Abstract
The gadolinium-based contrast agent (GdBCA) Omniscan activates human macrophages through Toll-like receptor (TLR)-4 and TLR-7 signalling. To explore the mechanisms responsible we compared the ability of linear and macrocyclic GdBCA to induce a type I interferon signature and a proinflammatory/profibrotic phenotype in normal human monocytes in vitro. Expression of genes associated with type I interferon signalling and inflammation and production of their corresponding proteins were determined. Both linear and macrocyclic GdBCA stimulated expression of multiple type I interferon-regulated genes and the expression of numerous chemokines, cytokines and growth factors in normal human peripheral blood monocytes. There was no correlation between the magnitude of the measured response and the Gd chelate used. To explore the mechanisms responsible for GdBCA induction of fibrosis in nephrogenic systemic fibrosis (NSF) in vitro, normal human dermal fibroblasts were incubated with GdBCA-treated monocyte culture supernatants and the effects on profibrotic gene expression were examined. Supernatants from monocytes exposed to all GdBCA stimulated types I and III collagen, fibronectin and α-smooth muscle actin (α-SMA) expression in normal dermal fibroblasts. The results indicate that the monocyte activation induced by GdBCA may be the initial step in the development of GdBCA associated fibrosis in NSF.
Keywords: gadolinium-based contrast agent, macrophages, nephrogenic systemic fibrosis, tissue fibrosis, type I interferon
Introduction
Nephrogenic systemic fibrosis (NSF) is a systemic fibrosing disorder occurring in patients with chronic kidney disease (CKD) characterized by progressive fibrosis of skin, periarticular tissues, striated muscles and various internal organs 1–4. Histopathological studies of affected NSF skin demonstrate severe dermal and subdermal fibrosis, prominent mucin deposition, accumulation of activated fibroblasts and macrophages and strikingly increased in-situ expression of transforming growth factor beta (TGF-β) 3. Although the exact mechanisms responsible for NSF development are not known, the majority of reported cases occurred following exposure to high doses of Gd-based contrast agents (GdBCA) in the context of renal insufficiency 5–8. Gd accumulation in CKD patient tissues is thought to be the primary event in NSF pathogenesis. Indeed, numerous studies have described Gd deposition in affected tissues from NSF patients 9,10.
Two models have been proposed that differ in the nature of the bioactive form of Gd associated with NSF occurrence in CKD individuals 12–16. The transmetallation model posits that GdBCA accumulate in the extravascular space and tissues where endogenous free cations such as Zn2+, Ca2+, Cu2+ or Fe2+ displace Gd3+ from the chelate complex 12–15. The Gd3+ released from the chelate complex can induce activation of various profibrotic molecular pathways in one or more of the cell types present in fibrotic NSF lesions, such as macrophages, fibroblasts and fibrocytes. According to this model, linear GdBCA that are less thermodynamically stable than macrocyclic GdBCA are more prone to undergo transmetallation, thereby initiating the fibrotic process leading to NSF. The second model, the chelate model, proposes that intact Gd chelate complexes, rather than transmetallated Gd3+, are the bioactive agent triggering the NSF fibrotic process 16. This model suggests that certain intrinsic property(s) of the intact Gd chelate complex is(are) responsible for the disproportionately greater association of linear GdBCA with NSF development compared to macrocyclic GdBCA based on exposure rates to these classes of agents.
Activated macrophage infiltration into affected NSF skin and visceral tissues 3 and the induction of a proinflammatory/profibrotic phenotype in normal human peripheral blood monocytes and macrophages by the linear contrast agents Omniscan and Gd-DTPA have been described previously 17–18. Recently, it has been reported that Omniscan stimulation of production of proinflammatory/profibrotic cytokines, chemokines and growth factors is mediated by Toll-like receptor (TLR)-4 and TLR-7 in normal differentiated human macrophages 19. Numerous other studies have demonstrated that GdBCA can induce profibrotic responses in normal and NSF fibroblasts as well as in skin organ cultures 20–23. However, the induction of a type I interferon (IFN) signature and a detailed comparison of proinflammatory and profibrotic effects of various linear and macrocyclic GdBCA have not been performed. Thus, the purpose of the present study was to compare the ability of four different linear GdBCA and two different macrocyclic GdBCA to induce a type I IFN signature and the expression of a proinflammatory/profibrotic phenotype in normal human monocytes in vitro. Furthermore, the effects of supernatants from these cells on cultured normal human dermal fibroblasts were examined to explore the mechanisms that might be responsible for the differential association of various GdBCA agents with NSF development.
Experimental
Gd compounds
Dotarem® (Guerbet LLC, Bloomington, IL, USA), MultiHance® (Bracco Diagnostics, Milan, Italy), ProHance® (Bracco Diagnostics) and OptiMark® (Mallinckrodt Inc/Covidien, Hazelwood, IN, USA) were supplied as sterile, aqueous solutions each containing 500 mM of the Gd chelate. Omniscan® and gadodiamide (provided by GE Healthcare, Chalfont St Giles, UK) were supplied as sterile, aqueous solutions each containing 287 mg/ml (500 mM) gadodiamide. The Omniscan® solution contained an additional 12 mg/ml (25 mM) caldiamide sodium in water. Caldiamide (GE Healthcare) was supplied as a sterile aqueous solution containing 12 mg/ml (25 mM) caldiamide sodium in water. Gd-ethylenediamine tetraacetic acid (EDTA) (GE Healthcare) was supplied as a sterile aqueous solution containing 250 mM Gd-EDTA in water. Gd-citrate (250 μM) was prepared by mixing 1 ml of 250 μM gadolinium chloride and 1 ml of 500 μM sodium citrate solutions at pH 7·4 24. Gadolinium diethylenetriaminepentaacetic acid (Gd-DTPA; Sigma-Aldrich, St Louis, MO, USA), the Gd chelate used in Magnevist®, was dissolved in sterile phosphate-buffered saline (PBS) solution at 0·5 M concentration. The reagents employed for all the studies were tested and verified by the manufacturer to be free from endotoxin contamination. The absence of endotoxin contamination was confirmed further in our laboratories utilizing the Etoxate assay (Sigma-Aldrich), according to the manufacturer's instructions.
Monocyte isolation
Leucoreduction filters from three different normal donors were obtained from the Thomas Jefferson University Hospital Blood Bank following Institutional Review Board approval. Human peripheral blood mononuclear cells (PBMC) were isolated from the filters by Ficoll-Hypaque gradient centrifugation (Amersham Pharmacia Biotech, Piscataway, NJ, USA) and enriched for monocytes by adherence to plastic culture dishes for 2 h, as described previously 17–25.
Treatment of monocytes with Gd compounds
Equal numbers of monocytes were plated in 60 mm plastic culture dishes in RPMI-1640 media supplemented with 25 mM HEPES, L-glutamine and 10% fetal bovine serum (FBS). Lipopolysaccharide (LPS; 1 μg/ml) was the positive control and sterile saline was the negative control. Cells were exposed for 12 or 24 h to 5 mM of each of the six GdBCA or to 250 μM caldiamide, which corresponds to the amount present in the Omniscan® preparation. Total RNA was isolated from the cells exposed to GdBCA for 12 h, as described previously 17–18, and supernatants from each sample were collected and stored at −20°C until used.
Enzyme-linked immunosorbent assay (ELISA)
SearchLight ELISA proteome array analyses (Pierce Biotechnology, Woburn, MA, USA) were performed as described previously 17–18 to quantitate the levels of interleukin (IL)-4, IL-6, IL-13, IFN-γ, transforming growth factor (TGF)-β and vascular endothelial growth factor (VEGF) in supernatants from monocytes exposed to the Gd compounds for 24 h. Briefly, samples were diluted 1:2, 1:50 and 1:1000 and then incubated for 1 h on the array plates, which had been prespotted with capture antibodies specific for each protein. Plates were decanted and washed three times with PBS before addition of a mixture of biotinylated detection antibodies to each well. Following incubation with detection antibodies for 30 min, plates were washed three times and incubated for 30 min with streptavidin–horseradish peroxidase. Plates were again washed and SuperSignal Femto chemiluminescent substrate was added. The plates were imaged immediately using the SearchLight imaging system and data were analysed using ArrayVision software.
Real-time polymerase chain reaction (PCR)
Monocyte and fibroblast transcript levels were quantified using SYBR Green real-time PCR, as described previously 17–18. Primers were designed using Primer Express software (Applied Biosystems, Foster City, CA, USA) and validated for specificity. The primers employed are displayed in the Supporting information, Table S1. The differences in the number of mRNA copies in each PCR were corrected for human β-actin endogenous control transcript levels; levels in control experiments were set at 100 and all other values expressed as multiples of control values.
Treatment of cultured fibroblasts with conditioned media from peripheral blood monocytes exposed to Gd compounds
Two normal human dermal fibroblast cell lines (passages 4–10) from the Scleroderma Center Tissue Bank, Thomas Jefferson University were cultured in Dulbecco's modified Eagle's medium (MEM) supplemented with 10% FBS, antibiotics, 50 mM HEPES and glutamine until confluent.
For experiments, the cells were cultured in complete medium in six-well plastic plates until confluence. Following preincubation for 24 h with 40 μg/ml ascorbic acid (Sigma-Aldrich) to optimize collagen production, fibroblasts were incubated for 24 h with conditioned media from Gd-exposed peripheral blood monocytes at a 1:2 dilution. Fibroblasts were then were harvested with trypsin-EDTA, washed with PBS and then processed for RNA extraction using the RNeasy kit (Qiagen, Valencia, CA, USA), as described previously 17–18.
Statistical analysis
Real-time PCR values reflect the mean and standard deviation of three separate experiments each performed in triplicate with each of the three samples of normal human macrophages. The statistical significance of the real-time PCR data was assessed by Student's two-tailed t test. P-values less than 0·05 were considered statistically significant.
Results
Effects of Gd compounds on cytokine and growth factor gene expression levels in normal human monocytes
Expression levels of profibrotic/proinflammatory cytokines in normal human monocytes in response to 12 h exposure to Gd-containing agents were analysed by real-time PCR. IL-6 and IL-13 were increased markedly in response to all Gd compounds, whereas VEGF was increased by all Gd compounds except MultiHance and OptiMARK (Fig. 1). IL-4 was increased by all Gd compounds except Gd-DTPA, MultiHance and OptiMARK, whereas TGF-β was increased only in response to Omniscan, gadodiamide, Gd-EDTA and Gd-citrate. IFN-γ, however, was increased only in response to Gd-DTPA, Dotarem and ProHance. No correlation was observed between the level of induction of expression of various genes encoding the proinflammatory/profibrotic cytokines and growth factors and the structure of the GdBCA chelate molecule or the non-chelated Gd compound. Exposure of monocytes to caldiamide had no effect on the expression level of any gene measured.
Figure 1.
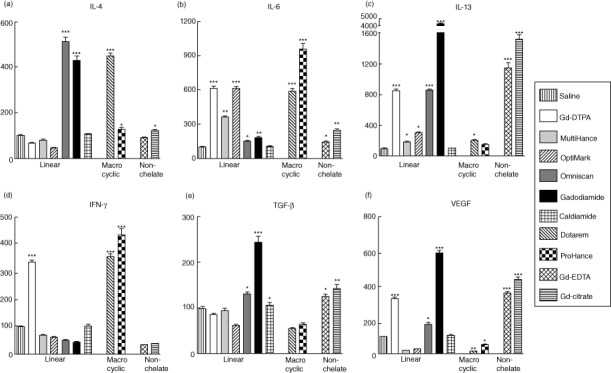
Effect of non-ionic and ionic linear and macrocyclic gadolinium-based contrast agents (GdBCA) on the expression levels of various genes encoding profibrotic/proinflammatory cytokines and growth factors in normal human monocytes. Values represent the mean (± standard deviation) expression levels of three replicates of three separate experiments with monocytes obtained from three different normal individuals exposed to the Gd compounds for 12 h. Cycle threshold [C(t)] values for cytokines were normalized with β-actin. The saline control levels were set arbitrarily at 100% expression at each time-point. Values for other samples are expressed relative to the saline control. Expression levels of (a) interleukin (IL)-4, (b) IL-6, (c) IL-13, (d) interferon (IFN)-γ, (e) transforming growth factor (TGF)-β and (f) vascular endothelial growth factor (VEGF) transcripts are displayed. Statistical significance of changes in cytokine and growth factor expression as determined by Student's t-test comparing each Gd compound to the saline control appears above the corresponding bar. *P < 0·05; **P < 0·01; ***P < 0·001. Caldiamide did not induce a significant effect for any cytokine or growth factor.
Effects of Gd compounds on chemokine gene expression levels by normal human monocytes
Expression levels of chemokine genes in normal human monocytes in response to 12 h exposure to Gd-containing agents were analysed by real-time PCR. CXCL9 and CXCL12 were increased markedly in response to all Gd compounds, whereas CCL2 was increased by all Gd compounds except Gd-DTPA, and CCL8 was increased by all Gd compounds except OptiMARK (Fig. 2). CXCL11 was increased by all Gd compounds except MultiHance and Dotarem, whereas CXCL10 was increased only in response to Gd-DTPA, Omniscan, gadodiamide, Gd-EDTA and Gd-citrate. As with expression levels of cytokines and growth factors, no correlation between the level of response attained and the structure of the chelate molecule of the GdBCA was observed.
Figure 2.
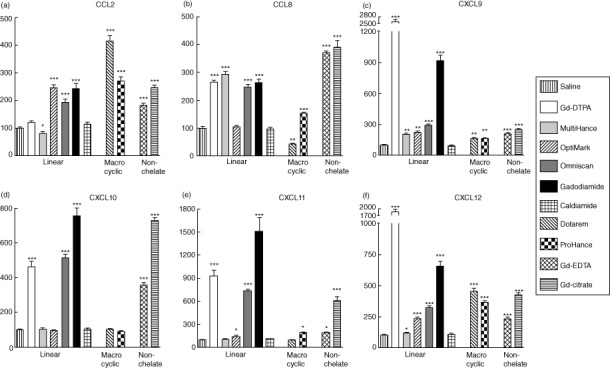
Effect of non-ionic and ionic linear and macrocyclic gadolinium-based contrast agents (GdBCA) on the expression levels of various chemokine genes in normal human monocytes. Values represent the mean (± standard deviation) expression levels of three replicates of three separate experiments with monocytes obtained from three different normal individuals exposed to the Gd compounds for 12 h. Cycle threshold [C(t)] values for chemokines were normalized with β-actin. The saline control levels were set arbitrarily at 100% expression at each time-point. Values for other samples are expressed relative to the saline control. Expression levels of (a) CCL2, (b) CCL8, (c) CXCL9, (d) CXCL10, (e) CXCL11 and (f) CXCL12 transcripts are displayed. Statistical significance of changes in chemokine expression as determined by Student's t-test comparing each Gd compound to the saline control appears above the corresponding bar. *P < 0·05; **P < 0·01; ***P < 0·001. Caldiamide did not induce a significant effect for any chemokine.
Effects of Gd compounds on normal human monocyte production of proinflammatory/profibrotic cytokines and chemokines
To assess whether the effects observed at the gene expression level were also reflected at the protein level, the amounts of cytokines and growth factors present in culture supernatants isolated from normal human monocytes incubated for 24 h with the Gd compounds were assayed by SearchLight proteome multiplex arrays (Fig. 3). The levels of IL-4, IL-13 and TGF-β produced were elevated significantly by all the GdBCA tested, regardless of the chelate class (Fig. 3), and all agents except OptiMARK induced increased VEGF levels, whereas only Omniscan, gadodiamide and ProHance induced increased production of IL-6 significantly. Omniscan- and gadodiamide-treated cells produced the highest levels of IL-4, IL-6 and IL-13, whereas Omniscan, gadodiamide and MultiHance produced the highest levels of VEGF.
Figure 3.
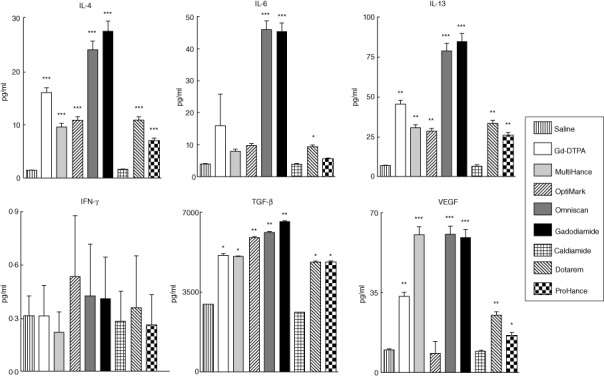
Quantitative measurement of various cytokines and growth factors present in the culture media of Gd-compound-exposed normal human monocytes employing multiplex proteome array analyses. Cells were incubated with either saline, 5 mM gadolinium diethylenetriaminepentaacetic acid (Gd-DTPA), MultiHance, Omniscan, gadodiamide, Dotarem or ProHance or 2·5 mM caldiamide for 24 h and the culture media was assayed. Values are expressed in pg/ml and represent the mean (± standard deviation) value of values obtained from duplicate results of pooled samples of three individuals at three dilutions: 1:2, 1:50 and 1:1000. Statistical significance of changes in cytokine and growth factor production as determined by Student's t-test comparing each Gd compound to the saline control appears above the corresponding bar. *P < 0·05; **P < 0·01; ***P < 0·001. Caldiamide did not induce a significant effect for any cytokine or growth factor.
Conditioned media from Gd-stimulated normal human monocytes induce a profibrotic phenotype in normal human dermal fibroblasts
Fibroblasts cultured with supernatants from monocytes that had been exposed to linear and macrocyclic GdBCA or to non-chelated Gd compounds displayed increased expression of types I and III human collagen (COL1A and COL3A). All monocyte supernatants caused collagen gene expression stimulation with similar efficacy (Fig. 4). Similarly, α-smooth muscle actin (α-SMA) expression, a marker of myofibroblast activation, and fibronectin (FN1) expression were up-regulated by all the GdBCA, although supernatants from cells exposed to the linear chelate GdBCA showed a slightly greater level of up-regulation of α-SMA expression compared to those induced by macrocyclic GdBCA. In contrast, supernatants from monocytes exposed to caldiamide alone did not affect expression of any of the genes examined in the cultured fibroblasts.
Figure 4.
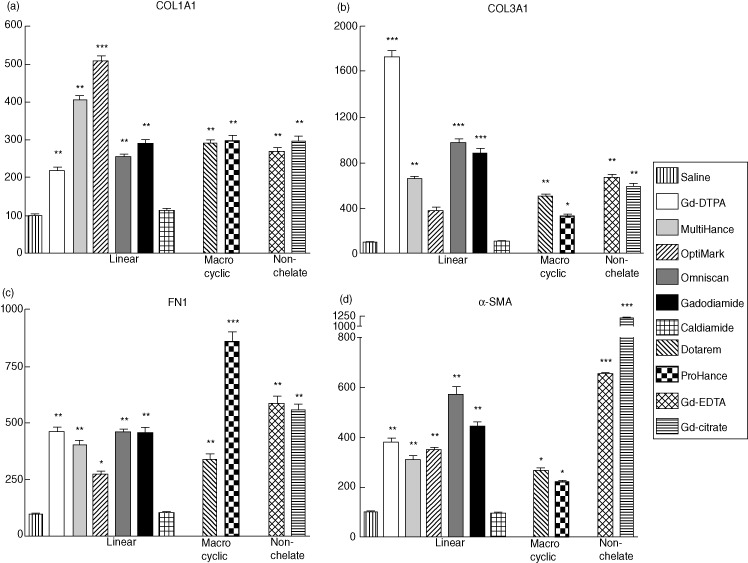
Effects of supernatants from monocytes exposed to gadolinium (Gd) compounds on expression of types I and III collagen, fibronectin (FN1) and α-smooth muscle actin (α-SMA) in normal dermal human fibroblasts. Fibroblasts were incubated for 24 h in media containing a 1:2 dilution of culture supernatants isolated from monocytes exposed to Gd compounds for 12 h. Expression levels of (a) type I human collagen (COL1A)1, (b) type III human collagen (COL3A)1, (c) FN1 and (d) α-SMA transcripts determined by real-time polymerase chain reaction (PCR). The results shown are representative of three separate experiments, each performed in triplicate. The saline control levels were set arbitrarily at 100%. Values for the samples are expressed as the mean (± standard deviation) percentage increase over the saline control value. Statistical significance of changes in gene expression as determined by Student's t-test comparing each Gd compound to the saline control appears above the corresponding bar. *P < 0·05; **P < 0·01; ***P < 0·001. Supernatants from monocytes exposed to caldiamide did not induce a significant effect on fibroblast gene expression.
Effects of Gd compounds on expression levels of type I IFNs and of genes involved in induction of nuclear factor kappa B (NF-κB) by normal human monocytes
Expression levels of type I IFNs (IFN-α and IFN-β) and of the NF-κB activator genes, IFN regulatory factor (IRF)-1, IRF-4, IRF-9 and X-linked inhibitor of apoptosis (XIAP) were analysed in normal human monocytes following 12 h exposure to Gd-containing agents. All linear chelates and non-chelated Gd compounds induced striking increases in the expression of all six genes (Fig. 5). In contrast, Dotarem induced increased expression of IFN-α, IFN-β, IRF-4 and IRF-9, but not of IRF-1 or XIAP, and ProHance increased expression of only IFN-α, IFN-β and IRF-9.
Figure 5.
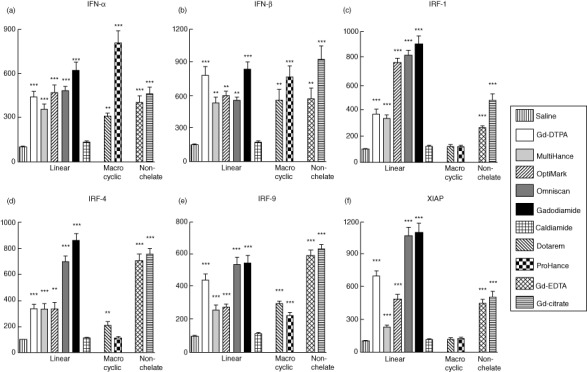
Effects of gadolinium (Gd) compounds on expression of interferon (IFN)-α, IFN-β, X-linked inhibitor of apoptosis (XIAP) and interferon regulatory factor (IRF) family genes in normal human monocytes. Values represent the mean (± standard deviation) expression levels of three replicates of three separate experiments with monocytes obtained from three different normal individuals. Cycle threshold [C(t)] values for type I IFNs, IRF genes and XIAP were normalized with β-actin. The saline control levels were set arbitrarily at 100% expression at each time-point. Values for other samples are expressed relative to the saline control. Expression levels of (a) IFN-α, (b) IFN-β, (c) IRF-1, (d) IRF-4, (e) IRF-9 and (f) XIAP transcripts are displayed. Statistical significance of changes in gene expression as determined by Student's t-test comparing each Gd compound to the saline control appears above the corresponding bar. *P < 0·05; **P < 0·01; ***P < 0·001. Caldiamide did not induce a significant effect.
Effects of Gd compounds on gene expression levels of IFN-responsive genes by normal human monocytes
Analysis of expression levels of IFN-responsive genes in normal human monocytes in response to 12 h exposure to Gd-containing agents were analysed next. Expression of CCL20, IFN-inducible 44-like (IFI44L), IFN-induced protein with tetratricopeptide repeats 1 (IFIT1), IL-8, IL-18 and immunoresponsive gene 1 (IRG1) were all increased markedly in response to all GdBCA (Fig. 6). No correlation was observed between the level of response attained and the structure of the chelate molecule of the GdBCA with all linear and macrocyclic GdBCA. We also found that all non-chelated Gd compounds induced expression of all IFN-responsive genes.
Figure 6.
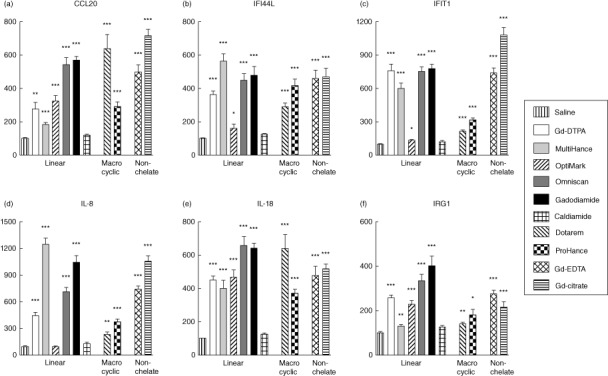
Effects of gadolinium (Gd) compounds on expression of type I interferon responsive genes in normal human monocytes. Values represent the mean (± standard deviation) expression levels of three replicates of three separate experiments with monocytes obtained from three different normal individuals. Cycle threshold [C(t)] values for IFN-responsive genes were normalized with β-actin. The saline control levels were set arbitrarily at 100% expression at each time-point. Values for other samples are expressed relative to the saline control. Expression levels of (a) CCL20, (b) interferon-inducible 44-like (IFI44L), (c) interferon-induced protein with tetratricopeptide repeats 1 (IFIT1), (d) interleukin (IL-8), (e) IL-18 and (f) immunoresponsive gene (IRG)1 transcripts are displayed. Statistical significance of changes in gene expression as determined by Student's t-test comparing each Gd compound to the saline control appears above the corresponding bar. *P < 0·05; **P < 0·01; ***P < 0·001. Caldiamide did not induce a significant effect.
Discussion
Exposure to GdBCA is a crucial event in NSF pathogenesis, but the mechanisms that trigger this severe fibrotic disease are poorly understood. Multiple, non-mutually exclusive hypotheses to explain the roles of a variety of molecular pathways and possible co-factors in NSF pathogenesis have been proposed 12–27. One area of debate concerns the form of the active Gd molecule. The transmetallation hypothesis proposes that free Gd3+ is the active form 12–15, whereas the chelate hypothesis posits the intact Gd chelate molecule instead 16. The present study was designed to compare the relative abilities of linear and macrocyclic GdBCA to induce a proinflammatory/profibrotic phenotype in normal human monocytes. A second goal of the studies described was to examine the ability of the linear and macrocyclic GcBCA to affect expression levels of type I IFNs and type I IFN responsive genes, as well as genes involved in NF-κB expression and NF-κB-mediated transcriptional activation. These studies were carried out in order to provide a mechanistic link between the previously described GdBCA signalling through TLR-4 and TLR-7 19 and the induction of the profibrotic/proinflammatory phenotype responsible for NSF development.
The results demonstrate that, in normal human monocytes, both classes of GdBCA as well as non-chelated Gd compounds induce the expression and secretion of numerous cytokines, chemokines and growth factors generally recognized as important mediators of the initiation and/or progression of the fibrotic process. Further, culture supernatants isolated from monocytes exposed to either linear or macrocyclic agents or to non-chelated Gd compounds were shown to stimulate types I and III collagens and α-SMA and FN1 expression in normal human dermal fibroblasts. While we recognize that HEPES buffer may affect B lymphocyte DNA replication negatively, several studies have found that this effect is observed only in the absence of CO2 28 and that in the presence of CO2 HEPES offers greater buffering capacity and ensures better cell survival and stimulation of cellular proliferation 29. Indeed, in a previous study we observed comparable levels of stimulation of gene expression by GdBCA in human macrophages cultured in the absence of HEPES to the results described here in human monocytes cultured in the presence of HEPES 19.
The changes in expression of numerous proinflammatory/profibrotic chemokines, cytokines and growth factors and of type I IFN responsive genes were consistent in three separate experiments utilizing monocytes derived from three different normal individuals. However, the magnitude of the observed effects was variable among the cells from the three individuals, owing possibly to genetic heterogeneity and variability in the sensitivity of monocytes from different donors to the activating stimuli. These observations are consistent with epidemiological studies showing that NSF development occurs infrequently, even among the large population of renal insufficiency patients exposed to GdBCA.
There was no correlation between the induction of proinflammatory/profibrotic gene expression in normal human monocytes with the dynamic stability of the GdBCA, as would be expected if the active agent was free Gd3+. While some free Gd3+ release cannot be ruled out entirely for ProHance, the thermodynamic stability of Dotarem at the acidic pH levels found in macrophage lysosomal compartments is ∼338 h 27. Therefore, if free Gd3+ is required for the induction of profibrotic molecular pathways, as proposed in the transmetallation hypothesis, then the macrocyclic agents should have had minimal or no detectable effects on cytokine and growth factor expression. Thus, the greater ability of certain GcBCA to induce NSF cannot be explained simply by their enhanced susceptibility to undergo transmetallation. It seems more likely that a unique characteristic of certain linear GdBCA, or of gadodiamide in particular, such as its shape, molecular pattern or molecular surface reactivity, is responsible for the disproportionately high frequency of NSF cases associated with their use.
It has been reported recently that Omniscan and gadodiamide induce TLR-4 and TLR-7-dependent expression of profibrotic/proinflammatory cytokines and growth factors in normal human differentiated macrophages 19. The results described here demonstrate that all Gd compounds examined were capable of inducing expression of genes associated with TLR signalling, including XIAP, which activates NF-κB expression in response to TGF-β signalling 30, and of IRF-1, IRF-4 and IRF-9, which are components of downstream intracellular signalling complexes mediating NF-κB target gene regulation and induction of type I IFN expression 31–34. Interestingly, exposure to all Gd compounds tested resulted in a striking induction of the expression of type I IFN-responsive genes CCL20, IFI44L, IFIT, IL-8, IL-18 and IRG1. These observations are consistent with the notion that GdBCA molecular pattern recognition by the TLR as well as a direct effect of free Gd3+ participate in NSF pathogenesis.
These results, therefore, suggest the establishment of a signalling pathway in which GdBCA as well as non-chelated Gd compounds signal through TLR-4 and TLR-7, inducing expression of XIAP and IRF genes that mediate NF-κB activation. NF-κB activation and signalling, in turn, induce the expression of type I IFN-responsive genes that mediate the establishment of a proinflammatory/profibrotic phenotype characterized by the expression and production of cytokines, chemokines and growth factors capable of triggering the activation of quiescent fibroblasts into myofibroblasts. This putative pathway is illustrated in Fig. 7. These results are of relevance to other fibrotic disorders, such as systemic sclerosis (SSc), in which activation of a type I IFN pathway has been reported 35–41.
Figure 7.
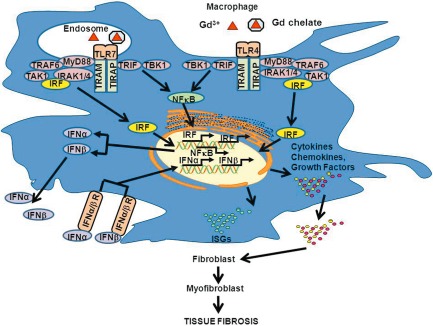
Pathways mediating gadolinium-based contrast agent (GdBCA) induction of a proinflammatory/profibrotic phenotype in normal human macrophages. Cellular recognition of either free Gd3+ (transmetallation model) or of the intact gadolinium chelate (chelate model) is mediated through Toll-like receptor (TLR)-4 and TLR-7 signalling. The signalling events result in up-regulation of X-linked inhibitor of apoptosis (XIAP) and interferon regulatory factor (IRF) genes and activation of nuclear factor kappa B (NFκB), which co-operate to up-regulate type I interferons, that in turn activate type I interferon signature genes (ISGs) resulting in production of proinflammatory and profibrotic chemokines, cytokines and growth factors. These factors are secreted by activated macrophages and can then act to promote the transdifferentiation of quiescent fibroblasts to activated myofibroblasts, characterized by initiation of α-smooth muscle actin (α-SMA) expression and in dysregulated and sustained extracellular matrix production leading to tissue fibrosis. Figure modified from 19.
In conclusion, the results described here demonstrate that GdBCA, regardless of chelate class and thermodynamic stability, induce in normal human monocytes remarkably potent expression of numerous genes encoding well-characterized profibrotic/proinflammatory chemokines, cytokines and growth factors and of type I IFNs and IFN-responsive genes, resulting in their increased production and release. Furthermore, conditioned medium from monocytes exposed to the different Gd-containing compounds caused a potent stimulation of expression of the profibrotic markers types I and III collagen, fibronectin and α-SMA in normal human dermal fibroblasts. The observations that both linear and macrocylic classes of Gd chelates as well as the non-chelated Gd-containing compounds induce these potent cellular phenotypic and gene expression changes in normal human monocytes indicate that GdBCA thermodynamic stability is not likely to be the primary factor influencing the relative activity of these compounds in the induction of NSF.
Acknowledgments
This work was supported by an Investigator Initiated grant from GE Healthcare and by a grant from NIH (R21 AR-061680-01to S. A. J.). We thank Dr Ben Newton for supplying GdBCA and for stimulating discussions and constructive critical reading of the manuscript.
Disclosures
The authors have no financial conflicts of interest.
Supporting Information
Additional Supporting information may be found in the online version of this article at the publisher's website:
Primer sequences utilized for reverse transcription-polymerase chain reaction (RT-PCR).
References
- 1.Cowper SE, Robin HS, Steinberg SM, Su LD, Gupta S, LeBoit PE. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet. 2001;356:1000–1001. doi: 10.1016/S0140-6736(00)02694-5. [DOI] [PubMed] [Google Scholar]
- 2.Cowper SE, Su LD, Bhawan J, Robin HS, LeBoit PE. Nephrogenic fibrosing dermopathy. Am J Dermatopathol. 2001;23:383–393. doi: 10.1097/00000372-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Jimenez SA, Artlett CM, Sandorfi N, et al. Dialysis-associated systemic fibrosis (nephrogenic fibrosing dermopathy) Arthritis Rheum. 2004;50:2660–2666. doi: 10.1002/art.20362. [DOI] [PubMed] [Google Scholar]
- 4.Mendoza FA, Artlett CM, Sandorfi N, Latinis K, Piera-Velazquez S, Jimenez SA. Description of twelve cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum. 2006;35:238–249. doi: 10.1016/j.semarthrit.2005.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grobner T. Gadolinium – a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21:1745. doi: 10.1093/ndt/gfk062. [DOI] [PubMed] [Google Scholar]
- 6.Prince MR, Zhang H, Morris M, et al. Incidence of nephrogenic systemic fibrosis at two large medical centers. Radiology. 2008;248:807–816. doi: 10.1148/radiol.2483071863. [DOI] [PubMed] [Google Scholar]
- 7.Altun E, Martin DR, Wertman R, Lugo-Somolinos A, Fuller ER, III, Semelka RC. Nephrogenic systemic fibrosis: change in incidence following a switch in gadolinium agents and adoption of a gadolinium policy – report from two U.S. universities. Radiology. 2009;253:689–696. doi: 10.1148/radiol.2533090649. [DOI] [PubMed] [Google Scholar]
- 8.Kendrick-Jones JC, Voss DM, De Zoysa JR. Nephrogenic systemic fibrosis, in patients with end-stage renal disease on dialysis, in the greater Auckland region, from 2000–2006. Nephrology (Carlton) 2011;16:243–248. doi: 10.1111/j.1440-1797.2010.01397.x. [DOI] [PubMed] [Google Scholar]
- 9.High WA, Ayers RA, Chandler J, Zito G, Cowper SE. Gadolinium is detectable within the tissue of patients with nephrogenic systemic fibrosis. J Am Acad Dermatol. 2007;56:21–26. doi: 10.1016/j.jaad.2006.10.047. [DOI] [PubMed] [Google Scholar]
- 10.Boyd AS, Zic JA, Abraham JL. Gadolinium deposition in nephrogenic fibrosing dermopathy. J Am Acad Dermatol. 2007;56:27–30. doi: 10.1016/j.jaad.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 11.Kay J, Bazari H, Avery LL, Koreishi AF. Case records of the Massachusetts General Hospital. Case 6-2008. A 46-year-old woman with renal failure and stiffness of the joints and skin. N Engl J Med. 2008;358:827–838. doi: 10.1056/NEJMcpc0708697. [DOI] [PubMed] [Google Scholar]
- 12.Cacheris WP, Quay SC, Rocklage SM. The relationship between thermodynamics and the toxicity of gadolinium complexes. Magn Reson Imaging. 1990;8:476–481. doi: 10.1016/0730-725x(90)90055-7. [DOI] [PubMed] [Google Scholar]
- 13.Idee JM, Port M, Raynal I, Schaefer M, Le Greneur S, Corot C. Clinical and biological consequences of transmetallation induced by contrast agents for magnetic resonance imaging: a review. Fundam Clin Pharmacol. 2006;20:563–576. doi: 10.1111/j.1472-8206.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- 14.Kuo PH. Gadolinium-containing MRI contrast agents: important variations on a theme for NSF. J Am Coll Radiol. 2008;5:29–35. doi: 10.1016/j.jacr.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Morcos SK. Extracellular gadolinium contrast agents: differences in stability. Eur J Radiol. 2008;66:175–179. doi: 10.1016/j.ejrad.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 16.Newton BB, Jimenez SA. Mechanism of NSF: new evidence challenging the prevailing theory. J Magn Reson Imaging. 2010;30:1277–1283. doi: 10.1002/jmri.21980. [DOI] [PubMed] [Google Scholar]
- 17.Wermuth PJ, Del Galdo F, Jimenez SA. Induction of the expression of profibrotic cytokines and growth factors in normal human peripheral blood monocytes by gadolinium contrast agents. Arthritis Rheum. 2009;60:1508–1518. doi: 10.1002/art.24471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del Galdo F, Wermuth PJ, Addya S, Fortina P, Jimenez SA. NFκB activation and stimulation of chemokine production in normal human macrophages by the gadolinium-based magnetic resonance contrast agent Omniscan: possible role in the pathogenesis of nephrogenic systemic fibrosis. Ann Rheum Dis. 2010;69:2024–2033. doi: 10.1136/ard.2010.134858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wermuth PJ, Jimenez SA. Gadolinium compounds signaling through TLR4 and TLR7 in normal human macrophages: establishment of a proinflammatory phenotype and implications for the pathogenesis of nephrogenic systemic fibrosis. J Immunol. 2012;189:318–327. doi: 10.4049/jimmunol.1103099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edward M, Quinn JA, Burden AD, Newton BB, Jardine AG. Effect of different classes of gadolinium-based contrast agents on control and nephrogenic systemic fibrosis-derived fibroblast proliferation. Radiology. 2010;256:735–743. doi: 10.1148/radiol.10091131. [DOI] [PubMed] [Google Scholar]
- 21.Varani J, DaSilva M, Warner RL, et al. Effects of gadolinium-based magnetic resonance imaging contrast agents on human skin in organ culture and human skin fibroblasts. Invest Radiol. 2009;44:74–91. doi: 10.1097/RLI.0b013e31818f76b5. [DOI] [PubMed] [Google Scholar]
- 22.Perone PA, Weber SL, DaSilva M, et al. Collagenolytic activity is suppressed in organ-cultured human skin exposed to a gadolinium-based MRI contrast agent. Invest Radiol. 2010;45:42–48. doi: 10.1097/RLI.0b013e3181bf95eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edward M, Quinn JA, Mukherjee S, et al. Gadodiamide contrast agent ‘activates’ fibroblasts: a possible cause of nephrogenic systemic fibrosis. J Pathol. 2008;4:584–593. doi: 10.1002/path.2311. [DOI] [PubMed] [Google Scholar]
- 24.Cheng Y, Maozi L, Rongchang L, Wang C, Bai C, Wang K. Gadolinium induces domain and pore formation of human erythrocyte membrane: an atomic force microscopic study. Biochim Biophys Acta. 1999;1421:249–260. doi: 10.1016/s0005-2736(99)00125-x. [DOI] [PubMed] [Google Scholar]
- 25.Davies JQ, Gordon S. Isolation and culture of human macrophages. Methods Mol Biol. 2005;290:105–116. doi: 10.1385/1-59259-838-2:105. [DOI] [PubMed] [Google Scholar]
- 26.Idee JM, Port M, Medina C, et al. Possible involvement of gadolinium chelates in the mechanism of nephrogenic systemic fibrosis: an update. Radiol Clin North Am. 2009;47:855–869. doi: 10.1016/j.rcl.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Port M, Idee JM, Medina C, Robic C, Sabatou M, Corot C. Efficiency, thermodynamic and kinetic stability of marketed gadolinium chelates and their possible clinical consequences: a critical review. Biometals. 2008;21:469–490. doi: 10.1007/s10534-008-9135-x. [DOI] [PubMed] [Google Scholar]
- 28.Chakrabarti R, Chakrabarti R. Novel role of extracellular carbon dioxide in lymphocyte proliferation in culture. J Cell Biochem. 2001;83:200–203. doi: 10.1002/jcb.1218. [DOI] [PubMed] [Google Scholar]
- 29.Darzynkiewicz Z, Jacobson B. HEPES-buffered media in lymphocyte cultures. Proc Soc Exp Biol Med. 1971;136:387–393. doi: 10.3181/00379727-136-35271. [DOI] [PubMed] [Google Scholar]
- 30.Lu M, Lin SC, Huang Y, et al. XIAP induces NF-kappaB activation via the BIR1/TAB1 interaction and BIR1 dimerization. Mol Cell. 2007;26:689–702. doi: 10.1016/j.molcel.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pitha PM. Innate antiviral response: role in HIV-1 infection. Viruses. 2011;3:1179–1203. doi: 10.3390/v3071179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu Y, Xie C, Yan M, Joh JW, Lu C, Mohan C. The lipopolysaccharide-triggered mesangial transcriptome: evaluating the role of interferon regulatory factor-1. Kidney Int. 2005;67:1350–1361. doi: 10.1111/j.1523-1755.2005.00212.x. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Basagoudanavar SH, Wang X, et al. NF-κB RelA subunit is crucial for early IFN-beta expression and resistance to RNA virus replication. J Immunol. 2010;185:1720–1729. doi: 10.4049/jimmunol.1000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang WL, Liu W, Gong HY, Lin CC, Wu JL. Activation of cytokine expression occurs through the TNFα/NF-κB-mediated pathway in birnavirus-infected cells. Fish Shellfish Immunol. 2011;31:10–21. doi: 10.1016/j.fsi.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 35.Higgs BW, Liu Z, White B, et al. Patients with systemic lupus erythematosus, myositis, rheumatoid arthritis and scleroderma share activation of a common type I interferon pathway. Arthritis Rheum. 2011;70:2029–2036. doi: 10.1136/ard.2011.150326. [DOI] [PubMed] [Google Scholar]
- 36.Liu S, Mayes MD, Tan FK, et al. Correlation of interferon-inducible chemokine plasma levels with disease severity in systemic sclerosis. Arthritis Rheum. 2013;65:226–235. doi: 10.1002/art.37742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tao J, Li L, Tan Z, et al. Up-regulation of CC chemokine ligand 20 and its receptor CCR6 in the lesional skin of early systemic sclerosis. Eur J Dermatol. 2011;21:731–736. doi: 10.1684/ejd.2011.1469. [DOI] [PubMed] [Google Scholar]
- 38.Degrandi D, Hoffmann R, Beuter-Gunia C, Pfeffer K. The proinflammatory cytokine-induced IRG1 protein associates with mitochondria. J Interferon Cytokine Res. 2009;29:55–67. doi: 10.1089/jir.2008.0013. [DOI] [PubMed] [Google Scholar]
- 39.McDermott JE, Vartanian KB, Mitchell H, Stevens SL, Sanfillippo A, Stenzel-Poore MP. Identification and validation of Ifit1 as an important innate immune bottleneck. PLOS ONE. 2012;7:e:36465. doi: 10.1371/journal.pone.0036465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fineschi S, Goffin L, Rezzonico R, et al. Antifibroblast antibodies in systemic sclerosis induce fibroblasts to produce profibrotic chemokines, with partial exploitation of Toll-like receptor 4. Arthritis Rheum. 2008;58:3913–3923. doi: 10.1002/art.24049. [DOI] [PubMed] [Google Scholar]
- 41.Meldrum KK, Zhang H, Hile KL, Moldower LL, Dong Z, Meldrumd DR. Profibrotic effect of interleukin-18 in HK-2 cells is dependent on stimulation of the Toll-like receptor 4 (TLR4) promoter and increased TLR4 expression. J Biol Chem. 2012;287:40391–40399. doi: 10.1074/jbc.M112.402420. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primer sequences utilized for reverse transcription-polymerase chain reaction (RT-PCR).


