Abstract
Salting out constants for triglycine were calculated for a series of Hofmeister salts using molecular dynamics simulations. Three variants of the peptide were considered with both termini capped, just the N-terminus capped, and without capping. The simulations were supported by NMR and FTIR measurements. The data provide strong evidence that earlier experimental values of salting out constants assigned to the fully capped peptide (as previously assumed) should have been assigned to the half-capped peptide instead. Therefore, these values cannot be used to directly establish Hofmeister ordering of ions at the peptide backbone, since they are strongly influenced by interactions of the ions with the negatively charged C-terminus.
Keywords: ions, Hofmeister series, triglycine, NMR, molecular dynamics
The action of cosolvents, such as salt ions or osmolytes, on aqueous biological molecules can be treated in a thermodynamically rigorous way by Kirkwood-Buff theory.1 This theory connects microscopic structural properties in the form of radial distribution functions with macroscopic observables like excess chemical potentials and solubilities. In particular, it enables one to derive salting-out constants from molecular simulations and to connect them with experimentally observed values.2 Accurately determined salting-out constants for a given protein or peptide allow for quantitatively establishing Hofmeister ordering3, 4 of the investigated salt ions, which is a prerequisite for understanding ion specific effects on biomolecules.5
In the early 1970s, Nandi and Robinson measured the salting-out constants of short oligoglycines.6 In order to establish Hofmeister ordering of ions at the peptide bond, they tried to suppress the effects of the charged terminal groups of the oligopeptides by attempting to cap both the C- and N-termini. The salting-out constants they found for acetyltriglycine ethyl ester in aqueous salt solutions6 are reproduced in Table 1 together with the corresponding values for uncapped triglycine (GGG).7 As can be seen, capping the terminal groups has a dramatic effect on the salting out constants. Specifically, strongly hydrated ions display a salting-out effect on the capped peptide and weakly hydrated ions salt it in. In contrast, all investigated ions salt in the uncapped peptide. This is consistent with our recent findings that ion pairing with charged termini can be quite strong for short peptides and may overwhelm the interactions of ions with the peptide bond.8
Table 1.
Experimental6, 7 solubilities (g of peptide/100g of solution) and salting out constant ks for acetyltriglycine ethyl ester and triglycine in various Hofmeister salts at molar concentrations Cs of 1 M. The experimental definition of ks based on peptide solubility in water S(0) and in salt solution S(Cs) is as follows: ksCs = log(S(0)/ S(Cs)).
| acetyltriglycine ethyl ester6 | triglycine7 | |||
|---|---|---|---|---|
| salt | solubility | ks (M−1) | solubility | ks (M−1) |
| Water | 0.648 | 0 | 6.41 | 0 |
| Na2SO4 | 0.18 | 0.55 | --- | --- |
| NaAc | --- | --- | 7.44 | -0.06 |
| NaF | 0.35 | 0.27 | --- | --- |
| NaCl | 0.53 | 0.09 | 9.09 | -0.15 |
| NaBr | 0.57 | 0.05 | 9.48 | -0.17 |
| NaI | 0.87 | -0.13 | --- | --- |
| NaSCN | 1.05 | -0.21 | --- | --- |
| NaClO4 | 1.05 | -0.21 | --- | --- |
Recently, the salting-out constants for oligoglycines by Nandi and Robinson6 have been used to derive partitioning coefficients of individual ions in the Hofmeister series for the amide group forming the peptide bond at the protein backbone.9, 10 Such an approach can be justified provided the effects of the termini on ion binding are small, such as is the case when the termini are properly capped (and thus neutralized). However, we have found recently that while the N-terminus can be easily acetylated, it is actually rather difficult to cap the C-terminus of oligoglycines.8 As a matter of fact, we were not able to effectively cap the C-terminus by the esterification procedure described in Ref. 6. This led us to employ molecular dynamics (MD) simulations in order to extract salting out constants for fully capped vs. uncapped and half-capped (only at the N-terminus) GGG to find out which results correlate better with those from the earlier experiments.6
We have thus simulated capped, half-capped, and uncapped GGG in aqueous solutions of seven sodium salts with a series of Hofmeister anions - Na2SO4, NaF, NaCl, NaBr, NaI, NaSCN, and NaClO4. Using the simulations, we related the salting out constant, ks, to the calculated preferential binding coefficient, Γs, (for definition see Ref. 8) of a given salt at concentration Cs to the peptide molecule:
| (1) |
This relation, which assumes that the preferential binding coefficient Γs is independent of the peptide concentration and an ideal behavior of the solution, can be very accurate for molecules with low solubilities (such as the capped and half-capped GGG), and is approximately valid for more soluble molecules like GGG as well.2 The calculated salting-out constants can be then directly compared to the experimental values obtained by Nandi and Robinson.6
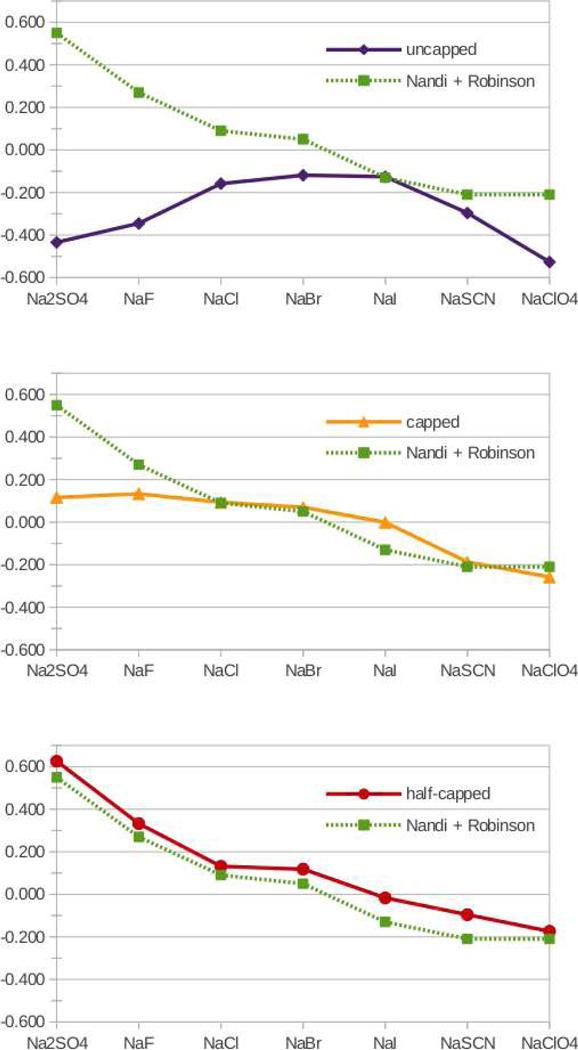
Figure 1 presents such a comparison for all three investigated variants of GGG, i.e., the uncapped, capped, and half-capped peptide. First and foremost, we see from Figure 1 that the simulations of the half-capped system agree quantitatively with the experiment, while there are significant discrepancies between the calculations and measurements for the other two systems. The present results thus strongly indicate that Nandi and Robinson6 actually did not succeed in capping the C-terminus. This observation, supported by the experimental fact that we were also unable to effectively cap the C-terminus of GGG by their esterification procedure (but instead had to use another synthetic route),8 has important implications for molecular interpretations of the Hofmeister ordering of ions, as discussed below.
Figure 1.
Salting out constants ks for uncapped, capped, and half-capped GGG in Hofmeister salt solutions as obtained from MD simulations and compared to the experimental data.6
The half-capped GGG system is negatively charged, having only the N-terminus neutralized. Anions, therefore, tend to be repelled from it, while sodium is attracted. For anions, which are more strongly hydrated than sodium, in particular sulfate and fluoride, anionic repulsion dominates over cationic attraction, leading to a positive salting-out constant (Figure 1, bottom). The opposite is true for weakly hydrated anions, such as iodide, thiocyanate, or perchlorate, which are also attracted to the peptide backbone.5, 8 As a result, sodium salts of these anions exhibit negative salting out constants (Figure 1, bottom). It is important to note that for the half-capped GGG molecule, salting-out or -in is a net effect of interactions of both cations and anions with the backbone and the C-terminus, which cannot be interpreted simply in terms of ion-backbone interactions only.9
In order to focus on the Hofmeister effects of ions on the peptide backbone, the properly capped GGG should be considered. Indeed, by capping both termini, the ion-peptide interactions become dominated by those with the backbone. Sodium cations, as well as strongly hydrated anions, interact weakly with the backbone leading to modestly positive (close to zero) values of the salting out constant (Figure 1, middle). Note that this is in clear disagreement with the Nandi and Robinson results, which again points to the fact that they did not, in fact, investigate the fully capped peptide.6 In contrast to strongly hydrated anions, weakly hydrated anions are attracted to the backbone, specifically to a site consisting of the amide NH group and the adjacent α-carbon,5 which leads to negative salting out coefficients (Figure 1, middle).
As discussed already in our previous study,8 ion specific effects on uncapped GGG are dominated by the charged termini. This leads, particularly for strongly hydrated ions, to large negative salting out coefficients (Figure 1, top), implying a salting-in effect. For weakly hydrated anions, additional attraction to the backbone plays an important role, which explains their negative salting out coefficients (Figure 1, top). As a result, there is no apparent Hofmeister ordering for uncapped GGG, with the corresponding curve for salting-out coefficients being non-monotonous.
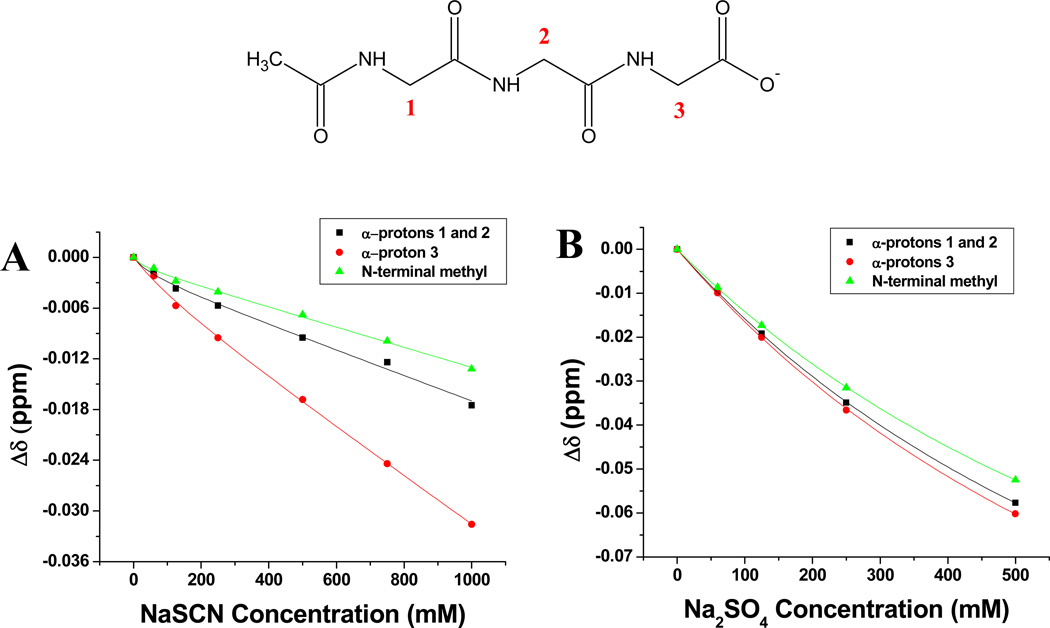
To experimentally verify the results found through simulations, we also performed proton NMR titrations of the N-capped peptide with NaSCN and Na2SO4. The chemical shift changes of the three backbone methylene protons as well as the N-terminal methyl protons of the capping group were measured as function of salt concentrations (Figure 2). As can be seen, the chemical shift decreased in a non-linear fashion in all cases. Had binding occurred, one would have expected a downward curve for these concentration dependent slopes, rather than the upward curve seen here.5, 8 In other words, none of the methylene units in the half-capped GGG backbone moieties displayed any apparent anion binding in the presence of NaSCN (Figure 2a). This is consistent with the anionic nature of the half capped GGG, which should repel SCN− anions compared with its fully capped and uncapped counterparts. Moreover, SO4 2− gave rise to even greater upward curve (Figure 2b), which is consistent with even greater anion repulsion. Such results for the half capped GGG molecule are in agreement with the solubility and MD simulation results described above.
Figure 2.
Relative chemical shift changes of the aliphatic protons of 50 mM N-Ac-GGG-CO2− (half capped GGG) as function of (A) NaSCN concentration and (B) Na2SO4 concentration. The data points are connected with lines as a guide to the eye.
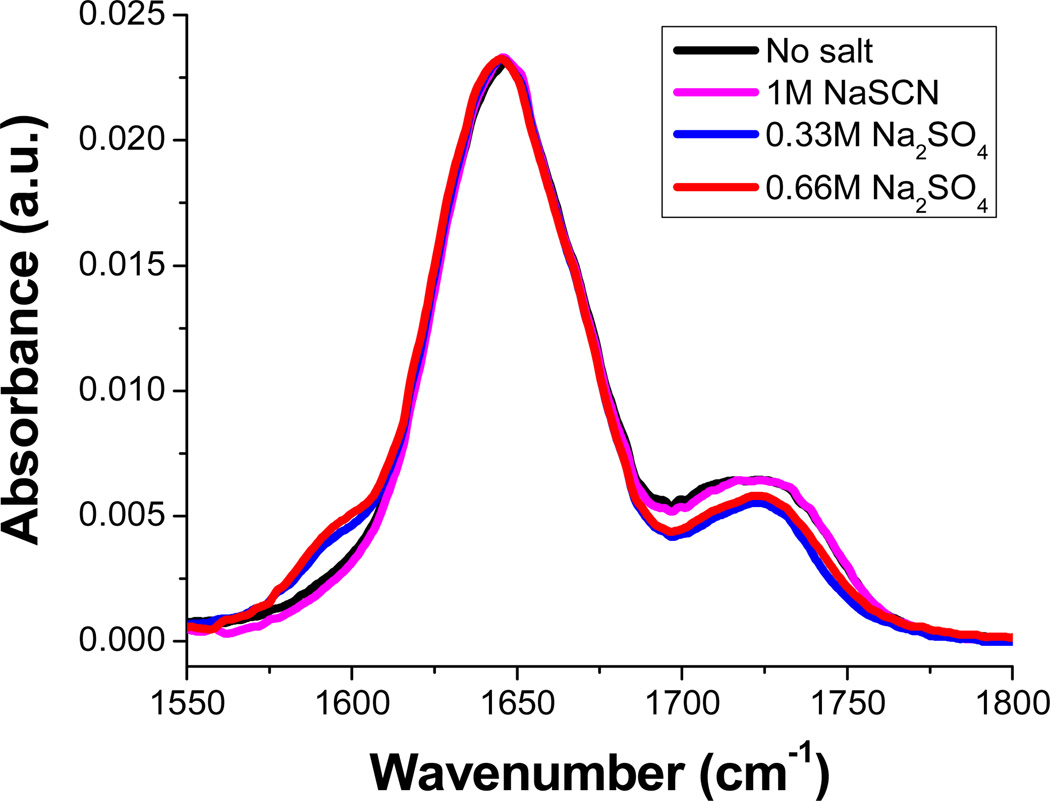
It should be noted that large salting out constants, ks, (table 1) could potentially be associated with structural changes to the peptide or peptide aggregation.11–13 To rule out this possibility, FTIR measurements were taken with half-capped GGG molecules in the presence of Na2SO4 and NaSCN as well as without any added salt. As can be seen, the peaks in the amide I band region occur at 1646 cm−1 and 1723 cm−1 in the absence of salt (Figure 3). These bands do not change at all after 1 M NaSCN is introduced into solution. However, adding increasing concentrations of Na2SO4 does lead to the rise of a very small new peak at 1592 cm−1 and the decrease in the higher frequency peak. Such changes are consistent with a very modest amount of NH hydrogen bonding to the carbonyl.14 This is again consistent with the strong salting-out behavior of Na2SO4.
Figure 3.
The attenuated total refection-Fourier transformed infrared spectra (ATR-FTIR) of 100 mM half-capped GGG in various D2O salt solutions. The spectra were collected with the same procedure and the instrumentation employed in Refs. 8 and 15. Peaks in the spectra can be assigned to amide I bands and combination bands of terminal carboxylate group and the amide carbonyl groups.8, 16
In summary, we have performed MD simulation supported by NMR and ATR-FTIR measurements that indicate strong ion interactions with the charged C-terminus of an aqueous peptide. The present calculations for fully capped triglycine show that strongly hydrated anions interact very weakly with the peptide backbone, while weakly hydrated anions exhibit an appreciable affinity to it. For the half-capped and uncapped peptide, these ion specific effects get, however, convoluted with attractive and repulsive interactions by salt ions with the charged peptide termini. All of these effects are, of course, ion specific as well.
Methods
Three variants of the triglycine molecule were simulated – uncapped, half-capped (with the N-terminus terminated by an acetyl group) and fully-capped (with the N-terminus terminated by an acetyl group and the C-terminus by an N-methylamide group). Each of the three variants of the tripeptide was solvated in 1 M NaX (X = F, Cl, Br, I, SCN, or ClO4) or in 0.333 M Na2SO4 (i.e., employing the same ionic strength for all of the solutions) and described with the polarizable version of the parm99SB force field.17 Compared to our previous studies,8 we changed the capping of the C-terminus, investigated additional salts, and evaluated the salting out coefficients in order to be able to directly compare with the experiments of Nandi and Robinson.6
The simulation was performed with 2503 water molecules (POL3,18 except for NaF and NaClO4 solutions, where we used the SPC/E19 model primarily due to convergence problems with the polarizable water force field), one tripeptide, 45 sodium cations and 45 anions (or 30 cations and 15 anions for sulfate) in a 43 A × 43 A × 43 A box. We applied 3D periodic boundary conditions with an 8 Å cutoff for long-range interactions using the particle mesh Ewald (PME) method.20 All bonds containing hydrogens were constrained using the SHAKE algorithm.21 A Berendsen barostat and thermostat were employed to maintain ambient temperature (300 K) and pressure (1 atm).22 We analyzed the data for each salt solution during a 100 ns production run after a 1 ns equilibration time with time steps of 1 fs, calculated using the AMBER11 program.23 Salting out constants were then evaluated using Eq. 1.
Spectra for NMR titrations with half capped GGG were acquired on a 400 MHz spectrometer equipped with a 5 mm TXI probe (Bruker, Billerica, MA) at a temperature of 298 K using W5 watergate for water suppression.24 Sample spectra were externally referenced to sodium 2,2-dimethyl-2-silapentane-5-sulfonate (DSS) (Cambridge Isotope Laboratories) in pure D2O (99.9% D, Cambridge Isotope Laboratories) in NMR tubes adapted with coaxial inserts (Wilmad-Lab Glass). The DSS was always in the inner of the concentric tubes, while the peptide sample was in the outer tube to ensure the DSS control was never exposed to the peptide or salt. Chemical shift assignments of the peptide were made by employing a 400 MHz spectrometer with [1H,1H]- NOSEY (100 ms mixing time) and [1H,1H]-TOCSY (100 ms mixing time),25 as well as with [13C, 1H]-HSQC and [13C, 1H]- HMBC (J-filter range between 5 and 14 Hz) experiments.26 TopSpin software (Bruker) was used for data processing.
The NaSCN and Na2SO4 employed in the ATR-FTIR and NMR experiments were purchased from Sigma Aldrich (St. Louis,MO). Na2SO4 was >99% pure and NaSCN was at least 98% pure. 18.2 MSΩ·cm purified water from a NANO pure Ultrapure Water system (Dubuque, IA) was used to prepare both salt and peptide solutions. Stock solutions of salts and peptides were made separately at double the desired concentration and mixed together in a 1:1 (volume to volume) ratio to obtain the desired sample concentration. The total peptide concentration in all experiments was 50 mM. The preparation of the half-capped peptide has been described previously.5, 8
Acknowledgment
We thank the Czech Science Foundation (Grant P208/12/G016) for support. P.J. acknowledges the Academy of Sciences for the Praemium Academie award. J. Hl. acknowledges support from the International Max-Planck Research School for Dynamical Processes in Atoms, Molecules and Solids in Dresden. J.He. acknowledges funding from the Alexander-von-Humboldt Stiftung. P.C. thanks the National Institutes of Health (GM-070622) for funding.
References
- 1.Kirkwood JG, Buff FP. The Statistical Mechanical Theory of Solutions 1. J. Chem. Phys. 1951;19:774–777. [Google Scholar]
- 2.Smith PE, Mazo RA. On the theory of solute solubility in mixed solvents. J. Phys. Chem. B. 2008;112:7875–7884. doi: 10.1021/jp712179w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hofmeister F. Zur Lehre von der Wirkung der Salze. Arch. Exp. Pathol. Pharmakol. 1888;24:247–260. [Google Scholar]
- 4.Kunz W, Henle J, Ninham BW. 'Zur Lehre von der Wirkung der Salze' (about the science of the effect of salts): Franz Hofmeister's historical papers. Current Opinion Colloid & Interface Sci. 2004;9:19–37. [Google Scholar]
- 5.Rembert KB, Paterova J, Heyda J, Hilty C, Jungwirth P, Cremer PS. Molecular Mechanisms of Ion-Specific Effects on Proteins. J. Am. Chem. Soc. 2012;134:10039–10046. doi: 10.1021/ja301297g. [DOI] [PubMed] [Google Scholar]
- 6.Nandi PK, Robinson DR. Effects of Salts on Free-Energy of Peptide Group. J. Am. Chem. Soc. 1972;94:1299–1308. doi: 10.1021/ja00759a042. [DOI] [PubMed] [Google Scholar]
- 7.Venkatesu P, Lee MJ, Lin HM. Transfer free energies of peptide backbone unit from water to aqueous electrolyte solutions at 298.15 K. Biochem. Eng. J. 2006;32:157–170. [Google Scholar]
- 8.Paterova J, Rembert KB, Heyda J, Kurra Y, Okur HI, Liu WR, Hilty C, Cremer PS, Jungwirth P. Reversal of the Hofmeister Series: Specific Ion Effects on Peptides. J. Phys. Chem. B. 2013;117:8150–8158. doi: 10.1021/jp405683s. [DOI] [PubMed] [Google Scholar]
- 9.Pegram LM, Record MT. Thermodynamic origin of Hofmeister ion effects. J. Phys. Chem. B. 2008;112:9428–9436. doi: 10.1021/jp800816a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pegram LM, Wendorff T, Erdmann R, Shkel I, Bellissimo D, Felitsky DJ, Record MT. Why Hofmeister effects of many salts favor protein folding but not DNA helix formation. Proc. Nat. Acad. Sci. USA. 2010;107:7716–7721. doi: 10.1073/pnas.0913376107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chi EY, Krishnan S, Randolph TW, Carpenter JF. Physical stability of proteins in aqueous solution: Mechanism and driving forces in nonnative protein aggregation. Pharmaceutical Res. 2003;20:1325–1336. doi: 10.1023/a:1025771421906. [DOI] [PubMed] [Google Scholar]
- 12.Record MT, Guinn E, Pegram L, Capp M. Introductory Lecture: Interpreting and predicting Hofmeister salt ion and solute effects on biopolymer and model processes using the solute partitioning model. Faraday Discuss. 2013;160:9–44. doi: 10.1039/c2fd20128c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho YH, Zhang YJ, Christensen T, Sagle LB, Chilkoti A, Cremer PS. Effects of Hofmeister Anions on the Phase Transition Temperature of Elastin-like Polypeptides. J. Phys. Chem. B. 2008;112:13765–13771. doi: 10.1021/jp8062977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barth A, Zscherp C. What vibrations tell us about proteins. Quarterly Rev. Biophys. 2002;35:369–430. doi: 10.1017/s0033583502003815. [DOI] [PubMed] [Google Scholar]
- 15.Okur HI, Kherb J, Cremer PS. Cations Bind Only Weakly to Amides in Aqueous Solutions. J. Am. Chem. Soc. 2013;135:5062–5067. doi: 10.1021/ja3119256. [DOI] [PubMed] [Google Scholar]
- 16.Kim MK, Martell AE. Infrared Spectra od Aqueous solutions 4. Glycine and Glycine Peptides. J. Am. Chem. Soc. 1963;85:3080–3083. [Google Scholar]
- 17.Wang JM, Wolf RM, Caldwell JW, Kollman PA, Case DA. Development and testing of a general amber force field. J. Comput. Chem. 2004;25:1157–1174. doi: 10.1002/jcc.20035. [DOI] [PubMed] [Google Scholar]
- 18.Caldwell JW, Kollman PA. Structure and Properties of Neat Liquids Using Nonadditive Molecular-Dynamics - Water, Methanol, and N-Methylacetamide. J. Phys. Chem. 1995;99:6208–6219. [Google Scholar]
- 19.Berendsen HJC, Grigera JR, Straatsma TP. The Missing Term in Effective Pair Potentials. J. Phys. Chem. 1987;91:6269–6271. [Google Scholar]
- 20.Essmann U, Perera L, Berkowitz ML, Darden T, Lee H, Pedersen LG. A Smooth Particle Mesh Ewald Method. J. Chem. Phys. 1995;103:8577–8593. [Google Scholar]
- 21.Ryckaert JP, Ciccotti G, Berendsen HJC. Numerical-Integration of Cartesian Equations of Motion of a System with Constraints - Molecular-Dynamics of N-Alkanes. J. Comput. Phys. 1977;23:327–341. [Google Scholar]
- 22.Berendsen HJC, Postma JPM, Vangunsteren WF, Dinola A, Haak JR. Molecular-Dynamics with Coupling to an External Bath. J. Chem. Phys. 1984;81:3684–3690. [Google Scholar]
- 23.Case DAD, Cheatham TE, III, Simmerling CL, Wang J, Duke RE, Luo R, Walker RC, Zhang W, Merz KM, Roberts B, et al. AMBER. Vol. 11. San Francisco: University of California; 2010. [Google Scholar]
- 24.Liu ML, Mao XA, Ye CH, Huang H, Nicholson JK, Lindon JC. Improved WATERGATE pulse sequences for solvent suppression in NMR spectroscopy. J. Magnetic Resonance. 1998;132:125–129. [Google Scholar]
- 25.Wuthrich K. NMR of Proteins and Nucleic Acids. New York: Wiley-Interscience; 1986. [Google Scholar]
- 26.Hadden CE, Martin GE, Krishnamurthy VV. Improved performance accordion heteronuclear multiple-bond correlation spectroscopy - IMPEACH-MBC. J. Magnetic Resonance. 1999;140:274–280. doi: 10.1006/jmre.1999.1840. [DOI] [PubMed] [Google Scholar]