Abstract
Objectives To examine the quality of reporting of harms in systematic reviews, and to determine the need for a reporting guideline specific for reviews of harms.
Design Systematic review.
Data sources Cochrane Database of Systematic Reviews (CDSR) and Database of Abstracts of Reviews of Effects (DARE).
Review methods Databases were searched for systematic reviews having an adverse event as the main outcome, published from January 2008 to April 2011. Adverse events included an adverse reaction, harms, or complications associated with any healthcare intervention. Articles with a primary aim to investigate the complete safety profile of an intervention were also included. We developed a list of 37 items to measure the quality of reporting on harms in each review; data were collected as dichotomous outcomes (“yes” or “no” for each item).
Results Of 4644 reviews identified, 309 were systematic reviews or meta-analyses primarily assessing harms (13 from CDSR; 296 from DARE). Despite a short time interval, the comparison between the years of 2008 and 2010-11 showed no difference on the quality of reporting over time (P=0.079). Titles in fewer than half the reviews (proportion of reviews 0.46 (95% confidence interval 0.40 to 0.52)) did not mention any harm related terms. Almost one third of DARE reviews (0.26 (0.22 to 0.31)) did not clearly define the adverse events reviewed, nor did they specify the study designs selected for inclusion in their methods section. Almost half of reviews (n=170) did not consider patient risk factors or length of follow-up when reviewing harms of an intervention. Of 67 reviews of complications related to surgery or other procedures, only four (0.05 (0.01 to 0.14)) reported professional qualifications of the individuals involved. The overall, unweighted, proportion of reviews with good reporting was 0.56 (0.55 to 0.57); corresponding proportions were 0.55 (0.53 to 0.57) in 2008, 0.55 (0.54 to 0.57) in 2009, and 0.57 (0.55 to 0.58) in 2010-11.
Conclusion Systematic reviews compound the poor reporting of harms data in primary studies by failing to report on harms or doing so inadequately. Improving reporting of adverse events in systematic reviews is an important step towards a balanced assessment of an intervention.
Introduction
A balanced assessment of interventions requires analysis of both benefits and harms. Systematic reviews or meta-analyses of randomised controlled trials are the preferred method to synthesise evidence in a comprehensive, transparent, and reproducible manner. Randomised controlled trials rarely assess harms as their primary outcome; therefore, they typically lack the power to detect differences in harms between groups (table 1). Usually designed to evaluate treatment efficacy or effectiveness, randomised controlled trials are often done over a short period of time, with a relatively small number of participants. These trials are known to be poor at identifying and reporting harms, which can lead to a misconception that a given intervention is safe, when its safety is actually unknown.1 2 3 4 5 6 7 8 Systematic reviews with a primary objective to assess harms represent fewer than 10% of all systematic reviews published yearly.9 10 Systematic reviews of harms can provide valuable information to describe adverse events (frequency, nature, seriousness), but they are hampered by a lack of standardised methods to report these events and the fact that harms are not usually the primary outcome of included studies.9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28
Table 1.
Glossary of terms
| Term | Definition |
|---|---|
| Adverse drug reaction29 | An adverse effect specific to a drug |
| Adverse effect25,29 | An unfavourable outcome that occurs during or after the use of a drug or other intervention but is not necessarily caused by it |
| Adverse event25,29 | An unfavourable outcome that occurs during or after the use of a drug or other intervention but is not necessarily caused by it |
| Complication29 | An adverse event or effect following surgical and other invasive interventions |
| Harm8 | The totality of possible adverse consequences of an intervention or therapy; they are the direct opposite of benefits |
| Safety8 | Substantive evidence of an absence of harm. The term is often misused when there is simply absence of evidence of harm |
| Side effect29 | Any unintended effect, adverse or beneficial, of a drug that occurs at doses normally used for treatment |
| Toxicity8 | Describes drug related harms. The term may be most appropriate for laboratory determined measurements, although it is also used in relation to clinical events. The disadvantage of the term “toxicity” is that it implies causality. If authors cannot prove causality, the terms “abnormal laboratory measurements” or ‘laboratory abnormalities’ are more appropriate to use |
Several studies have identified challenges when developing a systematic review of adverse events.9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 These include: the poor quality information on harms reported on original studies, difficulties in identifying relevant studies on adverse events when using standard systematic searches techniques, and the lack of a specific guideline to perform a systematic review of adverse events. The need for better reporting on harms in general1 2 3 4 5 6 7 8—and in systematic reviews9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 in particular—has been voiced. In a previous review28 of systematic reviews from the Cochrane Database of Systematic Reviews (CDSR) and Database of Abstracts of Reviews of Effects (DARE), our team identified a significantly increased number of reviews of adverse events over the past 17 years (P<0.001); however, the proportion of these reviews out of the total number of reviews was unchanged at 5%.11 14 28 Some positive points were noted—for example, the increased number of databases searched per review and the reduction in number of systematic reviews limiting their search strategies by date or language—but appropriate reporting of search strategies was still a problem.28
The PRISMA30 (preferred reporting items for systematic review and meta-analysis) statement was developed to deal with suboptimal reporting in systematic reviews. Thus far, PRISMA has mainly focused on efficacy and not on harms. A reporting guideline specific for systematic reviews of harms is crucial to provide a better assessment of adverse events of interventions. The first step for successful guideline development is to document the quality of reporting in published research articles to justify the need for the guideline.30 The goal of this review was to determine whether there is a need for a guideline specific for reviews of harms30 through assessment of the quality of reporting in systematic reviews of harms published between January 2008 and April 2011, from two major databases.
Methods
Development of the checklist items
To assess the quality of reporting in systematic reviews of harms, we developed a set of items (table 2) to be reported in these reviews.
Table 2.
Definitions of reporting items
| Item and number | Definition of “yes” |
|---|---|
| Title | |
| 1a) Specifically mentions “harms” or other related term | Should contain a word or phrase related to harm, such as “adverse effect,” “adverse event,” “complications,” and “risk” |
| 1b) Clarifies whether both benefits and harms are examined or only harms | Mentions whether benefits are reviewed or not |
| 1c) Mentions the specific intervention being reviewed | Mentions the intervention being reviewed |
| 1d) Refers to specific patient group or conditions (or both) in which harms have been assessed | Clearly states the specific group of patients or conditions being reviewed |
| Abstract | |
| 2a) Specifically mentions “harms” or other related terms | Should contain a word or phrase related to harm, such as “adverse effect,” “adverse event,” “complications,” and “risk” |
| 2b) Clarifies whether both benefits and harms are examined or only harms | Mentions whether benefits are reviewed or not |
| 2c) Refers to a specific harm assessed | Clearly states the adverse event being reviewed. General descriptions are accepted, for example, “cardiovascular events” or “maternal complications.” Also acceptable if the report is searching for any adverse event and it does not refer to a specific harm |
| 2d) Specifies what type of data was sought | Clearly names the kind of studies searched (for example, randomised controlled trials, cohort studies, case reports only, all types of data) |
| 2e) Specifies what type of data was included | Clearly names the kind of studies included in the analysis or results (that is, randomised controlled trials, cohort studies, case reports, all types of data) |
| 2f) Specifies how each type of data has been appraised | Clearly states the method of quality appraisal for included studies (that is, Jadad score, Cochrane risk of bias tool, Newcastle-Ottawa scale) |
| Introduction | |
| 3a) Explains rationale for addressing specific harm(s), condition(s) and patient group(s) | Reports reasons for proceeding with the systematic review |
| 3b) Clearly defines what events or effects are considered harms in the context of the intervention(s) examined | Clearly states the adverse event being reviewed. General definitions are acceptable (for example, “cardiovascular events” or “maternal complications”). Also acceptable if the report is searching for any adverse events, as in a scoping review, and does not refer to a specific harm |
| 3c) Describes the rationale for type of harms systematic review done: hypothesis generating versus hypothesis testing | Clearly states whether a specific adverse event is being reviewed (hypothesis testing review; for example, cardiovascular deaths) or the authors are searching for any adverse events related to the intervention (hypothesis generating) |
| 3d) Explains rationale for selection of study types or data sources and relevance to focus of the review | Clearly states which study designs are included (for example, randomised controlled trials, cohorts, case reports). Rationale is not necessary for “yes” |
| Objectives | |
| 4a) Provides an explicit statement of questions being asked with reference to harms | Clearly defines, preferably at the end of introduction section, the intervention and the adverse events being reviewed. For this review, it was considered acceptable if intervention and outcome were clearly stated |
| Methods | |
| Protocol and registration | |
| 5a) Describes whether protocol was developed in collaboration with someone with clinical expertise for field or intervention under study | Mentions whether a protocol was developed previously. For this review, it was not necessary to state the clinical expertise of who developed it |
| Eligibility criteria | |
| 6a) Clearly defines what events or effects are considered harms in the context of the intervention(s) examined | Provides a clear definition of the adverse event being reviewed. If a general description was provided previously (that is, “cardiovascular events”), now the specific events need to be defined |
| 6b) Specifies type of studies on harms to be included | Defines which kind of study designs will be included (for example, randomised controlled trials, cohort studies, case-control studies) |
| Information sources | |
| 7a) States whether additional sources for adverse events were searched (for example, regulatory bodies, industry); if so, describes the source, terms used, and dates searched | Reports whether any other sources of adverse events (other than regular peer reviewed journals) were searched. For this review, it was not necessary to have the terms used and dates searched |
| Search | |
| 8a) Presents the full search strategy if additional searches were used to identify adverse events | Reports search strategy used. Adverse events terms and databases searched |
| Study selection | |
| 9a) States what study designs were eligible and provide rationale for their selection | Reports the study designs included in the review (for example, randomised controlled trials, cohort studies, case-control studies). No rationale is required |
| 9b) Defines if studies were screened on the basis of the presence or absence of harms related terms in title or abstract | Reports whether the study screening is based on the report of adverse events or not (for example, review of mortality associated with antiglycaemic drugs; only studies reporting on mortality were included) |
| Data collection process | |
| 10a) Describes method of data extraction for each type of study or report | States whether a data extraction form is used, and how it is done (in duplicate, checked by a second author) |
| Data items | |
| 11a) Lists and defines variables for which data were sought for individual therapies | Reports variable(s) sought for the intervention reviewed (for example, whether the outcome is kidney failure, what variable was used to define kidney failure—creatinine level, creatinine clearance, need of dialysis) |
| 11b) Lists and defines variables for which data were sought for patient underlying risk factors | States whether any potential patient risk factors or confounders are sought (for example, age, sex, comorbidities, previous events) |
| 11c) Lists and defines variables for which data were sought for practitioner training or qualifications | States any variable(s) sought for healthcare personnel (practitioner) (for example, relevant degree, years of experience, other qualifications) |
| 11d) Lists and defines harms for individual therapies | Provides the definition for the harm(s) sought (for example, side effects of propranolol and atenolol defined as severe bradycardia (heart rate ≤45 beats per min) and severe hypotension (systolic blood pressure <80 mm Hg) |
| Risk of bias in individual studies | |
| 12a) For uncontrolled studies, describes whether causality between intervention and adverse event was adjudicated and if so, how | Specifies whether review authors adjudicate if the intervention can cause the harm (for example, use of Bradford Hill criteria for causality32 |
| 12b) Describes risk of bias in studies with incomplete or selective report of adverse events | Describes how studies not reporting adverse events are handled regarding risk of bias. For example, adverse events reported in a randomised controlled trial investigating glucose control in type 2 diabetes included hypoglycaemia but not mortality. Did the authors assess whether the event did not occur (zero deaths), was not measured (and may have occurred), or was measured but not reported |
| Summary measures | |
| 13a) If rare outcomes are being investigated, specifies which summary measures will be used (for example, event rate, events or person time) | Defines the summary measures used for rare events |
| Synthesis of results | |
| 14a) Describes statistical methods of handling with the zero events in included studies | Clearly defines how studies with no adverse events reported are reported and analysed (for example, when zero was an outcome for a 2×2 table, reviewers added a 0.5 value to it) |
| Additional analysis | |
| 16a) Describes additional analysis with studies with high risk of bias | Describes whether studies with high risk of bias are analysed separately (for example, subgroup analysis with high and low risk of bias studies analysed separately) |
| Results | |
| Study selection | |
| 17a) Provides process, table, or flow for each type of study design | Provides a table (or text) containing included studies and a clear reason for exclusion of studies |
| Study characteristics | |
| 18a) Reports study characteristics, such as patient demographics or length of follow-up that may have influenced the risk estimates for the adverse outcome of interest | Reports any potential confounder or patient risk factors that can affect the outcome (adverse event) |
| 18b) Describes methods of collecting adverse events in included studies (for example, patient report, active search) | Reports how adverse events are investigated in included studies (for example, voluntary report, active search) |
| 18c) For each primary study, lists and defines each adverse event reported and how it is identified | Clear definition of adverse event under investigation and how it is identified (method of measurement, assessment or identification) |
| Conclusion | |
| 26a) Provides balanced discussion of benefits and harms with emphasis on study limitations, generalisability, and other sources of information on harms | Clearly discusses the benefits of the interventions and the harms identified in the review |
The items were originally based on a draft generated from analysis of a systematic review of harms conducted previously.33 During the development of the data extraction form for this current review, several items were added. The wording and content were further refined over telephone meetings, by a group of experts in systematic reviews and guideline development.8 31 32 34 35 36 37 38 39
Not every PRISMA item has a corresponding harms item, and a few have more than one suggestion per item. PRISMA items 15, 19-25, and 27 did not have any specific harms related items.
Search strategy
We searched the CDSR (via the Cochrane Library) and DARE (via the Centre for Reviews and Dissemination and the Cochrane Library) databases for systematic reviews having an adverse event as the primary outcome measured. DARE is compiled through rigorous weekly searches of bibliographic databases (including Medline, Embase, PsycINFO, PubMed, and the Cumulative Index to Nursing and Allied Health Literature). It also involves less frequent searches of the Allied and Complementary Medicine Database and the Education Resource Information Center, hand searching of key journals, grey literature, and regular searches of the internet. CDSR includes all the systematic reviews published by the Cochrane Collaboration. We selected the combination of these two databases because they are likely to represent the most comprehensive collection of systematic reviews published in healthcare.28
The search was limited to a 40 month period between 1 January 2008 and 25 April 2011. The web appendix shows the search strategy used. The dates were selected to include recent reviews in order to describe the current state of reporting in systematic reviews of harms.
Eligibility criteria
Reviews were selected if the primary outcome investigated was exclusively an unintended effect or effects of an intervention. It could be an adverse event, adverse effect, adverse reaction, harms, or complications (table 1) associated with any healthcare intervention (such as pharmaceutical interventions, diagnostic procedures, surgical interventions, or medical devices). Articles with a primary aim to investigate the complete safety profile of an intervention were included. Reviews were not excluded on the basis of their results or conclusions.
We excluded reviews assessing both beneficial and harmful effects (reviews of both efficacy and harms), reviews of desirable side effects of drugs, or reviews of prevention or reduction of unintended or adverse effects. No limitations on interventions, patient groups, or language were applied.
Screening and data extraction
Relevant studies were screened by title and abstract (when available) independently by two authors (LZ and SG). Any disagreements were resolved by consensus; if consensus could not be reached, disagreements were resolved with a third author (SV). Abstracts that were identified as potentially meeting the DARE criteria but were not assessed were called “provisional abstracts.” The full text was retrieved for these abstracts.
The data extraction was based on the items developed, piloted, and refined (table 2). Each field received a “yes” if the item was reported as defined, or “no” if not reported. The data were extracted by one author (LZ) and verified by a second author (YL). Disagreements were resolved by consensus.
Outcome
The main outcome assessed was the quality of reporting in reviews of harms for each of the 37 items. We also measured the proportion of each “yes” response for each year of the search: 2008, 2009, and 2010-11 (12 reviews published in the first four months of 2011 were combined with the reviews published in 2010). The quality of reporting was compared between the earliest and latest years reviewed (2008 v 2010-11) to assess any improvement in quality of reporting during the study period.
We deliberately decided not to include an intermediate category (such as “unclear”). If the item was not clearly reported, it was considered as a “no” response and the unclear category would simply be duplication.
The present review did not aim to evaluate the reason behind the review author’s decisions to examine harms. Our goal was to measure the quality of reporting on those reviews— answering the question “is the item clearly reported?” The goal was not to judge whether a methodologically appropriate decision was made (for example, statistical tests used, data extraction, data pooling), but to ensure clarity in reporting regarding the choices made. Information on the types of interventions, nature of included study designs, as well as search strategies and databases searched in systematic reviews published between 1994 and 2011 was previously reported by our team.28
This study does not intend to measure the effect of the PRISMA statement, for two reasons. Firstly, PRISMA focuses on efficacy; thus measurement of its effect would reasonably focus on systematic reviews of efficacy and not specifically of harms. Secondly, the 37 items measured in the present review were new items and not those found in PRISMA.
Data analysis
Data were collected as dichotomous outcomes (“yes” or “no”) for each item, and presented as proportions of reviews for each category (reported from 0 to 1) and as proportions divided by the database that the reviews were identified from (CDSR and DARE). We also provided 95% confidence intervals for each proportion based on methods described by Wilson and Newcombe40 41 using a correction for continuity.
An overall reporting quality rate was provided through an unweighted average of proportions of items with good reporting. P≤0.05 was considered statistically significant. We did statistical calculations using StataIC-13.
Results
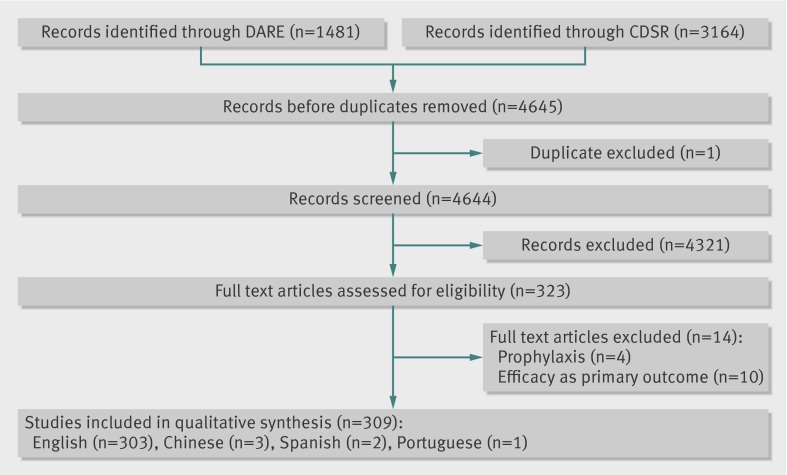
The search yielded 4644 unique references. After screening and retrieving full text articles, an extra 14 papers were excluded as they did not fulfil the inclusion criteria. A total of 309 reviews were identified as systematic reviews of meta-analyses primarily assessing harms, of which 13 were identified at CDSR and 296 at DARE (fig 1). Disagreements at the inclusion and exclusion criteria were discussed by LZ and SG and consensus was reached after discussion. Three of the included papers were published in Chinese, two in Spanish, and one in Portuguese. Table 3 provides detailed information on the reporting of each item.
Fig 1 PRISMA flow diagram
Table 3.
Summary of findings
| Item and number | Proportion (%) and No of reviews with “yes” responses | ||
|---|---|---|---|
| Total reviews (n=309) | CDSR reviews (n=13) | DARE reviews (n=296) | |
| Title | |||
| 1a) Specifically mentions “harms” or other related term | 53 (n=165) | 54 (n=7) | 53 (n=158) |
| 1b) Clarifies whether both benefits and harms are examined or only harms | 76 (n=234) | 92 (n=12) | 75 (n=222) |
| 1c) Mentions the specific intervention being reviewed | 98 (n=303) | 100 (n=13) | 98 (n=290) |
| 1d) Refers to specific patient group or conditions (or both) in which harms have been assessed | 58 (n=178) | 92 (n=12) | 56 (n=166) |
| Abstract | |||
| 2a) Specifically mentions “harms” or other related terms | 84 (n=261) | 85 (n=11) | 85 (n=250) |
| 2b) Clarifies whether both benefits and harms are examined or only harms | 92 (n=285) | 92 (n=12) | 92 (n=273) |
| 2c) Refers to a specific harm assessed | 97 (n=299) | 100 (n=13) | 97 (n=289) |
| 2d) Specifies what type of data was sought | 50 (n=156) | 92 (n=12) | 49 (n=144) |
| 2e) Specifies what type of data was included | 49 (n=151) | 92 (n=12) | 47 (n=139) |
| 2f) Specifies how each type of data has been appraised | 6 (n=19) | 23 (n=3) | 5 (n=16) |
| Introduction | |||
| 3a) Explains rationale for addressing specific harm(s), condition(s) and patient group(s) | 99 (n=307) | 100 (n=13) | 99 (n=294) |
| 3b) Clearly defines what events or effects are considered harms in the context of the intervention(s) examined | 94 (n=289) | 92 (n=12) | 94 (n=277) |
| 3c) Describes the rationale for type of harms systematic review done: hypothesis generating versus hypothesis testing | 99 (n=306) | 100 (n= 13) | 99 (n=293) |
| 3d) Explains rationale for selection of study types or data sources and relevance to focus of the review | 39 (n=120) | 54 (n=7) | 38 (n=113) |
| Objectives | |||
| 4a) Provides an explicit statement of questions being asked with reference to harms | 86 (n=265) | 100 (n=13) | 85(n=252) |
| Methods | |||
| Protocol and registration | |||
| 5a) Describes if protocol was developed | 11 (n=35) | 100(n=13) | 7.4 (n=22) |
| Eligibility criteria | |||
| 6a) Clearly defines what events or effects are considered harms in the context of the intervention(s) examined | 75 (n=231) | 92 (n=12) | 74 (n=219) |
| 6b) Specifies type of studies on harms to be included | 74 (n=230) | 92 (n=12) | 74 (n=218) |
| Information sources | |||
| 7a) States whether additional sources for adverse events were searched (for example, regulatory bodies, industry) | 17 (n=54) | 54 (n=7) | 16 (n=47) |
| Search | |||
| 8a) Presents the full search strategy if additional searches were used to identify adverse events | 69 (n=214) | 100 (n=13) | 68 (n=201) |
| Study selection | |||
| 9a) States what study designs were eligible | 74 (n=229) | 100 (n=13) | 73 (n=216) |
| 9b) Defines if studies were screened on the basis of the presence or absence of adverse events | 71 (n=218) | 92 (n=12) | 70 (n=206) |
| Data collection process | |||
| 10a) Describes method of data extraction for each type of study or report | 64 (n=199) | 100 (n=13) | 63 (n=186) |
| Data items | |||
| 11a) Lists and defines variables for which data were sought for individual therapies | 50 (n=154) | 23 (n=3) | 51 (n=151) |
| 11b) Lists and defines variables for which data were sought for patient underlying risk factors | 42 (n=129) | 23 (n=3) | 43 (n=126) |
| 11c) Lists and defines variables for which data were sought for practitioner training or qualifications | 1 (n=4) | 0 (n=0) | 1 (n=4) |
| 11d) Lists and defines harms for individual therapies | 68 (n=210) | 69 (n=9) | 68 (n=201) |
| Risk of bias in individual studies | |||
| 12a) For uncontrolled studies, describes whether causality between intervention and adverse event was adjudicated | 3 (n=10) | 23 (n=3) | 2 (n=7) |
| 12b) Describes risk of bias in studies with incomplete or selective report of adverse events | 2 (n=6) | 0 (n=0) | 2 (n=6) |
| Summary measures | |||
| 13a) If rare outcomes are being investigated, specifies which summary measures will be used (for example, event rate, events or person time) | 73 (n=227) | 100 (n=13) | 72 (n=214) |
| Synthesis of results | |||
| 14a) Describes statistical methods of handling with the zero events in included studies | 13 (n=41) | 46 (n=6) | 12 (n=35) |
| Additional analysis | |||
| 16a) Describes additional analysis with studies with high risk of bias | 20 (n=61) | 77 (n=10) | 17 (n=51) |
| Results | |||
| Study selection | |||
| 17a) Provides process, table, or flow for each type of study design | 79 (n=244) | 92 (n=12) | 78 (n=232) |
| Study characteristics | |||
| 18a) Reports study characteristics, such as patient demographics or length of follow-up that may have influenced the risk estimates for the adverse outcome of interest | 55 (n=171) | 85 (n=11) | 54 (n=160) |
| 18b) Describes methods of collecting adverse events in included studies (for example, patient report, active search) | 62 (n=190) | 85 (n=11) | 61 (n=179) |
| 18c) For each primary study, lists and defines each adverse event reported and how it is identified | 22 (n=68) | 23 (n=3) | 22 (n=65) |
| Discussion | |||
| 26a) Provides balanced discussion of benefits and harms with emphasis on study limitations, generalisability, and other sources of information on harms | 83 (n=257) | 92 (n=12) |
83 (n=245) |
The 309 systematic reviews and meta-analyses with harms as a primary outcome focused on the following interventions:
Drugs (223 studies, proportion of reviews 0.72 (95% confidence interval 0.66 to 0.77))
Surgery or other procedures (67 studies, 0.21 (0.17 to 0.26))
Devices (13 studies, 0.04 (0.02 to 0.07))
Blood transfusion (two studies, 0.006 (0.001 to 0.022))
Enteral nutrition (two studies, 0.006 (0.001 to 0.022))
Isolation rooms (one study, 0.003 (0.001 to 0.01))
Surgical versus medical treatment (one study, 0.003 (0.001 to 0.01)).
Titles and abstracts
Titles in close to half of included systematic reviews and meta-analyses of harms did not mention any harm related terms (proportion of reviews 0.46 (95% confidence interval 0.40 to 0.52)) and had no report of a patient population or condition under review (0.42 (0.36 to 0.48)). Twenty five reviews (0.08 (0.05 to 0.11) used the word “safety” to identify a review of harms. In the abstract section, reviews often used harm related terms (0.84 (0.79 to 0.88)). For other terms, one in every 6.5 reviews did not have any harms related word in the abstract, and half of reviews (0.5 (0.44 to 0.56)) did not report the study designs sought or included.
Introduction and rationale
Introductions were well written overall, explaining the rationale for the review and providing information on harms being reviewed. Fifty one reviews (proportion of reviews 0.16 (95% confidence interval 0.12 to 0.21)) were performed to investigate any adverse event associated with an intervention, rather than focusing on a specific event. As per our definition, these reviews provided “an explicit statement of questions being asked with reference to harms” as this broad goal was reported.
Methods
Protocol and registration
Consistent with Cochrane requirements for authors, all included systematic reviews conducted through the Cochrane Collaboration (Cochrane reviews) had a protocol. Cochrane reviews did not refer to a protocol in their full text reviews, but this item was considered “yes” for the reviews for which a protocol could be found. By contrast, only 22 reviews (proportion of reviews 0.07 (95% confidence interval 0.04 to 0.11)) identified through the DARE database reported the use of a protocol. Reporting clinical expertise was not deemed necessary to receive “yes” for this item (table 2).
Eligibility criteria
Almost one third of DARE reviews (proportion of reviews 0.26 (95% confidence interval 0.22 to 0.31)) did not have a clear definition of the adverse events reviewed, nor did they specify the study designs selected for inclusion in their methods section. All Cochrane reviews had a clear report of their search strategy, eligible study designs, and methods of data extraction.
Information sources
Authors did not usually search outside the peer reviewed literature for additional sources of adverse events; for example, only 54 reviews (proportion of reviews 0.17 (95% confidence interval 0.13 to 0.22)) searched databases of regulatory bodies or similar sources. Seven of 13 Cochrane reviews (0.53 (0.25 to 0.80)) searched for data from regulatory bodies or industry, compared with 47 of 296 (0.15 (0.11 to 0.20)) DARE reviews.
Study selection and data items
At the screening phase, most reviews only included studies if the harms being searched were reported (proportion of reviews 0.70 (95% confidence interval 0.65 to 0.75)). Of 67 reviews of complications related to surgery or procedures, only four (0.05 (0.01 to 0.14)) reported professional qualifications of the individuals involved.
Study characteristics
Reports of any possible patient related risk factors—such as age, sex, or comorbidities—were sought in less than half of reviews (proportion of reviews 0.41 (0.36 to 0.47)). Furthermore, only 10 reviews adjudicated whether the adverse event could be biologically, pharmacologically, or temporally caused by the intervention, as measured by item 12a.
Results
Fewer than half the reviews (132 of 309; proportion of reviews 0.42 (95% confidence interval 0.37 to 0.48)) only included controlled clinical trials (randomised or not). Only 59 reviews (0.19 (0.14 to 0.23)) included both clinical trials and observational studies. Only observational studies (prospective or retrospective) were included in 109 reviews (0.35 (0.29 to 0.40)), case series or case reports were included in 24 (0.07 (0.05 to 0.11)). Three of 13 Cochrane reviews (0.23 (0.05 to 0.53)) included observational studies compared with 165 of 296 DARE reviews (0.55 (0.49 to 0.61)). After reviewing the full text, nine reviews did not report the designs included anywhere in the text. Length of follow-up or patient demographics were only reported in just over half of reviews (170 of 309 reviews; 0.55 (0.49 to 0.60)).
In a retrospective analysis, we compared the quality of reporting between the years of 2008 and 2010-11 to measure any possible improvement over time on the quality of reporting. There were no significant differences (P=0.079) on the proportion of good reporting between the earlier and later years.
The 13 CDSR reviews had overall better reporting than DARE reviews in the abstracts, methods, and results sections; the other categories had similar levels of reporting quality. Almost half of DARE reviews (proportion of reviews 0.46 (95% confidence interval 0.40 to 0.51)) had poor reporting in the results section. Because the number of reviews was considerably different between databases, we considered it inappropriate to proceed with any formal tests to compare CDSR and DARE reviews.
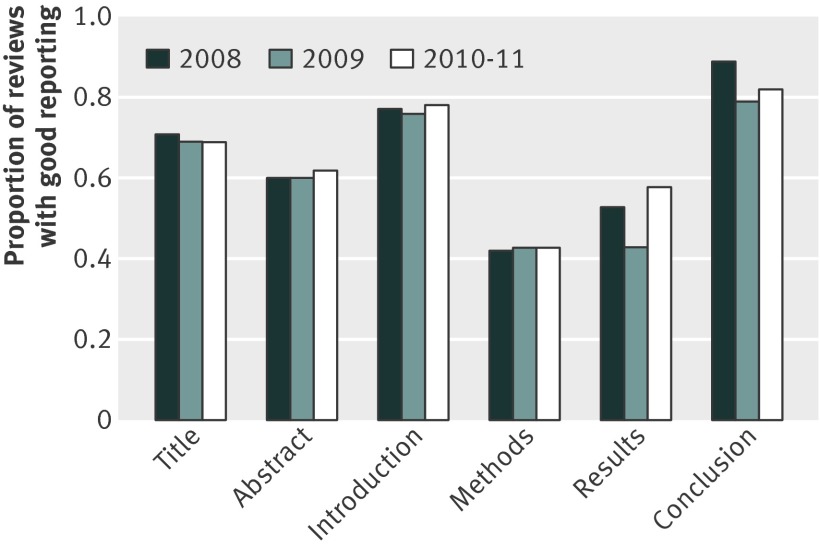
Figure 2 provides a graphic trend in the proportion of reviews with good reporting over time (2008, 2009, and 2010-11), by each item reviewed. Reviews had a poor report quality on methods and results, with an average of half of items being poorly reported on those sections.
Fig 2 Proportion of good reporting by subheadings
The overall proportion of reviews with good reporting was 0.56 (95% confidence interval 0.55 to 0.57). The corresponding proportions were 0.55 (0.53 to 0.57) in 2008, 0.55 (0.54 to 0.57) in 2009, and 0.57 (0.55 to 0.58) in 2010-11.
Discussion
Principal findings
We conducted this systematic review to assess the quality of reporting in systematic reviews of harms as a primary outcome using a set of proposed reporting items. This is the first step for the development of PRISMA Harms, a reporting guideline specifically designed for reviews of harms.30 There is a substantial difference between the number of systematic reviews measuring an adverse event as the main outcome identified through DARE and those identified through CDSR. CDSR reviews comprised only a small fraction of the total reviews included. This distinction may compromise any direct comparison between the two databases. Reviews of harms as a secondary outcome or a coprimary outcome were not included in this review, which could be one reason for the large dissimilarity. Despite the small number of reviews of harms published in the CDSR, they were better reported overall than DARE reviews, probably owing to the clear guidelines provided by the Cochrane Collaboration29 and the more flexible word limits allowed than those in other peer reviewed journals.
Several items were poorly reported in the included reviews, but a few are especially important when reviewing harms, because the lack of reporting on these could lead to misinterpretations of findings. In a systematic review, the screening phase is crucial and the exclusion of studies due to the absence of harms could overestimate the events and perhaps generate a biased review. Two thirds of reviews of harms only included studies if at least one adverse event was reported in the included studies. In a review of harms, “zero” is an important value, and studies with “no adverse events” are possibly as relevant to the review as those with reported adverse events. Nevertheless, zero events in studies require careful interpretation, because the lack of reported harms may have different reasons: they may not have occurred (that is, a zero event), they may not have been investigated (that is, unknown if zero or no events occurred), or they may have been detected but not reported (that is, unknown if zero or no events occurred). The lack of reporting can be thought of as a measurement bias or reporting bias and should be considered as such.1 2 3 4 5 6 7 8 9 10 11 12 24 25 26
All these scenarios have different implications for readers who need to judge whether an intervention may cause harm. Almost half of the included reviews of harms as a primary outcome did not consider patient risk factors or length of follow-up when reviewing adverse events of an intervention. Readers cannot properly judge whether there is an association between intervention and harms if these critical data are not reported. Most of the poorly reported items were identified at methods and results. The clarity on methods and results are essential to provide a clear picture of author’s intentions and limitations of findings and transparency is warranted.
Strengths and limitations
This review was unique by including more than 300 reviews, from two major databases, that looked at harms as a main outcome; each review was evaluated in depth using a novel set of 37 items to measure the quality of reporting. A limitation of this review was the lack of a reporting guideline specific for systematic reviews of harms; different formats of reporting were found, and assessing whether the reporting was adequate was challenging. The reviewers were generous in their assessment, accepting a range of reports as a “yes” to the presence of an item, which could have underestimated the degree of the problem.
Our review was limited exclusively to systematic reviews where harms were the primary focus. We believed that measuring the quality of reporting of adverse events in reviews specifically designed to evaluate adverse events would generate a pure sample focusing on the reporting of such events. The documenting of poor reporting on these reviews would imply poor reporting on adverse events in general. At this stage, we also decided to be inclusive and measure all potentially relevant items. In the future PRISMA Harms extension, we will limit these to the minimum set of essential items for reporting harms in a systematic review.
Comparison with other studies
Hopewell and colleagues14 reviewed a sample of 59 Cochrane reviews, of which only one was a harms review. The remaining 58 reviews focused primarily on benefit, with adverse events as secondary or tertiary considerations. Hopewell and colleagues reported that 32 (54%) reviews had fewer than three paragraphs of information on adverse events, and 11 (19%) had fewer than five sentences on adverse events.
Hammad and colleagues42 reviewed a sample of 27 meta-analyses primarily assessing drug safety and identified that more than 85% of the PRISMA items were reported in the majority of the reviews. However, most reviews did not report the 20 items specifically developed by the authors to address drug safety assessment. Several of the items considered important by Hammad and colleagues where similar to the ones developed by our team. The present review adds to the voice of many authors highlighting the poor reporting in reviews of adverse events.9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 42
Policy implications
Systematic reviews may compound the poor reporting of harms data in primary studies by failing to report on harms or doing so inadequately.9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 We recognise the need to optimise quality of reporting on harms in primary studies, and attempts to enhance it have already been made.6 At this phase, we measured the report quality in systematic reviews. As a future step, we intend to compare the quality of reporting in included studies with the reporting in the systematic review.
Despite their status as the preferred method for knowledge synthesis, systematic reviews can present an incomplete picture to readers by not representing a reliable assessment of a given treatment. Authors of the systematic review have a unique vantage point and can evaluate the entire evidence base under review, including deficits in reporting harms at the primary study level. This vantage point should be used to flag deficits in primary reporting. The goal over time should be to improve the quality and clarity of reporting in systematic reviews as well as the primary studies they evaluate. Although we are glad to see increasing numbers of systematic reviews of harms being published, we are also aware that the validity of the results can be heavily influenced by reviewers’ decisions during conduct of the review—even more so than in reviews of benefit because we are dealing with sparse data and secondary outcomes.43 44 45 Hence, we emphasise here the crucial importance of transparent reporting of the methods used in systematic reviews of harms.
Conclusions
Systematic reviews of interventions should put equal emphasis on efficacy and harms. Improved reporting of adverse events in systematic reviews is one step towards providing a balanced assessment of an intervention. Patients, healthcare professionals, and policymakers should base their decisions not only on the efficacy of an intervention, but also on its risks. Guidance on a minimal set of items to be reported when reviewing harms is needed to improve transparency and informed decision making, thereby greatly enhancing the relevance of systematic reviews to clinical practice.
This review used a set of proposed reporting items to assess reporting, and the findings indicate that specific aspects of harms reporting could be improved. The items will be further refined with the aim of developing a final set of criteria that would constitute the PRISMA Harms. The PRISMA statement30 is a living document, open to criticism and suggestions; these same principles will be shared by the PRISMA Harms. The development of a standardised format for reporting harms in systematic reviews will promote clarity and help ensure that readers have the basic information necessary to make an informed assessment of the intervention under review.
What this paper adds
There is room for improved reporting in systematic of harms, including clear definition of the events measured, length of follow-up, and patient risk factors
Comparisons of reviews of harms have shown no improvement in the quality of reporting over time
Lack of detail or transparency in the reporting of systematic reviews of harms could hinder proper assessment of validity of findings
What is already known on this topic
The number of systematic reviews of adverse effects has increased significantly over the past 17 years
Harms are poorly reported in randomised controlled trials, but it is unclear whether there are weaknesses in the reporting of systematic reviews of harms
Although the PRISMA statement aims to provide guidance on transparent reporting in systematic reviews, its recommendations are mainly focused on studies of beneficial outcomes
Contributors: All authors participated in the study conception and design, and analysis and interpretation of data; drafted the article or revisited it critically for important intellectual content; and approved the final version to be published. SV is the study guarantor.
Funding: No specific funding was given for this study.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: No ethics approval was necessary because this was a review of published literature. No patient data or confidential information were used in this manuscript.
Data sharing: The dataset is available from the corresponding author at svohra@ualberta.ca.
This manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Cite this as: BMJ 2014;348:f7668
Web Extra. Extra material supplied by the author
Web appendix: Search strategy
References
- 1.Ioannidis JP. Adverse events in randomized trials: neglected, restricted, distorted, and silenced. Arch Intern Med 2009;169:1737-9. [DOI] [PubMed] [Google Scholar]
- 2.Sigh S, Loke YK. Drug safety assessment in clinical trials: methodological challenges and opportunities. Trials 2012;13:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papanikolau PN, Ioannidis JP. Availability of large-scale evidence on specific harms from systematic reviews of randomized trials. Am J Med 2004;117:582-9. [DOI] [PubMed] [Google Scholar]
- 4.Pitrou I, Boutron I, Ahmad N, Ravaud P. Reporting of safety results in published reports of randomized controlled trials. Arch Intern Med 2009;169:1756-61. [DOI] [PubMed] [Google Scholar]
- 5.Loke YK, Derry S. Reporting of adverse drug reactions in randomized controlled trials—a systematic survey. BMC Clin Pharmacol 2001;1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ioannidis JP, Lau J. Completeness of safety reporting in randomized trials: an evaluation of 7 medical areas. JAMA 2001;285:437-43. [DOI] [PubMed] [Google Scholar]
- 7.Yazici Y. Some concerns about adverse event reporting in randomized clinical trials. Bull NYU Hosp Jt Dis 2008;66:143-5. [PubMed] [Google Scholar]
- 8.Ioannidis JP, Evans SJ, Gøtzsche PC, O’Neill RT, Altman DG, Schultz K, et al. Better reporting of harms in randomized clinical trials: an extension of the CONSORT statement. Ann Int Med 2004;141:781-8. [DOI] [PubMed] [Google Scholar]
- 9.McIntosh HM, Woolacott NF, Bagnall AM. Assessing harmful effects in systematic reviews. BMC Med Res Methodol 2004;4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ernst E, Pittler MH. Assessment of therapeutic safety in systematic reviews: literature review. BMJ 2001;323:546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ernst E, Pittler MH. Systematic reviews neglect safety issues. Arch Intern Med 2001;161:125-6. [DOI] [PubMed] [Google Scholar]
- 12.Aronson JK, Derry S, Loke YK. Adverse drug reactions: keeping up to date. Fundam Clin Pharmacol 2002;16:49-56. [DOI] [PubMed] [Google Scholar]
- 13.Moseley AM, Elkins MR, Herbert RD, Maher CG, Sherrington C. Cochrane reviews used more rigorous methods than non-cochrane reviews: survey of systematic reviews in physiotherapy. J Clin Epidemiol 2009;62:1021-30. [DOI] [PubMed] [Google Scholar]
- 14.Hopewell S, Wolfenden L, Clarke M. Reporting of adverse events in systematic reviews can be improved: survey results. J Clin Epidemiol 2007;61:597-602. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Tetzlaff J, Tricco AC, Sampson M, Altman DG. Epidemiology and reporting characteristics of systematic reviews. PloS Med 2007;4:e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Golder S, Loke YK, McIntosh HM. Room for improvement? A survey of the methods used in systematic reviews of adverse effects. BMC Med Res Methodol 2006;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vandermeer B, Bialy L, Johnston B, Hooton N, Hartling L, Klassen TP, et al. Meta-analyses of safety data: a comparison of exact versus asymptotic methods. Stat Methods Med Res 2009;18:421-32. [DOI] [PubMed] [Google Scholar]
- 18.Loke YK, Golder S, Vandenbroucke J. Comprehensive evaluations of the adverse effects of drugs: importance of appropriate study selection and data sources. Ther Adv Drug Saf 2011;2:59-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loke YK, Price D, Herxheimer A. Systematic reviews of adverse effects: framework for a structured approach. BMC Med Res Methodol 2007;7:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golder S, Loke YK, McIntosh HM. Poor reporting and inadequate searches were apparent in systematic reviews of adverse effects. J Clin Epidemiol 2008;61:440-8. [DOI] [PubMed] [Google Scholar]
- 21.Golder S, McIntosh HM, Loke YK. Identifying systematic reviews of the adverse effects of health care interventions. BMC Med Res Methodol 2006;6:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Derry S, Loke YK, Aronson JK. Incomplete evidence: the inadequacy of databases in tracing published adverse drug reactions in clinical trials. BMC Med Res Methodol 2001;1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Golder S, Loke Y. Is there evidence for biased reporting of published adverse effects data in pharmaceutical industry-funded studies? Br J Clin Pharmacol 2008;66:767-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chou R, Helfand M. Challenges in systematic reviews that assess treatment harms. Ann Intern Med 2005;142:1090-9. [DOI] [PubMed] [Google Scholar]
- 25.Chou R, Aronson N, Atkins D, Ismaila AS, Santaguida P, Smith DH, et al. AHRQ series paper 4: assessing harms when comparing medical interventions: AHRQ and the effective health care program. J Clin Epi 2010;63:502-12. [DOI] [PubMed] [Google Scholar]
- 26.Cornelius VR, Perrio MJ, Shakir SA, Smith LA. Systematic reviews of adverse effects of drug interventions: a survey of their conduct and reporting quality. Pharmacoepidemiol Drug Saf 2009;18:1223-31. [DOI] [PubMed] [Google Scholar]
- 27.Warren FC, Abrams KR, Golder S, Sutton AJ. Systematic review of methods used in meta-analyses where a primary outcome is an adverse or unintended event. BMC Med Res Methodol 2012;12:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Golder S, Loke YK, Zorzela L. Some improvements are apparent in identifying adverse effects in systematic reviews from 1994 to 2011. J Clin Epidemiol 2013;66:253-60. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 [updated March 2011]. Cochrane Collaboration, 2011. www.cochrane-handbook.org.
- 30.Moher D, Liberati A, Tetzlaff J, Altman D. The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PloS Med 2009;6:e1000097. [PMC free article] [PubMed] [Google Scholar]
- 31.Moher D, Schulz K, Simera I, Altman D. Guidance for developers of health research reporting guidelines. PloS Med 2010;7:e1000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill AB. The environment and disease: association or causation? Proc Roy Soc Med 1965;58:295-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pilkington K, Boshnakova A. Complementary medicine and safety: a systematic investigation of design and reporting of systematic reviews. Complement Ther Med 2012;20:73-82. [DOI] [PubMed] [Google Scholar]
- 34.Campbell MK, Piaggio G, Elbourne DR, Altman DG. Consort 2010 statement: extension to cluster randomised trials. BMJ 2012;345:e5661. [DOI] [PubMed] [Google Scholar]
- 35.Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SJ. Reporting of non inferiority and equivalence randomized trials: an extension of the CONSORT statement. JAMA 2006;295:1152-60. [DOI] [PubMed] [Google Scholar]
- 36.Zwarenstein M, Treweek S, Gagnier JJ, Altman DG,Tunis S, Haynes B, et al. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ 2008;337:a2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gagnier JJ, Boon H, Rochon P, Moher D, Barnes J, Bombardier C. Reporting randomized, controlled trials of herbal interventions: an elaborated CONSORT statement. Ann Intern Med 2006;144:364-67. [DOI] [PubMed] [Google Scholar]
- 38.Boutron I, Moher D, Altman DG, Schulz K, Ravaud P. Methods and processes of the CONSORT group: example of an extension for trials assessing nonpharmacologic treatments. Ann Intern Med 2008;148:W60-6. [DOI] [PubMed] [Google Scholar]
- 39.MacPherson H, Altman DG, Hammerschlag R, Youping L, Taixiang W, White A, et al. STRICTA revision group. Revised standards for reporting interventions in clinical trials of acupuncture (STRICTA): extending the CONSORT statement. PLoS Med 2010;7:e1000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Newcombe, Robert G. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med 1998;17:857-72. [DOI] [PubMed] [Google Scholar]
- 41.Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc 1927;22:209-21. [Google Scholar]
- 42.Hammad T, Neyarapally G, Pinheiro S, Iyasu S, Rochester G, Dal Pan G. Reporting of meta-analyses of randomized controlled trials with a focus on drug safety: An empirical assessment. Clin Trials 2013;10:389-97. [DOI] [PubMed] [Google Scholar]
- 43.Diamond GA, Bax L, Kaul S. Uncertain effects of rosiglitazone on the risk for myocardial infarction and cardiovascular death. Ann Intern Med 2007;147:578-81. [DOI] [PubMed] [Google Scholar]
- 44.Hernandez AV, Walker E, Ioannides-Demos LL, Kattan MW. Challenges in meta-analysis of randomized clinical trials for rare harmful cardiovascular events: the case of rosiglitazone. Am Heart J 2008;156:23-30. [DOI] [PubMed] [Google Scholar]
- 45.Shuster JJ, Jones LS, Salmon DA. Fixed vs random effects meta-analysis in rare event studies: the rosiglitazone link with myocardial infarction and cardiac death. Stat Med 2007;26:4375-85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Search strategy