limiting dilution analysis has shown only small, variable fractions of T cells to be antigen-specific 6,7. Other studies using tetramers have revealed much higher antigen-specific responses in certain viral models; up to 80% of CD8+ T cells in the spleen of lymphochoriomeningovirus (LCMV)-infected mice have been shown to be specific for 10 different LCMV-derived epitopes at the peak of primary infection 9,10. Another study investigating newly HIV-infected humans showed activation of approximately 50% of the circulating T cells, only about 1% of which were HIV-specific 12. During influenza infection, it has been observed that up to 70% of lung infiltrating CD8+ T cells showed signs of activation, whereas only 30% of those CD8+ T cells also stained positive for specific major histocompatibility complex (MHC) tetramers, given the current repertoire of known viral epitopes 13. Together these findings indicate that the proportion of non-specific, proliferating CD8+ T cells may differ among viral infections. Thus, the dynamic responses of CD8+ T cells to virally infected cells should include the contribution of antigen non-specific CD8+ T cells with regard to the kinetics of infection and the time-point being assessed.
During various acute infections, it has been observed that antigen independently proliferating T cells did not up-regulate CD25 or CD69, which are known to be up-regulated following recent T cell receptor (TCR) engagement 14. The status of the T cell, whether naive or memory, also appears critical in the initial response to infection. Cytokines induced during infection, such as type I interferons (IFN I), or IFN I-inducing reagents, such as poly I : C, lead to proliferation of memory CD44highCD8+ T cells but not naive T cells 15. Furthermore, the IFN I-induced proliferation was shown to be independent of TCR interaction with MHC class I 14 and may be facilitated by interleukin (IL)-15 production by antigen-presenting cells 16. Resting CD44highCD8+ T cells express higher levels of the CD122 (the IL-2Rβ chain, which also serves as the receptor for IL-15), than naive, CD44low T cells allowing them to be more receptive to IL-15 induced proliferation. IL-15 is believed to be a mediator of memory T cell bystander proliferation, and is also critical for memory T cell maintenance 17.
Finally, it has been suggested that memory cells, which require less IL-2 to promote effector functions, may be particularly sensitive to such antigen non-specific induction 8. Thus, the bystander response may serve not only as an amplification response but may also play a role in providing early innate effectors. While efforts have been made to characterize and describe the purpose of bystander proliferation, the immunological significance, with regard to viral infection, remains elusive. CD4+ T helper type 1 (Th1) bystander cells seem to contribute to the immune response via cytokine production, whereas no major biological consequence has been attributed to CD8+ T cell bystander proliferation 18. A recent study challenged this finding showing that, in humans, CD8+ T cells specific for herpesvirus were antigen independently activated during infections with influenza, dengue, hepatitis B and adenovirus and contributed to the immune response through production of interferon (IFN)-γ 19.
In this study we characterize the memory CD8+ T cell bystander expansion in the primary immune response to influenza. We observed that, during influenza infection, antigen-independent CD44highCD8+ T cells up-regulate the activation marker NKG2D but not CD25. NKG2D is an activating receptor expressed on most natural killer (NK) cells and activated CD8+ T cells, which has been shown to trigger cytolytic activity following engagement on NK and γδ T cells 20. This phenotype is in marked contrast to T cells in which the TCR has been engaged. The phenotype and expansion of antigen non-specific CD8+ T cells following influenza infection closely resembled that of the phenotype described following systemic cancer immunotherapy 21. Finally, NKG2D blocking studies revealed a role for NKG2D, and by account NKG2D bystander cells, in the early control of influenza virus replication. Altogether, these data support the concept that local, early bystander memory CD8+ T cell expansion and activation contribute to the immune response after influenza infection and that NKG2D-mediated effector functions may play a role.
Materials and methods
Mice
C57BL/6 and congenic Ly5·1 C57BL/6 mice were purchased from the Animal Production Area of the National Cancer Institute (Frederick, MD, USA) or Jackson Laboratories (Bar Harbor, ME, USA). Female OVA-specific CD8 T cells (OT-I) [C57BL/6-Tg(TcraTcrb)1100Mjb/J] and C57BL/6 mice and wild-type controls were purchased from the Jackson Laboratory and were used at 8–24 weeks of age. All mouse studies were performed with the consent and approval of the University of California, Davis Institutional Animal Care and Use Committee, protocol number 15359.
Influenza infection
The A/Mem71 and A/PR/8 influenza virus strains were maintained as described previously 22–23. Mice were anaesthetized lightly by isoflurane inhalation and infected sublethally with 12 000 plaque-forming units (PFU) of A/Mem71 or 20 PFU influenza virus in 40 μl of sterile PBS or 40 μl of sterile phosphate-buffered saline (PBS) alone by intranasal inoculation.
Immunotherapeutic treatment
C57BL/6 and BALB/c mice were treated with agonistic rat anti-mouse anti-CD40 (clone FGK115B3) [85 or 65 μg intraperitoneally (i.p.) for 5 days, respectively] and recombinant human IL-2 (1 million units i.p. twice a week for 2 weeks), as described previously 24. The anti-mouse CD40 antibody (clone FGK115B3) was generated via ascites production in our laboratory, as described previously 24. Recombinant human IL-2 (IL-2; TECIN Teceleukin) was provided by the National Cancer Institute repository (Frederick, MD, USA).
Adoptive transfer experiments
Spleens and lymph node cells from OT-I mice were collected and processed into single-cell suspensions. CD8 T cells were purified by magnetic separation using a CD8 T cell-negative selection kit (StemCell Technologies, Vancouver, Canada). Purification was quantified using flow cytometry staining against CD45·2+CD8+Vα2+Vβ5·1/2+ cells. The cells were then concentrated to 5 × 106 OT-I CD8+ cells per 0·2 ml and injected intravenously. Each mouse received 5 × 106 OT–I cells in 0·2 ml. One day after transfer, recipients were vaccinated with ovalbumin (OVA)/incomplete Freund's emulsion to expand the memory T cell population. Specifically, OVA (Sigma, St Louis, MO, USA) was diluted at a concentration of 10 mg/ml in Dulbecco's PBS (D-PBS) (Mediatech, Herndon, VA, USA) and equal volumes (1 : 1) of OVA and incomplete Freund's adjuvant (Sigma) were subjected to water into oil emulsification. Two hundred microlitres of emulsions were injected immediately i.p. into recipient congenic (Ly5·1) C57BL/6 mice. Mice rested for at least 1 month after vaccination prior to use in subsequent experiments using the A/PR/8 strain or immunotherapy.
Flow cytometry and antibodies
Single-cell suspensions were labelled with Fc block (purified anti-mouse CD16) (BD Pharmingen, San Diego, CA, USA) and antibodies for 20 min and washed twice with a staining buffer consisting of 5% fetal bovine serum (FBS) (Gemini Bio-products, Sacramento, CA, USA) in D-PBS (Mediatech). Samples were analysed using a custom-configured Fortessa using FACSDiva® software (Becton Dickinson, San Jose, CA, USA). The BD Cytofix/Cytoperm™ kit (Becton Dickinson) was used for granzyme staining as per the manufacturer's instructions. Data were analysed using FlowJo software (TreeStar, Ashland, OR, USA). Antibodies included: phycoerythrin-cyanin 5 (PE-Cy5)-conjugated anti-mouse CD62L, allophycocyanin (APC)-Cy7-conjugated anti-mouse CD25 (clone PC61), PE-Cy5-conjugated anti-mouse CD44, PE and PE-Cy7-conjugated anti-mouse NKG2D, PE-conjugated anti-mouse TCR Vα2, PE-conjugated anti-mouse programmed death 1 (PD-1) (eBioscience, San Diego, CA, USA); fluorescein isothiocyanate (FITC)-conjugated anti-mouse bromodeoxyuridine (BrdU), FITC-conjugated anti-mouse TCR Vβ5·1/5·2, FITC-conjugated anti-mouse CD4 and APC-Cy7-conjugated CD25 (clone PC61) (BD Pharmingen, San Diego, CA, USA); Alexa Fluor 700-conjugated anti-mouse CD8, Pacific Blue-conjugated anti mouse CD44 and anti-mouse CD45·2 (BioLegend, San Diego, CA, USA) and PE-conjugated anti-granzyme B (Invitrogen, Carlsbad, CA, USA).
Tissue collection and processing
Lungs were dissected and flushed with PBS through the heart, cut into very small pieces and incubated with digesting media containing 2% bovine serum albumin (BSA), 1 μg/ml collagenase type I (Worthington, Lakewood, NJ, USA) and 0·1 μg/ml DNAse I (Worthington) in D-PBS for 90 min at 37°C on a shaker. Lung cells were then dissociated into single-cell suspensions through vigorous pipetting and filtered. Prior to counting, red blood cells were lysed and cells counted using a Z1 Particle Counter (Beckman Coulter, Brea, VA, USA). Mediastinal lymph nodes were collected in loco after complete removal of the thymus. Peripheral non-draining lymph nodes including the scapular, axilliary and inguinal nodes were collected. Lymph nodes were crushed, filtered and counted. Spleens were crushed, filtered, red blood cells lysed and counted.
Anti-NKG2D administration
The non-depleting, blocking anti-NKG2D monoclonal antibody (clone CX5; Lewis Lanier, University of California, San Francisco, CA, USA) was administered by intranasal inoculation at 100 μg/0·1 ml sterile PBS concurrent with A/PR8 influenza virus (days 0, 2 and 4) and mice were harvested for lung viral titre determination at 3 and 5 days post-infection. Control mice received rat immunoglobulin (Ig)G (Jackson Immunoresearch) instead.
Viral titre determination by quantitative reverse transcription–polymerase chain reaction (qRT–PCR)
Quantification of A/PR8 influenza virus using real-time PCR was performed as described previously [61]. Briefly, total RNA was extracted from aliquots of lung homogenates using a QIAamp Viral RNA Kit (Qiagen). Viral positive-stranded mRNA for nuclear protein (which indicates the presence of replicating virus) was amplified and quantified by single-round quantitative RT–PCR with an ABI 7900 real-time PCR system. Relative PFU were determined from lung samples by comparison to a standard curve established by amplification of serial dilutions of the positive control.
Statistics
Statistical analyses were performed using Prism software (GraphPad Software Inc., San Diego, CA, USA). For analysis of three or more groups, the non-parametric analysis of variance (anova) test was performed with Bonferroni's post-test. Analysis of differences between two normally distributed test groups was performed using Student's t-test. Data were tested for normality and variance. A P-value of <0·05 was considered significant. All experiments were performed at least twice for reproducibility.
Results
Phenotype of CD8+ T cells after primary influenza infection
Primary challenge of mice with sublethal influenza A infection, strain A/Mem/71 (refer to Supporting information Fig. S1a for infection model schema), induces a localized immune response which leads to clearance of the virus within 10–14 days 25–26. This immune response was characterized by significant increases in total numbers of CD8+ T cells within the lung, starting at day 5 13 (Fig. 1a). At this time, clear changes were occurring in the T cell compartment as the CD44high, effector/memory proportion became significantly expanded (Fig. 1b). These expanded CD8+ T cells were also highly activated, as evidenced by expression of the lytic molecule granzyme B (Fig. 1c).
Figure 1.
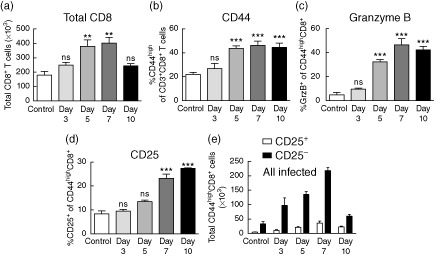
Activation and expansion of CD8+ T cells within the lung after influenza infection. Mice were infected intranasally with 1·2 × 104 plaque-forming units (PFU) A/Mem/71 and harvested at the various time-points indicated. Lungs were processed and stained for flow cytometry to evalulate the following parameters. (a) Total CD8+ T cells. (b) Percentage of CD44high of total CD8+ T cells. (c) Percentage of granzyme B+ and (d) CD25+ of CD44highCD8+ T cells. (e) Absolute numbers of CD25+/−CD44highCD8+ T cells. Data are representative of at least five independent experiments. Statistics performed using one-way analysis of variance (anova) with Bonferroni's post-test where appropriate. *P < 0·05; **P < 0·01; ***P < 0·001.
Further analysis of the activated CD8+ T cells revealed that the majority were negative for CD25 (Fig. 1d,e), a component of the high-affinity IL-2 receptor, which is up-regulated following TCR engagement 14–27. In fact, CD25+ (presumably antigen-specific) CD44highCD8+ T cells did not become expanded significantly until day 7 (Fig. 1d). Similar results were obtained using another influenza A strain (Supporting information Fig. S2a–c), A/PR/8 (Supporting information Fig. S1a), confirming that this was common during the course of infection and not an artefact of the A/Mem/71 strain. To confirm CD25 as an antigen-specific marker, we stained influenza-specific CD8+ T cells for CD25 and >90% expressed the marker (Supporting information Fig. S2e,f). These observations suggest that bystander activation is prevalent during the early stages (<7 days) of influenza infection. Antigen non-specific bystander activation has been well documented in a number of viral infections 12–28, including influenza 29–30. While others have observed bystander activation, little is known about the specific phenotype and/or function of these cells during infections. In a recent study, we characterized CD8+ T cell bystander activation following immunotherapeutic (IT) regimens for cancer on the basis of CD25, PD-1 and NKG2D expression. We observed that, in contrast to TCR engagement, which results in significant up-regulation of all three surface markers, bystander-activated cells up-regulate NKG2D yet do not significantly up-regulate CD25, and up-regulate PD-1 to a much lesser extent 21. We next sought to analyse the CD44highCD8+CD25− bystander-activated cells present in the lung during primary influenza infection for expression of PD-1 and NKG2D.
On day 5 after infection, the percentage of CD8+ T cells that were CD25+CD44high had not yet expanded (Fig. 1d) suggesting that, at this time-point, the majority of CD8+ T cells present at the site of infection were not antigen-specifically activated. Analysis of the CD44highCD8+CD25− population in the lung revealed that a significant proportion of these cells up-regulated PD-1 (Fig. 2a,b). To investigate this discrepancy further, we compared PD-1 expression in the CD25+ (antigen-specific) and CD25− (presumed bystander) fractions of the CD44highCD8+ T cells and observed that >80% of the CD25+ population expressed PD-1 while only ∼35% of the CD25− population expressed PD-1 (Fig. 2c), which was consistent with our previous observation that bystander-activated cells express PD-1 to a much lesser extent when compared with TCR triggering. Confirming our findings on bulk lung T cell populations, we also found PD-1 expression to be high (>85%) on influenza-specific, tetramer-positive CD8+ T cells (Supporting information Fig. S2e,f). While PD-1 is widely known for its function as an inhibitory mediator, it is also known to function as an acute activation marker present on effector/effector memory cells 31–32. Given that our previous studies involving immunotherapy focused primarily on secondary lymphoid organs, we reasoned that tissue-resident memory cells, such as those found in the lung, are generally of the effector memory phenotype and may up-regulate PD-1 in our immunotherapy-induced model as well. To investigate this, we treated mice with anti-CD40/IL-2 immunotherapy (IT) or infected with influenza and harvested on day 11 of IT or day 5 of infection. Consistent with previous data, PD-1 was not up-regulated in secondary lymphoid organs (spleen and lymph nodes) following IT (Supporting information Fig. S3a,b). However, it was up-regulated significantly in the lungs consistent with the influenza group (Fig. S3a,b).
Figure 2.
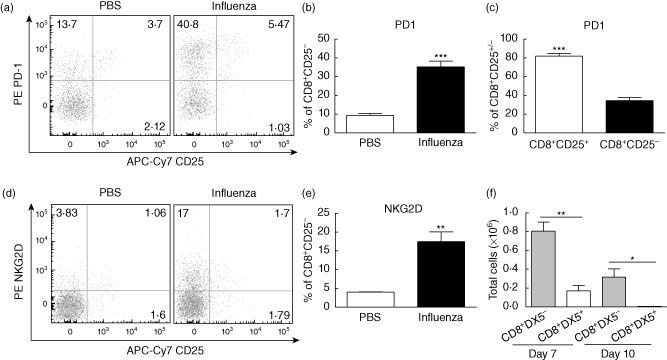
Characterization of phenotype of CD25−CD44highCD8+ T cells. Mice were harvested on day 5 following intranasal inoculation with 1·2 × 104 plaque-forming units (PFU) A/Mem/71 influenza unless noted otherwise. (a) Representative dot-plots depicting programmed death 1 (PD-1) and CD25 expression on CD8+ T cells and (b) percentages depicting PD-1 expression on CD8+CD25− cells. (c) PD-1 expression on CD8+CD25+ and CD8+CD25− populations. (d) Representative dot-plots depicting natural killer group 2D (NKG2D) and CD25 expression on CD3+CD8+ T cells and (e) percentages depicting NKG2D expression on CD3+CD8+CD25− cells. (f) Absolute numbers of DX5+ and DX5−CD8+NKG2D+ T cells. Statistics performed using Student's t-test where appropriate. Data are representative of at least three independent experiments. *P < 0·05; **P < 0·01; ***P < 0·001.
In addition to PD-1, NKG2D was also up-regulated significantly in the CD44highCD8+CD25− memory CD8+ T cell population (Fig. 2d,e). Similar results were observed in both strains of influenza (Supporting information Fig. S2d). NKG2D is expressed constitutively on NK cells and has been observed to be up-regulated on CD8+ T cells after activation 33. As NKG2D is expressed on the majority of NK T cells in the mouse, we wanted to confirm that the NKG2D+CD8+ cells were indeed conventional T cells and not NK T cells. Staining with an NK T cell-specific marker, DX5, showed that a portion of NKG2D up-regulation occurred in the NK T cell compartment, but the vast majority of the observed CD25-NKG2D+ memory CD8+ T cells were indeed conventional T cells (Fig. 2f).
Up-regulation of NKG2D and granzyme B on T cells with irrelevant TCR following influenza infection
The lack of CD25 expression on the surface of the majority of NKG2D+ memory CD8+ T cells, along with the extremely rapid kinetics associated with the local memory-phenotype T cell expansion after primary infection, suggested that these cells might be expanding independently of antigen stimulation through bystander proliferation. The polyclonal repertoire of antigen-specific cells in different influenza A viruses such as H1N1 has been characterized thoroughly in C57BL/6 mice. The beta chains of these antigen-dependent memory cells were described to be predominately vβ8·3 or vβ7 34–37. The A/Mem/71 strain of virus is a reassortment of A/Memphis/1/71 and A/Bellamy/42, which could potentially differ from the beta chains of the antigen-dependent memory cells described above 38. Conversely, the A/PR/8 influenza virus is related much more closely to the X31 reassortment virus, indicating a similar dependence on vβ8·3 or vβ7 antigen-specific recognition 35.
To characterize more clearly CD8+ T cells not specific to influenza, we focused on cells expressing the vβ5·1/2 chain of the TCR which, according to previous reports, should mark an irrelevant TCR in the A/PR/8 strain. We observed that the vβ5·1/2+CD44highCD8+ population expanded significantly after influenza infection on day 7 (Fig. 3a,b). Furthermore, those cells showed a similar activated phenotype compared to the total CD8 population regarding the increased expression of granzyme B (Fig. 3c) and the up-regulation of NKG2D (Fig. 3d). These data also support the concept that antigen non-specific memory CD8+ T cell expansion is occurring after primary infection.
Figure 3.
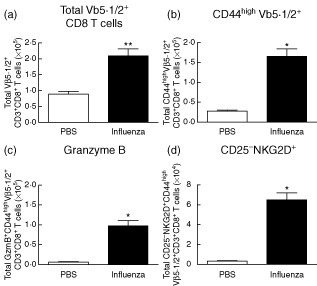
Expansion and up-regulation of natural killer group 2D (NKG2D) on CD8+ T cells expressing an irrelevant T cell receptor (TCR). Mice were infected intranasally with 20 plaque-forming units (PFU) A/PR8/8 and harvested at day 7. (a) Total numbers of Vβ 5·1/2+CD8 T cells at day 7 post-influenza infection. Total numbers of (b) CD44high, (c) granzyme B+ and (d) CD25–NKG2D CD44highVβ 5·1/2+ CD8 T cells at day 7 post-influenza infection. Data are representative of at least two independent experiments. Statistics performed using one- or two-way analysis of variance (anova) with Bonferroni's post-test where appropriate. *P < 0·05; **P < 0·01; ***P < 0·001.
To confirm further the antigen-independent nature of the memory NKG2D+CD25−CD44highCD8+ T cells after infection, we adoptively transferred purified OVA-specific CD8+ T cells from OT-I mice into congenic Ly5·1 C57BL/6 mice. Following transfer, congenic mice were vaccinated with OVA to enrich for memory OT-I CD8+ cells. Mice were then infected with A/PR/8 > 30 days post-vaccination with OVA and the phenotype of transgenic cells was assessed at day 7 following infection. As a positive control, a separate cohort of mice was treated with IT following adoptive transfer and vaccination, as we have shown previously expansion and up-regulation of NKG2D on OT-I cells using this model. Treatment schema and purity of adoptively transferred OT-I cells are shown in Supporting information Fig. S4. To ensure that adoptively transferred cells had matured into memory cells (as evidenced by up-regulation of CD44) and were not still activated from the vaccination, adoptively transferred OT-I cells were stained for markers associated with memory maturation and activation. As expected, the majority (>95%) of congenic OT-I cells were CD44high, indicating memory status, yet the majority (∼98%) lacked markers associated with activation (Supporting information Fig. S5a). As expected, OT-I cells expanded and up-regulated NKG2D in the spleen and lungs following IT, confirming the validity of the model with past studies (Fig. 4b–d). Following influenza infection, only OT-I cells present in the lung expanded and up-regulated NKG2D (but not CD25), consistent with the highly localized nature of influenza infection. Therefore, we demonstrate that local bystander memory CD8+ T cells expand and up-regulate NKG2D but not CD25 after primary influenza infection.
Figure 4.
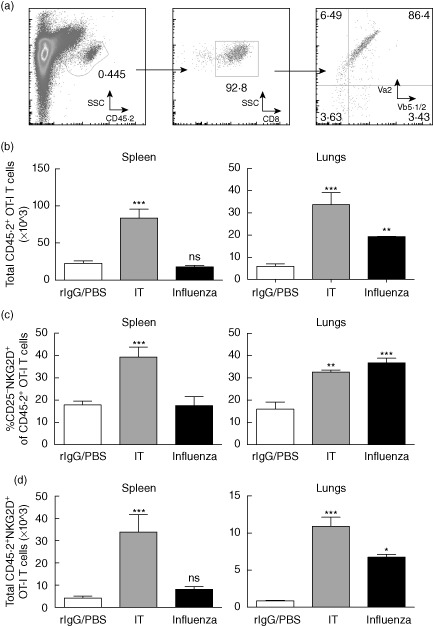
Expansion and up-regulation of bystander phenotype in ovalbumin (OVA)-specific CD8 T cells (OT-I) following influenza infection. OT-I CD8 T cells (CD45·2) were purified from OT-I T cell receptor (TCR) transgenic mice and transferred adoptively into congenic Ly5·1 (CD45·1) mice. Mice were vaccinated subsequently with OVA in incomplete Freund's adjuvant (IFA) and rested for >30 days. Mice were then challenged with A/PR/8 influenza or subjected to the immunotherapy regimen. For influenza mice were harvested on day 7 and for immunotherapy mice were harvested on day 11. (a) Representative gating schema for OT-I cells from congenic hosts. (b) Total numbers of congenic (CD45·2+) OT-I CD8+ T cells in spleen (left) and lungs (right). (c) Percentages and (d) numbers of bystander phenotype (CD25– natural killer group 2D (NKG2D)+) OT-I CD8+ T cells in spleen (left) and lungs (right). Data are representative of at least two independent experiments. Statistics performed using one- or two-way analysis of variance (anova) with Bonferroni's post-test where appropriate. *P < 0·05; **P < 0·01; ***P < 0·001.
Kinetics and localization of CD25−NKG2D+ bystander cells during infection
NKG2D+CD44highCD8+ T cells began to appear in the lung within 3 days and the number of those cells increased dramatically over the course of infection (Fig. 5a and Supporting information S2d). Significant expansion of CD25−NKG2D+CD44highCD8+ T cells in the spleen or lymph nodes, including the draining mediastinal lymph nodes, was not observed (Fig. 5b). We confirmed further the lung-specific expansion of bystander T cells following influenza infection in the OT-I model used in the previous figure and illustrated in Supporting information Fig. S4. Again, following influenza infection, CD25−NKG2D+ OT-I cells were expanded only in the lungs, both in percentage and number (Fig. 5c,d). OT-I cells from IT-treated mice were used as a positive control and were shown to expand and display the bystander phenotype (CD25–NKG2D+) in every organ surveyed, consistent with the systemic nature of the IT stimulus. Previous studies have indicated that the bystander T cells present in the lung during influenza infection are not tissue-resident cells but migrated into the lung from circulation 29. Due to the initial expansion of presumed bystander CD25−NKG2D+CD44highCD8+ T cells within the lung 3 days after infection, the persistent expansion throughout the course of infection and the absence of the CD25−NKG2D+ population in the mediastinal lymph node (from which activated cells are known to traffic to the site of infection) or any other lymphoid organ, we hypothesized that the bystander-activated CD8+ T cells arose from the tissue-resident memory T cell pool. It has been reported that influenza-specific CD8+ T cells become detectable within the lung between days 5–7 following infection, with the majority of cells appearing at day 6 and beyond 39. To assess more accurately whether these bystander-activated cells were already present in the lung or trafficked to the site during the course of infection, we examined the proliferation of CD8+ T cells within the lung prior to day 6, when the majority of activated, influenza-specific cells are first observed at this site. In-vivo analysis of proliferating cells by BrdU revealed that the overwhelming majority of the CD8+ T cells proliferating in the lung were in fact CD25−NKG2D+ (Fig. 5e). We confirmed proliferation of antigen non-specific cells using the OT-I model. As expected, OT-I cells indeed proliferated in the lungs of mice infected with influenza but not systemically, as occurs with immunotherapy (Fig. 5f,g). We therefore concluded that the local, tissue-resident memory CD8+ T cells are most probably the source of the activated CD25−NKG2D+CD44highCD8+ T cells within the lung.
Figure 5.
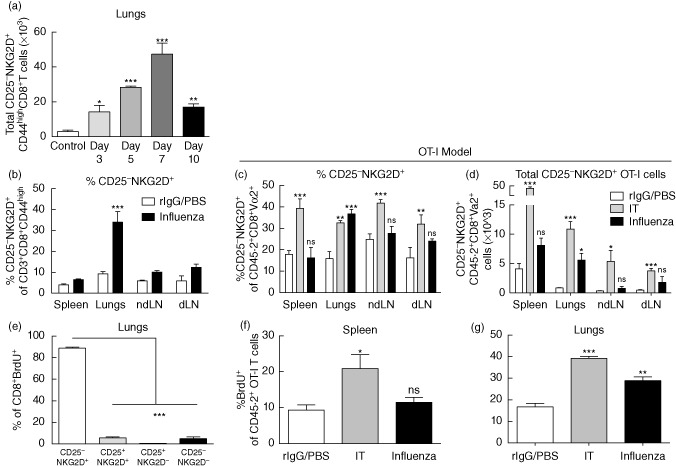
Timing and distribution of expanded CD25– natural killer group 2D (NKG2D)+CD8+ T cells after infection. Mice were infected with A/Mem/71 influenza and harvested on day 5 for wild-type studies. Ovalbumin (OVA)-specific CD8 T cell (OT-I) studies were performed as described in Fig. 4 and Supporting information Fig. S4. (a) Absolute numbers of CD25−NKG2D+CD44highCD8+T cells in the lungs at days 3, 5, 7 and 10 within the lung post-influenza infection. (b) Percentages of NKG2D+CD25−CD44highCD8+ T cells in the lungs, spleen, mediastinal and non-draining lymph nodes. (c) Percentages and (d) numbers of CD25−NKG2D+ OT-I CD8+ T cells in various organs following influenza infection or immunotherapy. (e) Comparison of CD25 and NKG2D expression in the lung CD8+bromodeoxyuridine (BrdU)+ population at day 5 of influenza infection. Percentage of BrdU+ of OT-I CD8+ T cells in (f) spleen and (g) lungs following influenza infection or immunotherapy. Data are representative of at least two independent experiments. Statistics performed using one- or two-way analysis of variance (anova) with Bonferroni's post-test where appropriate. *P < 0·05; **P < 0·01; ***P < 0·001.
NKG2D-dependent control of viral replication during early influenza infection
NKG2D is a NK cell-activating receptor known to be expressed on NK cells and certain subsets of T cells. In mice, the ligands for NKG2D include retinoic acid early transcript 1γ (Rae-1γ), murine UL16 binding protein (ULBP) transcript 1 (MULT1) and ULBP, which are stress ligands found on virally infected or transformed cells 40. NKG2D expression has been shown to endow T cells with the ability to recognize infected or transformed cells independent of their TCR specificity and therefore may enable cytokine-induced bystander T cells to contribute to cell-mediated immunity 41–42. Consistent with this, we have observed that following IT, local administration of NKG2D blockade at the tumour site partially abrogated bystander-mediated anti-tumour effects 21, further confirming a role for NKG2D in bystander function.
We hypothesized that because bystander CD44highCD8+ T cells with the NKG2D+CD25− phenotype arise early, prior to the migration of influenza-specific CD8 T cells, they may play an innate effector role in the control of early viral replication via NKG2D-mediated responses. To test this hypothesis, we infected mice with the A/PR/8 strain of influenza with or without concurrent administration of anti-NKG2D intranasally. NKG2D blocking antibody was given every 2 days to ensure blocking throughout the course of infection (refer to Supporting information Fig. S1c for schema depicting model). We then examined viral titres at days 3 and 5, times at which bystander CD8+ T cells were hypothesized to play a role in controlling viral replication. By day 3, there was a trend towards increased viral titres in the group receiving the NKG2D block (Fig. 6). At day 5, the viral titres in the NKG2D blocked group were significantly higher, thereby confirming a role for NKG2D in the control of early viral replication (Fig. 6).
Figure 6.
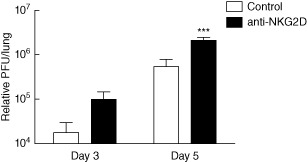
Natural killer group 2D (NKG2D)-dependent role in control of early viral replication. Mice were infected with 20 plaque-forming units (PFU) A/PR8 influenza and harvested on days 3 and 5 following infection. Some mice were given 100 μg anti-NKG2D or rat immunoglobulin (Ig)G intranasally on days 0, 2 and 4 following infection. Viral titres as determined by quantitative polymerase chain reaction (qPCR) on days 3 and 5 following influenza infection. Data are representative of at least two independent experiments. Statistics performed using one- or two-way analysis of variance (anova) with Bonferroni's post-test where appropriate. *P < 0·05; **P < 0·01; ***P < 0·001.
Discussion
Bystander proliferation of T cells after viral infection has been well documented in numerous model systems 15; however, the precise nature of these expanded T cells and their role in viral elimination has remained controversial 10,18. Nevertheless, significant antigen non-specific proliferation has been observed in several bacterial as well as viral studies 12,19, suggesting that the role of bystander cells in pathogenic control may also depend partially on the model being used.
We observed a significant expansion of the CD25−CD44highCD8+ T cell compartment in the lungs after influenza infection. The demonstration that the expanded population was negative for CD25, the high-affinity component of the IL-2R which is known to be up-regulated upon TCR engagement, suggested that this population was largely antigen non-specific. Although CD25 up-regulation is associated with TCR engagement, it has also been shown that duration of CD25 expression varies among antigen-specifically activated T cell clones 45. While it is unlikely that all the ∼40% of CD44high cells expressing NKG2D in the absence of CD25 are antigen-specific, it is possible that a small fraction of them may still be. While CD25 itself can be considered a controversial marker of TCR engagement, we observed similar expansions, in the absence of CD25 up-regulation, among subsets of T cells known to not recognize influenza A antigens (Vβ5+ and OVA-specific T cells), further illustrating the expansion of bystander cells and validating the use of lack of CD25 expression as one of many markers used to identify them. It is important to note that included within the CD25− population, non-activated memory CD8 T cells also reside, which may make the total population seem artificially larger than the antigen-specific (CD25+) population; therefore, it is important to not make judgements on numbers alone. Nevertheless, a proportion of the CD25− population expanded and up-regulated activation markers such as granzyme B, indicating a change in phenotype away from a resting state. We have recently characterized CD8+ bystander activation following cytokine-based immunotherapeutic regimens for cancer based on the expression of CD25, PD-1 and NKG2D; CD44highCD8+ bystander-activated cells were CD25negPD-1low/negNKG2D+ 21. Further analysis of the CD25− population following influenza infection revealed that the expression of PD-1, another marker associated with TCR engagement, was up-regulated but to a much lesser degree in this subset. It has been demonstrated that while PD-1 is indeed up-regulated markedly after TCR engagement, it can also be up-regulated in the presence of gamma-chain cytokines, although to a much lesser extent than following TCR engagement 46. Subsequent analysis of PD-1 expression in the CD8+CD25+ (TCR-triggered) and the CD8+CD25− (bystander-activated) populations confirmed that PD-1 was up-regulated to a much lesser extent in the CD25− population than the CD25+ population, further confirming our hypothesis that these cells were not activated through TCR triggering. While PD-1 induction in response to TCR engagement has been associated with inhibition and an exhausted phenotype 47–50, PD-1 expression induced by cytokine exposure has been shown to have different qualitative effects on T cell dynamics and function 46. It is possible that bystander-activated cells may have an advantage over TCR-activated cells during chronic inflammatory infections, in that they are less susceptible to PD-1-mediated inhibition. Consistent with our previous study, the influenza-induced bystander population also up-regulated NKG2D.
We observed the CD25−CD8+ expansion in the lung occurred primarily in the CD44high effector/memory compartment. While it has been shown that naive T cells may undergo bystander proliferation 18–44, the majority of studies report that, after infection, antigen non-specific proliferation occurs in the effector/memory compartment 14–53. CD44highCD8+ T cells are better equipped to respond to secreted cytokines compared to naive CD8+ T cells because of their enhanced expression of CD122 54–55. Furthermore, as memory T cells have previously encountered antigen in the periphery, antigen non-specific bystander proliferation can be regarded as ‘safer’, with decreased potential for autoreactive attack. Finally, as this population lacks expression of CD25, these expanded T cells are reliant upon cytokine-rich environments, such as those available at the site of infection, for their maintenance and are therefore self-limiting in nature.
We did not observe a significant up-regulation of NKG2D on the CD25−CD8+ T cells in any other organ except the lung during influenza infection. This is in sharp contrast to systemic IT, in which the same population is up-regulated ubiquitously throughout both lymphoid and non-lymphoid organs. This is due probably to the fact that influenza is, in most cases, a local infection; virus replication takes place primarily in the respiratory epithelium 26 and induces local cytokine production 39,56, whereas during systemic IT cytokines are abundant in circulation. The demonstration that NKG2D is not up-regulated in the mediastinal LN makes it likely that the NKG2D+CD44highCD8+ T cells are not recently activated effector cells generated from naive cells during the primary response. Furthermore, the demonstration that CD25−NKG2D+CD44highCD8+ T cells are not observed in the spleen or regional lymph nodes suggests that these cells are not migrating to the lung after activation in a peripheral organ but rather acting as innate effectors within the target organ itself. It has been known for some time that large numbers of tissue-resident T cells with a memory phenotype can be found in non-lymphoid organs such as the skin and lungs 58–59. Effector memory T cells have been shown to persist in non-lymphoid tissues and have been reported to possess a higher activation state than their splenic counterparts, enabling them to react faster to antigen encounter 60. Therefore, they are more likely to expand rapidly after cytokine stimulation and express effector molecules in an antigen-independent fashion, contributing to a more robust initial immune response.
The function and/or role of bystander T cell proliferation in the immune response to infection has been a matter of debate. It has been postulated that they are neutral ‘bystanders’, meaning that they do not contribute nor impair immune responses. It has also been suggested that they may down-regulate the immune response by suppressing the proliferation of naive T cells through a mechanism that mirrors the suppression of naive T cells by memory T cells 61. Alternatively, it has also been reported that antigen-independent proliferating CD8+ T cells enhance the immune response through production of IFN-γ and other mediators of effector functions 19,51. Our results indicate that these cells not only express lytic molecules but are also cytotoxic in nature, indicating that they may directly affect the initial immune response to influenza infection and help to shape the outcome.
In the present study we observed significant NKG2D up-regulation on activated antigen-independent CD44highCD8+ T cells in the lung. NKG2D is expressed on most NK cells and some NK T cells, on activated CD8+ T cells and, under certain conditions, on CD4+ T cells 33. Because NKG2D-deficient mice are more susceptible to tumorigenesis in vivo, NKG2D expression is believed to play a role in tumour surveillance 63. Various NKG2D ligands have been characterized in mice, including Rae1 and MULT1 64. Many different viruses have been shown to induce up-regulation of NKG2D ligands on infected cells in mice and humans 5–67. NKG2DL has also been shown to be relevant in influenza; in-vitro infection of human dendritic cells led to up-regulation of NKG2DL on the infected cells 68. While NKG2D is notably connected to NK cell-mediated killing, recent studies, by us and others, have also highlighted roles for NKG2D in both antigen-specific and non-specific T cell-mediated killing in models of autoimmunity, cancer and viruses 21,41. Specifically, we have shown NKG2D to play a significant role in clearance of primary tumour by antigen non-specific T cells during systemic immunotherapy 21. Therefore, it is conceivable that NKG2D-mediated killing of influenza-infected cells represents a mechanism by which antigen independently activated memory CD8+ T cells contribute to the anti-influenza immune response. Due to the lack of NKG2D expression in the draining LN, but presence of memory cells in the lung with up-regulation of NKG2D, we propose that NKG2D serves, in part, as an early non-specific local defence mechanism by resident memory cells after infection. To this effect, we have shown that blockade of NKG2D early during the course of infection results in significantly increased, albeit modest, viral replication in the lung, highlighting further the role of NKG2D in the control of early viral replication. A number of innate mechanisms have been put forward that also contribute during this time-frame, including broad-spectrum innate defence mechanisms (barrier, microbicidals, etc.), cytokine production (predominantly interferons) and innate immune cell clearance. Given that bystander-activated T cells are one of a number of contributing factors, one would not expect that blockade of the NKG2D pathway alone would achieve highly significant, ‘black and white’ differences in viral titre, as other mechanisms will probably provide some degree of compensation. Furthermore, we have shown previously that, at least during immunotherapy, the NKG2D pathway in bystander activation is only partially responsible for the observed immune effects 21. Therefore, it may be possible that bystander cells are utilizing other means (i.e. other NK-activating receptors such as NKp30, cytokine production, etc.) to impede virally infected cells as well. None the less, these results suggest that local antigen-independent expansion of memory T cells plays a role in the initial response to influenza infection which can help to keep viral replication under control while an antigen-specific response is being generated. This response would allow the immune system to use long-lived, tissue-resident memory T cells in both antigen-specific and non-specific roles.
While this publication has addressed a probable role for these cells in the context of acute viral infection, it is interesting to consider the broader implications of bystander activation and function during chronic infection or within other inflammatory environments. Due to their induction by cytokine exposure, it is likely that these cells persist throughout the course of infection, at least while high levels of cytokines are expressed. Indeed, data shown in Fig. 5 and Supporting information Fig. 2 would suggest that they are persisting throughout acute infection. Such a presence during chronic infection may signal a means by which deletion of virally infected cells through bystander-mediated elimination may function to keep some form of control over the infection while antigen-specific cells appear to become exhausted. Similarly, persistence during autoimmunity or graft-versus-host-disease (GVHD) may further fuel and lead to the snowball effects observed during flare-ups. To that effect, cells of a similar phenotype have been observed to be involved in the pathogenesis of coeliac disease 41. While such studies are beyond the scope of this paper, this study now provides a phenotypic framework for bystander activation such that these questions can be better addressed in the future.
Acknowledgments
This work was supported by a grant from the NIH R01 CA095572.
Disclosure
The authors have no competing interests to declare.
Supporting Information
Treatment schemas. (a) Mice were infected with 12 000 plaque-forming units (PFU) A/Mem/71 or 20 PFU A/PR/8 intranasally in 40 μl phosphate-buffered saline (PBS) and harvested at various time-points post-infection. (b) For immunotherapy experiments, mice were injected intra peritoneally (i.p.) with 80 μg/0·2 ml PBS agonistic anti-CD40 or rat immunoglobulin (Ig)G for 5 days. Mice were injected i.p. with 2 × 106 IU recombinant human interleukin (rhIL)-2 or 0·2 ml PBS twice a week for 2 weeks. Mice were harvested on day 11 post-immunotherapy. (c) Mice were infected with 20 PFU A/PR/8 influenza intranasally. Some groups received 100 μg anti-natural killer group 2D (NKG2D) or rat IgG intra nasally on days 0, 2 and 4. Mice were harvested at various time-points for viral titre analysis.
Time–course of CD8 T cells kinetics following A/PR/8 influenza infection mimics A/Mem/71 infection. Mice were infected with 20 plaque-forming units (PFU) A/PR/8 influenza and harvested at various time-points post-infection. (a) Total numbers of CD8+ T cells in the lung. Total numbers of (b) granzyme B+, (c) CD25+/−, (d) CD25−; natural killer group 2D (NKG2D+) in the lungs following influenza infection. (e) Representative dot-plots of influenza-specific (H-2Db/NP 366–374) CD8+ T cells. (f) Percentage of programmed death 1 (PD1) and CD25+ of CD8+Tet+CD44high T cells following influenza infection. Data are representative of at least two independent experiments. Statistics performed using one- or two-way analysis of variance (anova) with Bonferroni's post-test where appropriate. *P < 0·05; **P < 0·01; ***P < 0·001.
Programmed death 1 (PD1) expression on various organs following influenza infection and immunotherapy. Mice were infected with 12000 plague forming units (PFU) A/Mem/71 influenza infection or immunotherapy. (a) Representative dot plots of CD25 versus PD1 expression in various organs. (b) Quantification of PD1 expression on CD25−D44highCD8+ T cells in various organs. Data are representative of at least two independent experiments. Statistics performed using one or two way analysis of variance (anova) with Bonferroni's post-test where appropriate*P<0·05; **P<0·01; ***P<0·001.
Ovalbumin (OVA)-specific CD8 T cells (OT-I) adoptive transfer model. (a) Schematic for OT-I adoptive transfer model. Briefly, on day ?31, 5 × 106 magnet purified CD8+ T cells from OT-I mice (CD45·2) were transferred adoptively [intravenously (i.v.)] into congenic CD45·1 C57BL/6 mice. Mice were then immunized with 1 mg OVA in incomplete Freund's adjuvant (IFA) 24 h after the adoptive transfer. Mice were rested for >30 days and then either infected with 20 plaque-forming units (PFU) A/PR/8 influenza intranasally or treated with anti- CD40/interleukin (IL)-2 [immunotherapy (IT)]. Controls were given a regimen of recombinant human interleukin (rhIL)-2/phosphate-buffered saline (PBS) instead of IT. Mice were then harvested on day 7 following infection. (b) Staining of cells obtained from OT-I T cell receptor (TCR) transgenic mice pre- (left) and post- (right) CD8+ magnetic bead separation. Quantification of percentages of CD8+Vα2+Vβ5·1/5·2 (OT-I) T cells is shown in the far right panel.
Activation status of adoptively transferred ovaspecific CD8 T cells (OT-I) cells prior to infection or therapy. (a) Representative gating schema and staining from lymph nodes from control mice of adoptively transferred OT-I cells on day 37 after vaccination. Adoptively transferred OT-I cells (CD8+CD45·2+Va2+) were evaluated for expression of CD44 to determine memory status and [CD25, natural killer group 2D (NKG2D)] to determine activation status and baseline levels of these surface markers used to evaluate bystander activation.
References
- 1.Bi Z, Barna M, Komatsu T, Reiss CS. Vesicular stomatitis virus infection of the central nervous system activates both innate and acquired immunity. J Virol. 1995;69:6466–6472. doi: 10.1128/jvi.69.10.6466-6472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cousens LP, Orange JS, Biron CA. Endogenous IL-2 contributes to T cell expansion and IFN-gamma production during lymphocytic choriomeningitis virus infection. J Immunol. 1995;155:5690–5699. [PubMed] [Google Scholar]
- 3.Murata K, Garcia-Sastre A, Tsuji M, et al. Characterization of in vivo primary and secondary CD8+ T cell responses induced by recombinant influenza and vaccinia viruses. Cell Immunol. 1996;173:96–107. doi: 10.1006/cimm.1996.0255. [DOI] [PubMed] [Google Scholar]
- 4.Sunil-Chandra NP, Arno J, Fazakerley J, Nash AA. Lymphoproliferative disease in mice infected with murine gammaherpesvirus 68. Am J Pathol. 1994;145:818–826. [PMC free article] [PubMed] [Google Scholar]
- 5.Jacoby RO, Bhatt PN, Brownstein DG. Evidence that NK cells and interferon are required for genetic resistance to lethal infection with ectromelia virus. Arch Virol. 1989;108:49–58. doi: 10.1007/BF01313742. [DOI] [PubMed] [Google Scholar]
- 6.Doherty PC, Allan W, Eichelberger M, Carding SR. Roles of alpha beta and gamma delta T cell subsets in viral immunity. Annu Rev Immunol. 1992;10:123–151. doi: 10.1146/annurev.iy.10.040192.001011. [DOI] [PubMed] [Google Scholar]
- 7.Zinkernagel RM, Bachmann MF, Kundig TM, Oehen S, Pirchet H, Hengartner H. On immunological memory. Annu Rev Immunol. 1996;14:333–367. doi: 10.1146/annurev.immunol.14.1.333. [DOI] [PubMed] [Google Scholar]
- 8.Tripp RA, Hou S, McMickle A, Houston J, Doherty PC. Recruitment and proliferation of CD8+ T cells in respiratory virus infections. J Immunol. 1995;154:6013–6021. [PubMed] [Google Scholar]
- 9.Butz EA, Bevan MJ. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity. 1998;8:167–175. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murali-Krishna K, Altman JD, Suresh M, et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 11.Masopust D, Murali-Krishna K, Ahmed R. Quantitating the magnitude of the lymphocytic choriomeningitis virus-specific CD8 T-cell response: it is even bigger than we thought. J Virol. 2007;81:2002–2011. doi: 10.1128/JVI.01459-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doisne JM, Urrutia A, Lacabaratz-Porret C, et al. CD8+ T cells specific for EBV, cytomegalovirus, and influenza virus are activated during primary HIV infection. J Immunol. 2004;173:2410–2418. doi: 10.4049/jimmunol.173.4.2410. [DOI] [PubMed] [Google Scholar]
- 13.Lawrence CW, Ream RM, Braciale TJ. Frequency, specificity, and sites of expansion of CD8+ T cells during primary pulmonary influenza virus infection. J Immunol. 2005;174:5332–5340. doi: 10.4049/jimmunol.174.9.5332. [DOI] [PubMed] [Google Scholar]
- 14.Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 15.Sprent J, Zhang X, Sun S, Tough D. T-cell turnover in vivo and the role of cytokines. Immunol Lett. 1999;65:21–25. doi: 10.1016/s0165-2478(98)00119-9. [DOI] [PubMed] [Google Scholar]
- 16.McGill J, Van Rooijen N, Legge KL. IL-15 trans-presentation by pulmonary dendritic cells promotes effector CD8 T cell survival during influenza virus infection. J Exp Med. 2010;207:521–534. doi: 10.1084/jem.20091711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 18.Ehl S, Hombach J, Aichele P, Hengartner H, Zinkernagel RM. Bystander activation of cytotoxic T cells: studies on the mechanism and evaluation of in vivo significance in a transgenic mouse model. J Exp Med. 1997;185:1241–1251. doi: 10.1084/jem.185.7.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sandalova E, Laccabue D, Boni C, et al. Contribution of herpesvirus specific CD8 T cells to anti-viral T cell response in humans. PLOS Pathog. 2010;6:e1001051. doi: 10.1371/journal.ppat.1001051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogasawara K, Lanier LL. NKG2D in NK and T cell-mediated immunity. J Clin Immunol. 2005;25:534–540. doi: 10.1007/s10875-005-8786-4. [DOI] [PubMed] [Google Scholar]
- 21.Tietze JK, Wilkins DE, Sckisel GD, et al. Delineation of antigen-specific and antigen-nonspecific CD8(+) memory T-cell responses after cytokine-based cancer immunotherapy. Blood. 2012;119:3073–3083. doi: 10.1182/blood-2011-07-369736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baumgarth N, Brown L, Jackson D, Kelso A. Novel features of the respiratory tract T-cell response to influenza virus infection: lung T cells increase expression of gamma interferon mRNA in vivo and maintain high levels of mRNA expression for interleukin-5 (IL-5) and IL-10. J Virol. 1994;68:7575–7581. doi: 10.1128/jvi.68.11.7575-7581.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baumgarth N, Kelso A. In vivo blockade of gamma interferon affects the influenza virus-induced humoral and the local cellular immune response in lung tissue. J Virol. 1996;70:4411–4418. doi: 10.1128/jvi.70.7.4411-4418.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alderson KL, Zhou Q, Berner V, et al. Regulatory and conventional CD4+ T cells show differential effects correlating with PD-1 and B7-H1 expression after immunotherapy. J Immunol. 2008;180:2981–2988. doi: 10.4049/jimmunol.180.5.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doherty PC, Christensen JP. Accessing complexity: the dynamics of virus-specific T cell responses. Annu Rev Immunol. 2000;18:561–592. doi: 10.1146/annurev.immunol.18.1.561. [DOI] [PubMed] [Google Scholar]
- 26.Eichelberger MC, Wang ML, Allan W, Webster RG, Doherty PC. Influenza virus RNA in the lung and lymphoid tissue of immunologically intact and CD4-depleted mice. J Gen Virol. 1991;72(Pt 7):1695–1698. doi: 10.1099/0022-1317-72-7-1695. [DOI] [PubMed] [Google Scholar]
- 27.Baumgarth N, Egerton M, Kelso A. Activated T cells from draining lymph nodes and an effector site differ in their responses to TCR stimulation. J Immunol. 1997;159:1182–1191. [PubMed] [Google Scholar]
- 28.Pantaleo G, Koenig S, Baseler M, Lane HC, Fauci AS. Defective clonogenic potential of CD8+ T lymphocytes in patients with AIDS. Expansion in vivo of a nonclonogenic CD3+CD8+DR+CD25− T cell population. J Immunol. 1990;144:1696–1704. [PubMed] [Google Scholar]
- 29.Ely KH, Cauley LS, Roberts AD, Brennan JW, Cookenham T, Woodland DL. Nonspecific recruitment of memory CD8+ T cells to the lung airways during respiratory virus infections. J Immunol. 2003;170:1423–1429. doi: 10.4049/jimmunol.170.3.1423. [DOI] [PubMed] [Google Scholar]
- 30.Marshall DR, Turner SJ, Belz GT, et al. Measuring the diaspora for virus-specific CD8+ T cells. Proc Natl Acad Sci USA. 2001;98:6313–6318. doi: 10.1073/pnas.101132698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duraiswamy J, Ibegbu CC, Masopust D, et al. Phenotype, function, and gene expression profiles of programmed death-1(hi) CD8 T cells in healthy human adults. J Immunol. 2011;186:4200–4212. doi: 10.4049/jimmunol.1001783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hokey DA, Johnson FB, Smith J, et al. Activation drives PD-1 expression during vaccine-specific proliferation and following lentiviral infection in macaques. Eur J Immunol. 2008;38:1435–1445. doi: 10.1002/eji.200737857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Champsaur M, Lanier LL. Effect of NKG2D ligand expression on host immune responses. Immunol Rev. 2010;235:267–285. doi: 10.1111/j.0105-2896.2010.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belz GT, Stevenson PG, Doherty PC. Contemporary analysis of MHC-related immunodominance hierarchies in the CD8+ T cell response to influenza A viruses. J Immunol. 2000;165:2404–2409. doi: 10.4049/jimmunol.165.5.2404. [DOI] [PubMed] [Google Scholar]
- 35.Deckhut AM, Allan W, McMickle A, et al. Prominent usage of V beta 8.3 T cells in the H-2Db-restricted response to an influenza A virus nucleoprotein epitope. J Immunol. 1993;151:2658–2666. [PubMed] [Google Scholar]
- 36.La Gruta NL, Kedzierska K, Pang K, et al. A virus-specific CD8+ T cell immunodominance hierarchy determined by antigen dose and precursor frequencies. Proc Natl Acad Sci USA. 2006;103:994–999. doi: 10.1073/pnas.0510429103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moffat JM, Handel A, Doherty PC, Turner SJ, Thomas PG, La Gruta NL. Influenza epitope-specific CD8+ T cell avidity, but not cytokine polyfunctionality, can be determined by TCRbeta clonotype. J Immunol. 2010;185:6850–6856. doi: 10.4049/jimmunol.1002025. [DOI] [PubMed] [Google Scholar]
- 38.Deliyannis G, Jackson DC, Ede NJ, et al. Induction of long-term memory CD8(+) T cells for recall of viral clearing responses against influenza virus. J Virol. 2002;76:4212–4221. doi: 10.1128/JVI.76.9.4212-4221.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med. 2009;15:277–284. doi: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gonzalez S, Lopez-Soto A, Suarez-Alvarez B, Lopez-Vazquez A, Lopez-Larrea C. NKG2D ligands: key targets of the immune response. Trends Immunol. 2008;29:397–403. doi: 10.1016/j.it.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 41.Meresse B, Chen Z, Ciszewski C, et al. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity. 2004;21:357–366. doi: 10.1016/j.immuni.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 42.Verneris MR, Karami M, Baker J, Jayaswal A, Negrin RS. Role of NKG2D signaling in the cytotoxicity of activated and expanded CD8+ T cells. Blood. 2004;103:3065–3072. doi: 10.1182/blood-2003-06-2125. [DOI] [PubMed] [Google Scholar]
- 43.Zarozinski CC, Welsh RM. Minimal bystander activation of CD8 T cells during the virus-induced polyclonal T cell response. J Exp Med. 1997;185:1629–1639. doi: 10.1084/jem.185.9.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gilbertson B, Germano S, Steele P, Turner S, Fazekas de St Groth B, Cheers C. Bystander activation of CD8+ T lymphocytes during experimental mycobacterial infection. Infect Immun. 2004;72:6884–6891. doi: 10.1128/IAI.72.12.6884-6891.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal–effector differentiation in vivo. Immunity. 2010;32:91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 46.Kinter AL, Godbout EJ, McNally JP, et al. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J Immunol. 2008;181:6738–6746. doi: 10.4049/jimmunol.181.10.6738. [DOI] [PubMed] [Google Scholar]
- 47.Bennett F, Luxenberg D, Ling V, et al. Program death-1 engagement upon TCR activation has distinct effects on costimulation and cytokine-driven proliferation: attenuation of ICOS, IL-4, and IL-21, but not CD28, IL-7, and IL-15 responses. J Immunol. 2003;170:711–718. doi: 10.4049/jimmunol.170.2.711. [DOI] [PubMed] [Google Scholar]
- 48.Hofmeyer KA, Jeon H, Zang X. The PD-1/PD-L1 (B7-H1) pathway in chronic infection-induced cytotoxic T lymphocyte exhaustion. J Biomed Biotechnol. 2011;2011:451694. doi: 10.1155/2011/451694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jin HT, Anderson AC, Tan WG, et al. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci USA. 2010;107:14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sheppard KA, Fitz LJ, Lee JM, et al. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 2004;574:37–41. doi: 10.1016/j.febslet.2004.07.083. [DOI] [PubMed] [Google Scholar]
- 51.Chen HD, Fraire AE, Joris I, Brehm MA, Welsh RM, Selin LK. Memory CD8+ T cells in heterologous antiviral immunity and immunopathology in the lung. Nat Immunol. 2001;2:1067–1076. doi: 10.1038/ni727. [DOI] [PubMed] [Google Scholar]
- 52.Lodolce JP, Burkett PR, Boone DL, Chien M, Ma A. T cell-independent interleukin 15Ralpha signals are required for bystander proliferation. J Exp Med. 2001;194:1187–1194. doi: 10.1084/jem.194.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wan N, Dai H, Wang T, Moore Y, Zheng XX, Dai Z. Bystander central memory but not effector memory CD8+ T cells suppress allograft rejection. J Immunol. 2008;180:113–121. doi: 10.4049/jimmunol.180.1.113. [DOI] [PubMed] [Google Scholar]
- 54.Cho BK, Wang C, Sugawa S, Eisen HN, Chen J. Functional differences between memory and naive CD8 T cells. Proc Natl Acad Sci USA. 1999;96:2976–2981. doi: 10.1073/pnas.96.6.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 56.Hayden FG, Fritz R, Lobo MC, Alvord W, Strober W, Straus SE. Local and systemic cytokine responses during experimental human influenza A virus infection. Relation to symptom formation and host defense. J Clin Invest. 1998;101:643–649. doi: 10.1172/JCI1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perrone LA, Plowden JK, Garcia-Sastre A, Katz JM, Tumpey TM. H5N1 and 1918 pandemic influenza virus infection results in early and excessive infiltration of macrophages and neutrophils in the lungs of mice. PLOS Pathog. 2008;4:e1000115. doi: 10.1371/journal.ppat.1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clark RA, Chong B, Mirchandani N, et al. The vast majority of CLA+ T cells are resident in normal skin. J Immunol. 2006;176:4431–4439. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- 59.Purwar R, Campbell J, Murphy G, Richards WG, Clark RA, Kupper TS. Resident memory T cells (T(RM)) are abundant in human lung: diversity, function, and antigen specificity. PLOS One. 2011;6:e16245. doi: 10.1371/journal.pone.0016245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 61.Bourgeois C, Stockinger B. CD25+CD4+ regulatory T cells and memory T cells prevent lymphopenia-induced proliferation of naive T cells in transient states of lymphopenia. J Immunol. 2006;177:4558–4566. doi: 10.4049/jimmunol.177.7.4558. [DOI] [PubMed] [Google Scholar]
- 62.Das G, Vohra H, Saha B, Mishra GC. Th1-specific bystander costimulation imparts resistance against Mycobacterium tuberculosis infection. Scand J Immunol. 2000;52:515–518. doi: 10.1046/j.1365-3083.2000.00808.x. [DOI] [PubMed] [Google Scholar]
- 63.Guerra N, Tan YX, Joncker NT, et al. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28:571–580. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3:781–790. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 65.Walsh KB, Lanier LL, Lane TE. NKG2D receptor signaling enhances cytolytic activity by virus-specific CD8+ T cells: evidence for a protective role in virus-induced encephalitis. J Virol. 2008;82:3031–3044. doi: 10.1128/JVI.02033-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ward J, Bonaparte M, Sacks J, et al. HIV modulates the expression of ligands important in triggering natural killer cell cytotoxic responses on infected primary T-cell blasts. Blood. 2007;110:1207–1214. doi: 10.1182/blood-2006-06-028175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ogawa T, Tsuji-Kawahara S, Yuasa T, et al. Natural killer cells recognize Friend retrovirus-infected erythroid progenitor cells through NKG2D-RAE-1 interactions in vivo. J Virol. 2011;85:5423–5435. doi: 10.1128/JVI.02146-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Draghi M, Pashine A, Sanjanwala B, et al. NKp46 and NKG2D recognition of infected dendritic cells is necessary for NK cell activation in the human response to influenza infection. J Immunol. 2007;178:2688–2698. doi: 10.4049/jimmunol.178.5.2688. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Treatment schemas. (a) Mice were infected with 12 000 plaque-forming units (PFU) A/Mem/71 or 20 PFU A/PR/8 intranasally in 40 μl phosphate-buffered saline (PBS) and harvested at various time-points post-infection. (b) For immunotherapy experiments, mice were injected intra peritoneally (i.p.) with 80 μg/0·2 ml PBS agonistic anti-CD40 or rat immunoglobulin (Ig)G for 5 days. Mice were injected i.p. with 2 × 106 IU recombinant human interleukin (rhIL)-2 or 0·2 ml PBS twice a week for 2 weeks. Mice were harvested on day 11 post-immunotherapy. (c) Mice were infected with 20 PFU A/PR/8 influenza intranasally. Some groups received 100 μg anti-natural killer group 2D (NKG2D) or rat IgG intra nasally on days 0, 2 and 4. Mice were harvested at various time-points for viral titre analysis.
Time–course of CD8 T cells kinetics following A/PR/8 influenza infection mimics A/Mem/71 infection. Mice were infected with 20 plaque-forming units (PFU) A/PR/8 influenza and harvested at various time-points post-infection. (a) Total numbers of CD8+ T cells in the lung. Total numbers of (b) granzyme B+, (c) CD25+/−, (d) CD25−; natural killer group 2D (NKG2D+) in the lungs following influenza infection. (e) Representative dot-plots of influenza-specific (H-2Db/NP 366–374) CD8+ T cells. (f) Percentage of programmed death 1 (PD1) and CD25+ of CD8+Tet+CD44high T cells following influenza infection. Data are representative of at least two independent experiments. Statistics performed using one- or two-way analysis of variance (anova) with Bonferroni's post-test where appropriate. *P < 0·05; **P < 0·01; ***P < 0·001.
Programmed death 1 (PD1) expression on various organs following influenza infection and immunotherapy. Mice were infected with 12000 plague forming units (PFU) A/Mem/71 influenza infection or immunotherapy. (a) Representative dot plots of CD25 versus PD1 expression in various organs. (b) Quantification of PD1 expression on CD25−D44highCD8+ T cells in various organs. Data are representative of at least two independent experiments. Statistics performed using one or two way analysis of variance (anova) with Bonferroni's post-test where appropriate*P<0·05; **P<0·01; ***P<0·001.
Ovalbumin (OVA)-specific CD8 T cells (OT-I) adoptive transfer model. (a) Schematic for OT-I adoptive transfer model. Briefly, on day ?31, 5 × 106 magnet purified CD8+ T cells from OT-I mice (CD45·2) were transferred adoptively [intravenously (i.v.)] into congenic CD45·1 C57BL/6 mice. Mice were then immunized with 1 mg OVA in incomplete Freund's adjuvant (IFA) 24 h after the adoptive transfer. Mice were rested for >30 days and then either infected with 20 plaque-forming units (PFU) A/PR/8 influenza intranasally or treated with anti- CD40/interleukin (IL)-2 [immunotherapy (IT)]. Controls were given a regimen of recombinant human interleukin (rhIL)-2/phosphate-buffered saline (PBS) instead of IT. Mice were then harvested on day 7 following infection. (b) Staining of cells obtained from OT-I T cell receptor (TCR) transgenic mice pre- (left) and post- (right) CD8+ magnetic bead separation. Quantification of percentages of CD8+Vα2+Vβ5·1/5·2 (OT-I) T cells is shown in the far right panel.
Activation status of adoptively transferred ovaspecific CD8 T cells (OT-I) cells prior to infection or therapy. (a) Representative gating schema and staining from lymph nodes from control mice of adoptively transferred OT-I cells on day 37 after vaccination. Adoptively transferred OT-I cells (CD8+CD45·2+Va2+) were evaluated for expression of CD44 to determine memory status and [CD25, natural killer group 2D (NKG2D)] to determine activation status and baseline levels of these surface markers used to evaluate bystander activation.


