Abstract
RATIONALE
Nicotine enhancement of reward has been implicated as an important contributor to tobacco addiction. Despite the attention that reward enhancement has received, the behavioral mechanisms whereby nicotine enhances operant responding remain largely unknown. The present study sought to extend previous work by evaluating the effects of nicotine on responding for two qualitatively different rewards (visual stimulation (VS) and 4% sucrose solution) under fixed-ratio (FR) maintenance and extinction conditions.
METHOD
Sprague-Dawley rats were trained to press an active lever for VS (Experiment 1) or 4% sucrose solution (Experiment 2) and evaluated over 15 sessions on a FR5 schedule of reinforcement. Nicotine (0.4 mg base/kg, SC) or saline were administered 5 min before each session; the alternate solution was given in the home cage after the session. The effects of nicotine on extinction responding were then assessed over 5 sessions and rats were divided into 4 groups based on drug of injection received during FR-maintenance and extinction phases (Maintenance-Extinction): Nic-Nic, Nic-Sal, Sal-Sal, and Sal-Nic.
RESULTS
Nicotine increased active lever response rates for both VS and 4% sucrose under FR5 maintenance conditions. Nicotine also increased response rates in the Nic-Nic group relative to all other groups under extinction conditions in both experiments, though this effect had greater longevity following VS maintenance conditions than sucrose. Enhancement of responding during extinction does not appear dependent upon locomotor activation by nicotine.
Introduction
Tobacco use is the single greatest contributor to the global burden of disease and is the leading cause of preventable death and disease in the world (World Health Organization [WHO], 2004). The central nervous system effects of nicotine are often indicated as the primary motivator that drives addiction to tobacco products (USDHHS, 1988). The rewarding effects of nicotine are among the central nervous effects of nicotine that contribute to the tenacity of the smoking habit. These effects can be conceptualized in at least three fundamental ways: 1) nicotine possesses naturally rewarding properties that directly reinforce operant behavior (Corrigall and Coen, 1989; Donny et al., 1998); 2) stimuli or events that co-occur with nicotine acquire rewarding effects of their own by virtue of their association with the primary rewarding effects of nicotine (Caggiula et al., 2001; Rose et al., 2000; Wilkinson and Bevins, 2008); and 3) nicotine possesses reward-enhancing effects that make other rewarding stimuli more effective reinforcers (Bevins and Palmatier, 2004; Chaudhri et al., 2006a). Recent research has shown that the lattermost of these may be of particular importance in understanding the role of nicotine in tobacco addiction, as there is a growing body of evidence that suggests that the naturally rewarding properties of nicotine are relatively weak (Donny et al., 2003; Chaudhri et al., 2006a).
The reinforcement-enhancing effects of nicotine were well demonstrated by Donny and colleagues (2003). In that research, rats were trained to lever press for self-administered or yoked infusions of nicotine or saline, with or without an accompanying visual stimulus (VS). Rates of lever pressing were similar between groups that self-administered nicotine or saline without VS presentation. These results corroborate other findings that suggest that the primary rewarding effects of nicotine are relatively weak (Donny et al., 2003; Caggiula et al., 2009). Modest rates of lever pressing were maintained in the group receiving saline and a VS contingent upon lever pressing, suggesting that the VS functioned as a weak sensory reinforcer. Interestingly, rates of lever pressing were significantly higher for the group that received nicotine with response-contingent VS, regardless of whether delivery of nicotine was self-administered or controlled by a yoked partner (Donny et al., 2003). Several studies have since replicated this finding and have demonstrated that nicotine dose-dependently increases rates of operant responding for reinforcing stimuli in a fashion that is consistent with an interpretation of enhancement of reward (Barrett and Bevins, 2012; Barrett and Odum, 2011; Chaudhri et al., 2006b; 2007; Palmatier et al., 2006; 2007; Raiff and Dallery, 2006; 2008; 2009; Caggiula et al., 2009).
Although the reinforcement-enhancing effects of nicotine have received increased attention in recent years, there remains much that we do not understand regarding the nature of this enhancement. The large majority of the published works on reward-enhancement by nicotine employ procedures using rates of operant responding on a fixed-ratio (FR) schedule of reinforcement to measure changes in reinforcement value [for a review see Caggiula et al. (2009)]. Response rate maintained by simple schedules of reinforcement has a long tradition as a measure of response strength and reinforcement value. There are potential problems with relying on response rate under reinforcer maintenance conditions as a solitary measure of reinforcement value. Namely, response rate under reinforcer maintenance likely represents only a single facet of the many that characterize the constructs of response strength and reinforcement value (Killeen and Hall, 2001; Herrnstein, 1961; 1970; Nevin, 1974; 2012; Hursh and Silberberg, 2008). In fact, some manipulations that decrease response rates under maintenance conditions actually enhance responding using alternative measures believed to also index response strength and/or reinforcement value. For example, the delivery of noncontingent reinforcers in addition to response-contingent reinforcement decreases response rates under maintenance conditions. This same manipulation has also been shown to increase resistance to response disruption by session pre-feeding or extinction procedures [Nevin, 1974; 1983; 1990; Shull and Grimes, 2002; for a review see Nevin (2012)]. Similarly, the same manipulation of reinforcement value may have differing effects on response rates depending upon the maintaining schedule of reinforcement at the time of observation. For example, placing an economic constraint on the availability of reinforcers outside the context of the experimental session (i.e., open vs. closed economy) has been shown to have opposing effects on response rates for behavior maintained by different variable interval schedules (Hursh, 1980). Thus, using different measures or reinforcement contingencies can lead to different conclusions regarding the effect of a manipulation on response strength or reinforcement value.
One goal of the present experiments was to investigate whether nicotine administration enhances operant responding for two different kinds of reinforcers (visual stimuli and liquid sucrose) under ratio based schedules of reinforcement, and whether such enhancement will also be observed under conditions in which reinforcement is no longer forthcoming. While nicotine is known to increase response rates under conditions maintained by simple ratio schedules of reinforcement, it does not necessarily follow that a similar enhancement will continue when reinforcement is discontinued. Research by Raiff and Dallery (2008) using an observing response procedure suggests that nicotine enhancement of responding may not occur under extinction conditions. In that study, they found that nicotine enhanced responding maintained by a conditioned reinforcer (i.e., the response required to “observe” the signal for reinforcer availability). However, lever pressing maintained by food pellets and responding on the food reinforced lever under extinction conditions were not enhanced by nicotine (see Discussion).
In addition to increasing rates of operant responding on ratio-based schedules of reinforcement, nicotine has locomotor activating effects that tend to increase with repeated exposure (Bevins and Besheer, 2001; Bevins et al., 2001). Some investigators have suggested that the locomotor stimulant effects of nicotine may be responsible for the reward-enhancement effects of nicotine on operant responding (see Frenk and Dar, 2004). Although there is indirect evidence (e.g., pressing an inactive lever) challenging this locomotor account (e.g., Chaudhri et al., 2006b; Caggiula et al., 2009; Barrett and Bevins, 2012), the lack of a concomitant measure of general chamber activity along with lever pressing leaves open the question of whether nicotine-induced locomotor activity can explain at least some of the nicotine enhancement effect. Another goal of the present study was to more directly test this locomotor account by measuring lever pressing and chamber beam breaks in each experimental phase. If locomotor activation drives nicotine enhancement of operant responding, then activity and lever pressing when given nicotine should covary with one another.
Methods
Subjects
Forty eight male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing approximately 300 g at the start of the study were individually housed in clear polycarbonate tubs lined with wood shavings in a temperature- and humidity-controlled colony. Water was continuously available throughout the experiment and rats were given 20–25 g of laboratory chow daily (unless otherwise specified); previous studies from this laboratory have shown that rats maintain healthy rates of growth while still encouraging exploratory behavior under these feeding conditions. Sessions were conducted during the light phase of a 12:12 h light/dark cycle. Experimental protocols were approved by the University of Nebraska-Lincoln Institutional Animal Care and Use Committee.
Apparatus
Sessions were conducted in eight conditioning chambers (ENV-008CT; Med Associates, Inc., St. Albans, VT; measuring 30.5 × 24.1 × 21.0 cm, l × w × h) enclosed in light- and sound-attenuating cubicles fitted with a fan to mask noise and provide airflow. Sidewalls were aluminum; the ceiling and front and back walls were clear polycarbonate. One sidewall featured a dipper receptacle, occupying a 5.2 × 5.2 × 3.8 cm (l × w × h) recessed space, into which a dipper arm could provide 0.1 ml of sucrose solution when raised. Retractable response levers were featured on either side of the dipper receptacle, approximately 5 cm above the rod floor. White 28V DC (100 mA) lamps were located 3 cm above each lever, hereafter referred to as the right and left lever lights. Two external 28V DC (100 mA) lamps were also located above the conditioning chamber, but within the sound attenuating cubicle, hereafter referred to as the houselight. An infrared emitter/detector unit positioned 4 cm above the rod floor bisected the chamber 14.5 cm from the sidewall featuring the dipper receptacle and functioned to monitor chamber crosses (activity) during experimental sessions. Data collection and presentation of experimental events were controlled via personal computer with Med Associates interface and software (MedPC for Windows, IV) located in the same room as the chambers.
Drugs
(−)Nicotine tartrate salt (Sigma, St. Louis, MO) was dissolved in 0.9% saline and adjusted to a pH of 7.0 ± 0.2 with a dilute NaOH solution. Nicotine was diluted to 0.4 mg base/kg. Nicotine and saline injections were subcutaneous (SC) at 1 ml/kg. All injections were given 5 min before placement in the apparatus.
Procedure
Experiment 1
Male Sprague-Dawley rats (n=16) were first trained to acquire 26% liquid sucrose (w/v) from the dipper delivery system over 4 sessions. These sessions delivered 60 sucrose presentations over the 60-min session, arranged on a variable time (VT) 60-sec schedule with a 4-sec limited hold. Each rat was then trained to lever-press for sucrose via an autoshaping procedure over the next two sessions. These sessions consisted of 60 presentations of a lever for 15 sec, followed by 4-sec access to sucrose. Any response on the lever during the 15-sec presentation resulted in immediate lever retraction and 4-sec access to sucrose. Each lever, left or right, was inserted on half the trials in pseudo-random fashion with the constraint that the same lever would not be inserted more than two times in succession.
From the third day through the remainder of the experiment, no sucrose was available at anytime. Instead lever presses on the active lever (pseudo-randomly assigned within groups) resulted in 1-min termination of otherwise constant houselight illumination, concurrent with 5-sec illumination of both lever lights. This configuration is hereafter referenced as the visual stimulus (VS), and is similar to that used in previous studies (Donny et al., 2003; Chaudhri et al., 2006a). Presses on the inactive lever were recorded, but they had no programmed consequences. Over the following 20 sessions, the response requirement [i.e., fixed-ratio (FR) schedule] for the VS was increased from one response (FR1) to five responses per VS presentation (FR5); FR1 was in effect for 5 sessions, FR2 for the following 10 sessions, and FR5 for the following 5 sessions. Rats were then divided into two conditions by type of injection received daily 5 min before initiation of the next 15 sessions: 0.4 mg/kg nicotine or saline. The opposite injection was administered in the home cage 15 min after the session had finished. This injection controlled for total nicotine exposure and number of injections across the experiment.
Following this phase, responding for the VS was placed on extinction for 15 sessions. That is, lever presses were recorded but they no longer produced the VS. In the extinction phase, rats were divided into four groups designated by the injection solution received 5 min before the session during acquisition and extinction (Acquisition-Extinction): Nic-Nic, Nic-Sal, Sal-Sal, or Sal-Nic. Throughout the extinction phase, rats continued to receive the opposite injection 15 min after the session to control for total nicotine exposure.
Experiment 2
Experiment 2 was designed to replicate Experiment 1 with one major difference: liquid sucrose continued to be the reward for active lever pressing instead of the VS. The rationale for the change in procedure was two part: 1) to test the generality of effects from Experiment 1 by evaluating the effects of nicotine on responding for a qualitatively different reinforcer and 2) to observe the effects of nicotine on responding under extinction conditions following a higher baseline of response rates during maintenance conditions. Rats (n=32) were trained to lever press using the same two-session autoshaping procedure described in Experiment 1. On the third session, the contingency was changed such that presses on the active lever (psuedo-randomly assigned) were followed by 4-sec access to sucrose (26% w/v). Inactive lever presses were recorded but had no programmed consequences. The schedule of sucrose reinforcement was increased over 20 sessions, to an FR5 as described for Experiment 1.
Rats were then divided into two conditions by type of injection received 5 min before daily session initiation: 0.4 mg/kg nicotine or saline. Over 10 sessions, lever pressing was reinforced on a FR5 schedule of sucrose access. Across sessions, response rates did not differ between Nicotine and Saline conditions as they had in Experiment 1 (see Table 1); this presumably reflects a ceiling effect driven by the potency of 26% sucrose as a reinforcer (i.e., there was little room for reinforcer-enhancement and/or increases in rate of response above the saline condition). Therefore, we initiated a series of manipulations to identify a condition under which a difference would emerge with sucrose as the reinforcer. The first manipulation was to increase the response requirement. This shift occurred over 30 sessions: FR10 for 5 sessions, FR25 for 5 sessions, and FR50 for 20 sessions. No differences between groups emerged under any of these reinforcement schedules (see Table 1). Accordingly, the next manipulation was to move rats from 20–25g a day mild food restriction to unlimited access to food. Over 5 sessions, lever pressing was reinforced on a FR25 under these feeding conditions. Again no difference between the Nicotine and Saline conditions was seen (Table 1). The final manipulation was to decrease sucrose concentration. Over the next 15 sessions, rats lever pressed for 4% liquid sucrose (w/v) on a FR5 schedule of reinforcement. Here, a difference emerged and we shifted to the extinction phase of the experiment.
Table 1.
Mean number of sucrose presentations earned (± SEM) over last 5 sessions.
| FR5 | FR10 | FR25 | FR50 | Ad libitum | 4% Sucrose | |
|---|---|---|---|---|---|---|
|
| ||||||
| Nicotine | 119.8 ± 4.63 | 104.5 ± 7.27 | 48.1 ± 2.78 | 42.3 ± 6.70 | 21.0 ± 2.51 | 91.8 ± 3.04 |
| Saline | 121.5 ± 5.00 | 126.5 ± 3.95 | 60.4 ± 2.85 | 36.2 ± 24.7 | 29.4 ± 2.35 | 35.9 ± 2.84 |
Before the start of extinction, rats were divided into four groups by the type of injection they received before the session, as in Experiment 1. Extinction was in force over the next 5 sessions. Presses on either lever were recorded but had no programmed consequence. In all phases of Experiment 2 following initial autoshaping, rats received nicotine or saline 5 min before the session, and the opposite injection 15 min after the session to control for total nicotine exposure and number of injections.
Dependent Measures and Data Analysis
Number of active and inactive lever presses, as well as number of chamber beam breaks (locomotor activity) were measured throughout both experiments and served as primary dependent measures. During the 15 session maintenance phase, the effects of drug administration on lever pressing were analyzed via three-factor (Drug x Lever x Session) mixed-measures analysis of variance (ANOVA). Similarly, locomotor activity from this phase was analyzed via two-factor (Drug x Session) mixed-measures ANOVA. Lever pressing and locomotor activity during the extinction phase of both experiments were analyzed via three-factor (Group x Lever x Session) and two-factor (Group x Session) ANOVA, respectively, as in the preceding phase. Significance for all analyses was set at p<0.05, and pair-wise comparisons were conducted using Fischer’s LSD post-hoc tests using the minimum mean difference (LSDmmd).
Results
Experiment 1
Each rat learned to retrieve liquid sucrose from the dipper receptacle over the four sessions of training. The mean number of sucrose deliveries accessed on the fourth session was 53.9 (1.69 SEM) out of 60. Lever pressing was acquired over the two autoshaping sessions. The mean number sucrose presentations received by active lever responding on the second day of autoshaping was 48.3 (1.68 SEM).
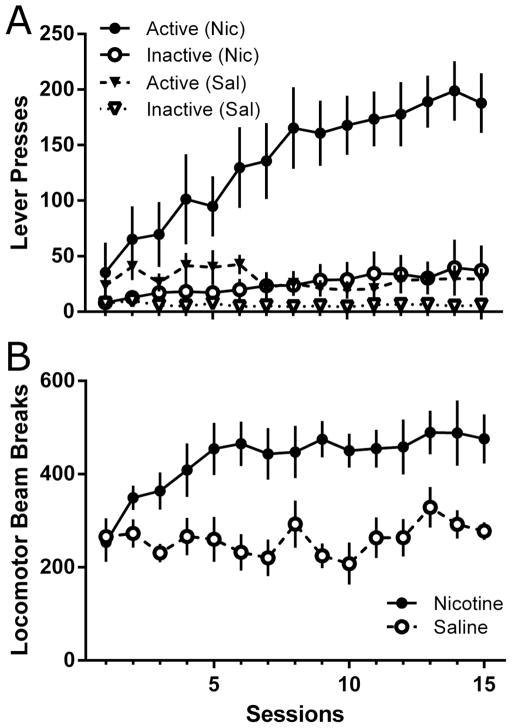
Nicotine administration increased lever pressing maintained by the VS across the 15 sessions preceding extinction (Figure 1A). A three-factor ANOVA revealed significant main effects of Lever [F(1,14)=44.2, p<0.001], of Drug [F(1,14)=10.2, p=0.006], and of Session [F(14,196)=14.7, p<0.001]. The Lever x Session x Drug [F(14,196)=6.53, p<0.001], Lever x Session [F(14, 196)=5.58, p<0.001], Session x Drug [F(14,196)=18.5, p<0.001], and Lever x Drug [F(1,14)=18.9, p=0.001] interactions were also significant. Post-hoc analyses revealed that nicotine significantly increased lever pressing maintained by the VS on all but the first two sessions. Albeit not as profound, nicotine also increased inactive lever pressing, beginning on the ninth session. Finally, active lever pressing was significantly higher than inactive lever pressing across all 15 sessions in the Nicotine condition, but only on sessions 2, and 4–6 in the Saline condition (LSDmmd=24.9).
Figure 1.
Total responses on each lever and locomotor activity across the 15-session VS-reinforcer maintenance phase of Experiment 1. Lever pressing data (Panel A) from the nicotine and saline conditions are represented as circles or triangles, respectively. Filled shaped represent active lever pressing, whereas open shapes represent inactive lever pressing. Locomotor activity data (Panel B) is represented as filled circles for the nicotine condition and as open circles for the saline condition. All data presented as mean ± SEM.
Nicotine increased locomotor activity across sessions 3–15 preceding extinction (Figure 1B). There were significant main effects of Drug [F(1,14)=13.9, p=0.002], and of Session [F(14,196)=2.90, p=0.001], as well as a significant Drug x Session interaction [F(14,196)=2.43, p=0.004]. Post-hoc analyses revealed that nicotine increased locomotor activity above saline beginning on the third session; this appeared to stabilize around the fifth session (LSDmmd=87.1).
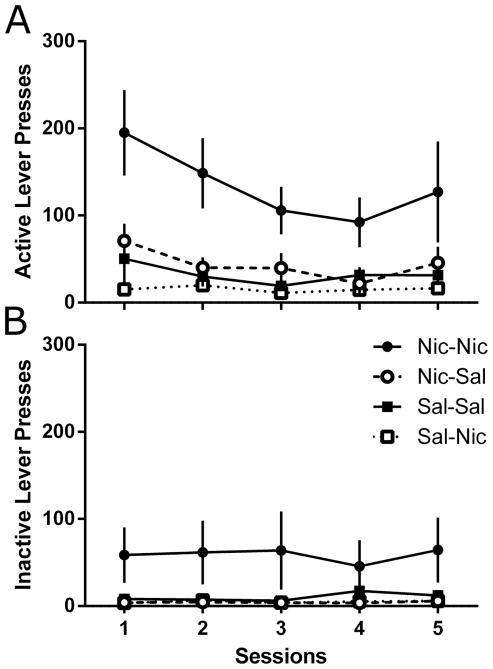
Analysis of lever pressing during the extinction phase (Figure 2A) revealed significant main effects of Group [F(3,12)=5.59, p=0.012], of Lever [F(1,12)=13.0, p=0.004], and of Session [F(4,48)=5.38, p=0.001]. A significant Lever x Session interaction was also found [F(4,48)=6.46, p<0.001]. Although no other interactions met the criterion for statistical significance [Fs<1.91, ps>0.05], the Group x Session interaction approached the cutoff [F(12,48)=1.94, p=0.052]. Post-hoc tests revealed significant differences between active and inactive lever responding (Figure 2B) on all extinction sessions in the Nic-Nic group, on all sessions but 4 for the Nic-Sal group, and on only session 1 for the Sal-Sal group; there were no differences for the Sal-Nic group (LSDmmd=26.9). Active lever pressing in the Nic-Nic group was higher than any other group across all five extinction sessions. Active lever pressing was significantly higher in the Sal-Sal group than the Sal-Nic group on the first extinction session only, and did not differ from the Nic-Sal group on any of the sessions. Responding on the active lever in the Nic-Sal group was higher than the Sal-Nic group on all but the fourth extinction session. Inactive lever pressing was found to be higher in the Nic-Nic group compared to all other groups across all five extinction sessions (LSDmmd=26.9).
Figure 2.
Active lever pressing (Panel A) and inactive lever pressing (Panel B) across the 5-session extinction phase between the Nic-Nic (filled circles), Nic-Sal (open circles), Sal-Sal (filled squares), and Sal-Nic (open squares) groups from Experiment 1. All data presented as mean ± SEM.
For locomotor activity during extinction sessions (Figure 3), there were no main effects of Group [F(3,48)=3.41, p=0.053] or of Session [F(4,48)=1.75, p=0.156]; the Group x Session interaction was not significant [F<1]. The main effect of Group approached conventional levels of significance (p=0.053), with a tendency for greater locomotor activity in the Nic-Nic group.
Figure 3.
Locomotor activity counts across the 5-session extinction phase between the Nic-Nic (filled circles), Nic-Sal (open circles), Sal-Sal (filled squares), and Sal-Nic (open squares) groups from Experiment 1. All data presented as mean ± SEM.
Experiment 2
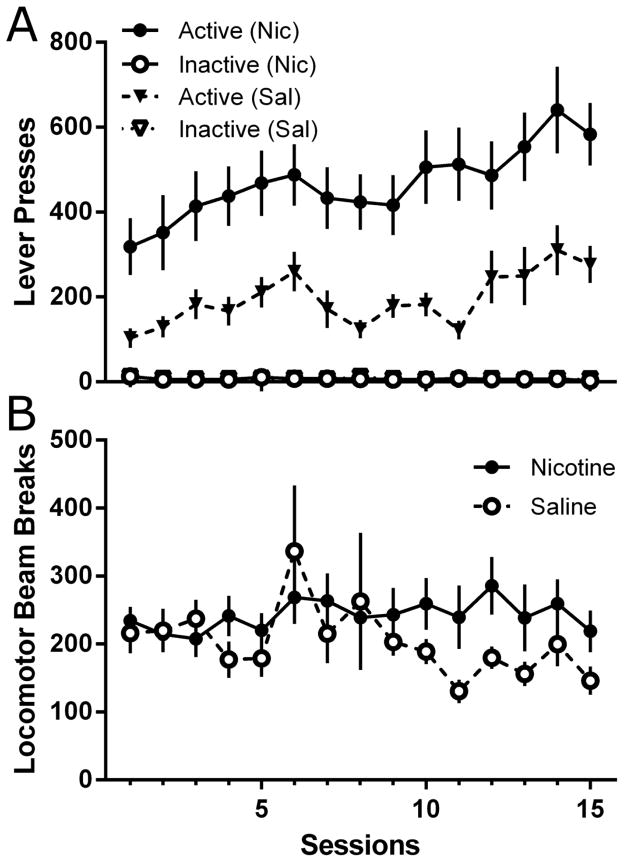
One rat from the Nicotine and one from the Saline conditions were removed from the study due to weight loss related to the development of tumors. Nicotine enhanced lever pressing for 4% sucrose on an FR5 schedule across the 15 session (Figure 4A). Analysis revealed a significant main effect of Lever [F(1,28)=93.6, p<0.001], of Drug [F(1,28)=16.4, p<0.001], and of Session [F(14,392)=5.20, p<0.001]. There were also significant Lever x Drug [F(1,28)=16.7, p<0.001] and Lever x Session interactions [F(14,392)=5.63, p<0.001]. Post-hoc tests revealed significantly higher rates of responding on the active lever than the inactive lever in both conditions (LSDmmd=83.2). The difference between response rates on active and inactive levers increased across sessions in both conditions, but the difference and rate of change were both greater in the Nicotine condition (LSDmmd=83.2).
Figure 4.
Total responses on each lever and locomotor activity across the 15-session sucrose-reinforcer maintenance phase of Experiment 2. Lever pressing data (Panel A) from the nicotine and saline conditions are represented as circles or triangles, respectively. Filled shaped represent active lever pressing, whereas open shapes represent inactive lever pressing. Locomotor activity data (Panel B) is represented as filled circles for the nicotine condition and as open circles for the saline condition. All data presented as mean ± SEM.
No systematic effects of nicotine administration were observed on locomotor activity during the 4% sucrose reinforcement phase (Figure 4B). Analysis revealed no significant main effects of Drug [F(1,28)=1.50, p=0.231] or of Session [F(14,392)=1.47, p=0.120], and no significant Drug x Session interaction [F(14,392)=1.13, p=0.331].
Analysis of lever pressing during the extinction phase (Figure 5A, active presses) revealed significant main effects of Lever [F(1,26)=175, p<0.001] and of Session [F(4,104)=93.5, p<0.001], but not of Group [F(3,26)=1.91, p=0.152]. There was a significant Lever x Group [F(3,104)=3.05, p=0.046] and Lever x Session [F(4,104)=82.1, p<0.001] interaction; no other interactions were significant [Fs<1]. Post-hoc tests revealed that in each group, significantly more presses were made on the active lever than on the inactive lever (Figure 5B) in the early extinction sessions. This difference disappeared by the end of extinction. Differences were observed between groups in terms of how many extinction sessions were required before discrimination between levers was no longer evident: 5 sessions for the Nic-Nic group, 3 sessions for the Sal-Nic and Sal-Sal groups, and 2 sessions for the Nic-Sal group. Post-hoc tests also revealed that active lever pressing was significantly higher in the Nic-Nic group compared to the Nic-Sal group on extinction sessions 1 through 4, higher than the Sal-Nic group on extinction sessions 1, 2 and 4, and higher than the Sal-Sal group on sessions 1 and 4. No significant differences in inactive lever pressing were observed between groups or across sessions (LSDmmd=17.9).
Figure 5.
Active lever pressing (Panel A) and inactive lever pressing (Panel B) across the 5-session extinction phase between the Nic-Nic (filled circles), Nic-Sal (open circles), Sal-Sal (filled squares), and Sal-Nic (open squares) groups from Experiment 2. All data presented as mean ± SEM.
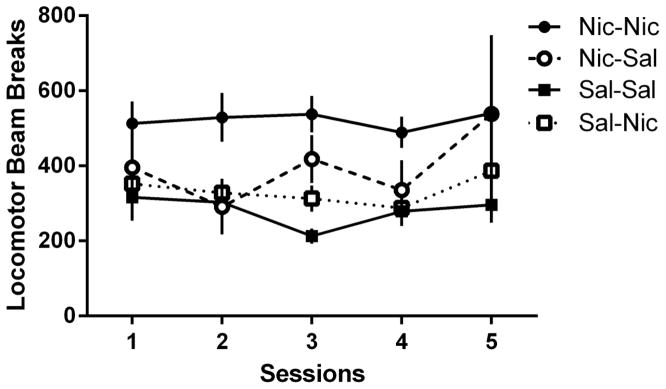
Analysis of the locomotor activity from the extinction phase (Figure 6) revealed a main effect of Group [F(3,104)=4.36, p=0.013] and of Session [F(4,104)=3.07, p=0.020], but no significant Group x Session interaction [F(12,104)=1.73, p=0.072]. Further analyses on the main effect of Group revealed that activity was significantly lower in the Sal-Sal group than all other groups. Activity was also higher in the Nic-Sal group compared to the Sal-Nic group (LSDmmd=47.1). Further analysis on the main effect of Session revealed that activity was higher in the first extinction session than all other sessions, and lower in session 3 compared to session 5 (LSDmmd=44.0).
Figure 6.
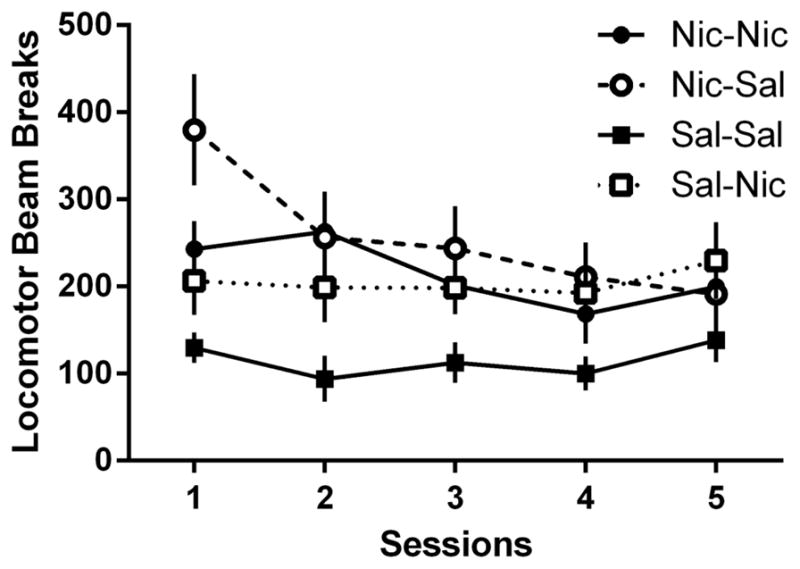
Locomotor activity counts across the 5-session extinction phase between the Nic-Nic (filled circles), Nic-Sal (open circles), Sal-Sal (filled squares), and Sal-Nic (open squares) groups from Experiment 2. All data presented as mean ± SEM.
Discussion
The present experiments demonstrate an effect of nicotine to enhance operant responding maintained by two qualitatively different reinforcers (VS and 4% sucrose) under FR5 schedules of reinforcement. In combination with similar findings from other laboratories, the present results indicate that nicotine enhances operant responding for weak to moderately reinforcing stimuli across a variety of routes of administration, reinforcement conditions, and contextual arrangements (Donny et al., 2003; Raiff and Dallery, 2008; Palmatier et al., 2012; Barrett and Bevins, 2012). The present experiments extend previous research by evaluating the effects of nicotine on responding under extinction conditions following maintenance on ratio based schedules of reinforcement. Nicotine enhanced response rates during extinction when rats had received nicotine under both maintenance and extinction conditions (Nic-Nic groups). This effect persisted across all extinction sessions when responding had been previously maintained by sensory reinforcement (Experiment 1). In contrast, enhanced levels of responding were only observed during the first extinction session when 4% sucrose had been the reinforcer (Experiment 2). The discrepancy between the persistence of responding between these two experiments may be informative of differences regarding the conditions under which reward-enhancement is expressed. However, careful parametric experimentation will be required to determine the confines of the conditions under which a reward-enhancement effect is observable. For instance, the finding that enhanced operant responding was not observable with higher concentrations of sucrose even on FR50 suggests that the baseline value of the maintaining reinforcer is a relevant variable for expression of the reward enhancement effects. Presumably, there exist high and low thresholds of baseline reinforcement value beyond which nicotine enhancement of reward may not be evident. Consistent with this suggestion, Palmatier et al. (2007) found that baseline reinforcement value of different sensory stimuli affected the extent to which nicotine could enhance responding maintained by these stimuli.
The present research also adds further evidence to a growing body of literature indicating that the enhancement effect is not solely driven by the locomotor-activating effects of nicotine. For example, during the 15 day maintenance phase of Experiment 2 there were no significant differences in activity between saline and nicotine-treated rats. Yet, lever-press responding maintained by 4% sucrose was enhanced nearly 3 fold in rats that were treated with nicotine before each session. Additionally, nicotine enhanced active lever responding but did not reliably increase locomotor activity or inactive lever pressing under extinction conditions. We also observed that nicotine enhanced responding under extinction conditions only in the Nic-Nic groups in both experiments. No enhancement of responding in extinction was ever observed in the Sal-Nic groups. This point is especially notable given that nicotine exposure was identical to the Nic-Nic group. Taken together, these findings suggest that the effects of nicotine on rates of operant responding cannot be adequately explained by a locomotor activation account.
Of course, we would not claim that locomotor activation by nicotine does not play any role in its reinforcement-enhancing effects. However, the extent to which locomotor activation is important to the expression of reward-enhancement is tenuous. As the examples in the previous paragraph highlight, an increase in chamber activity does not appear necessary for observing a reinforcement-enhancing effect. Even for examples where activity parallels enhanced lever pressing (compare Figure 1A and 1B), this similarity is a correlational observation and not a causal mechanism. To say that locomotor activation by nicotine plays a role in the expression of enhanced operant responding is merely to say that behaviorally-activated organisms produce more behavior and this behavior may translate into greater responding on the lever associated with reinforcement. Of course, a behaviorally active organism could also produce more behavior that does not translate into enhanced operant responding, but this does not seem to be the case in most experiments investigating the reinforcement-enhancing effects of nicotine. Several studies have now demonstrated that activation and reward-enhancement by nicotine may be correlated, the latter effect is not purely dependent on the former (Barrett and Bevins, 2012; Palmatier et al., 2012; Liu et al, 2006; Palmatier et al., 2006; Raiff and Dallery, 2008; Gancarz et al., 2012; Paterson et al., 2007). Therefore, to the extent that locomotor activation that translates into enhanced responding for rewards can be interpreted as an enhancement of reward, we argue for the abandonment (or in the very least, reevaluation) of arguments that dismiss the reinforcement-enhancing effects of nicotine as an artifact of its locomotor-activating effects (Frenk and Dar, 2004).
The present experiments fill a gap in the present reward-enhancement literature by extending the investigation of such effects to conditions of extinction and by evaluating the enhancement effect across two qualitatively different reinforcers. As noted earlier, Raiff and Dallery (2008) also investigated the effects of nicotine on responding maintained by food reinforcement and under conditions of extinction. However, there are notable differences between these two studies that contribute importantly to the literature. Briefly, Raiff and Dallery (2008) employed an observing response procedure which arranged alternating components of variable-interval food reinforcement and extinction on the same lever within the same session. On a second lever, responding on an independent variable-interval schedule produced visual cues which corresponded with the component in effect (reinforced or non-reinforced) on the other lever. One advantage of this procedure is that a measure of responding maintained by food, by conditioned reinforcement (the observing lever), and in extinction are obtained within the same session (Branch, 1973; Wyckoff, 1952). Raiff and Dallery (2008) found that nicotine enhanced responding on the observing lever, but not on the reinforced or extinction component of the other lever. As detailed by Raiff and Dallery (2008), their findings extend the nicotine enhancement effect to behavior maintained by conditioned reinforcement under an interval-based schedule of food reinforcement. In light of the findings from the present study, the question emerges as to what behavioral factors permit observation of nicotine enhancement of food-maintained responding and responding in extinction here, but not with the observing response protocol.
The results from the present experiments suggest a few variables that will be of interest in future studies to help explain these differences. Recall that the enhancement effect of nicotine was not evident in Experiment 2 when a higher concentration of sucrose (26%) was used to maintain lever pressing; even on an FR50. Perhaps the baseline value of the sucrose pellets used by Raiff and Dallery (2008) to maintain responding in the reinforced component was above a threshold for observing an enhancement effect by nicotine. Another possible behavioral factor of future interest will be the schedule of reinforcement. Differences between the pattern of behavior on fixed-ratio versus variable-interval schedules, as well as the potential differences in behavioral mechanisms responsible for these patterns, may account for the discrepancy between the two studies. Additionally, the availability of conditioned reinforcement on a concurrently available alternative (that was enhanced by nicotine) may interact in a yet to be identified manner that accounts for lack of enhancement of food-maintained or extinction responding in the observing procedure. In other words, the within-session alternating periods of extinction and variable-interval reinforcement in the observing procedure may affect expression of an enhancement effect by nicotine. Regardless, this discussion, and the present research, highlights the need for careful and rigorous parametric research identifying the conditions under which the nicotine enhancement effect is expressed.
The reinforcement-enhancing properties of nicotine hold some promise in explaining the tenacity of tobacco addiction. Certainly, reward potentiation by nicotine sheds some light on the paradox between the weak capacity of nicotine to function as a primary reinforcer and the virulent and ubiquitous nature of tobacco dependence (Bevins & Palmatier, 2004; Chaudhri et al., 2006a; Caggiula et al., 2009). The present study extends our previous understanding on the nature of reinforcement-enhancing effects by evaluating the effects of nicotine on operant responding both under maintenance and extinction conditions. The present findings demonstrate that the reinforcement-enhancing effects of nicotine are more complex than a simple increase in general reinforcement value, and that how nicotine affects the value of rewards depends, in part, on the reinforcement contingencies. Between both experiments, we demonstrated that nicotine will enhance responding for two qualitatively different rewards under maintenance conditions. When reinforcement was no longer forthcoming, nicotine only enhanced responding in rats that had a history of nicotine in the presence of reinforcement. Furthermore, this enhancement of responding in the Nic-Nic group persisted across extinction sessions when responding had been previously maintained by a weakly reinforcing VS, but was short-lived when 4% sucrose was the reinforcer. Determining the parameters under which an enhancement effect by nicotine is apparent should be the directive of future studies whose goal is the unraveling of the mechanisms by which the effect is maintained.
Highlights.
Nicotine enhanced operant responding for two qualitatively different rewards under both maintenance and extinction conditions.
Termination of nicotine administration resulted in an immediate decrease in rates of operant responding, supporting a non-associative mechanism for the enhancement effect.
A history of receiving nicotine in the context of reinforcement seems to be important for the expression of enhanced operant responding under extinction conditions.
Locomotor activation by nicotine does is not a sufficient explanation alone for the reward-enhancement effects of nicotine.
Acknowledgments
The research in this report was, in part, funded by USPHS grants DA018114 and DA034389. We wish to thank Stephanie Savala, Matthew Tracy and Sergios Charntikov for helping conduct experimental sessions. The Med-PC programs, or a slightly updated version, used in this research are available upon request.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barrett ST, Bevins RA. A quantitative analysis of the reward-enhancing effects of nicotine using reinforcer demand. Behavioural Pharmacology. 2012;23:781–789. doi: 10.1097/FBP.0b013e32835a38d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett ST, Odum AL. The effects of repeated exposure on the reward-enhancing effects of nicotine. Behavioural Pharmacology. 2011;22:283–290. doi: 10.1097/FBP.0b013e3283473c25. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J. Individual differences in rat locomotor activity are dminished by nicotine through stimulation of central nicotine acetylcholine receptors. Physiology and Behavior. 2001;72:237–244. doi: 10.1016/s0031-9384(00)00413-3. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J, Pickett KS. Nicotine-conditioned locomotor activity in rats: Dopaminergic and GABAergic influences on conditioned expression. Pharmacology, Biochemistry and Behavior. 2001;68:135–145. doi: 10.1016/s0091-3057(00)00451-2. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Palmatier MI. Extending the role of associative learning processees in nicotine addiction. Behavioral and Cognitive Neuroscience Reviews. 2004;3:143–158. doi: 10.1177/1534582304272005. [DOI] [PubMed] [Google Scholar]
- Branch MN. Observing responses in pigeons: Effects of schedule component duration and schedule value. Journal of the Experimental Analysis of Behavior. 1973;20:417–428. doi: 10.1901/jeab.1973.20-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF. The role of nicotine in smoking: A dual reinforcement model. In: Bevins RA, Caggiula AR, editors. The Motivational Impact of Nicotine and its Role in Tobacco Use. New York: Springer Science and Business Media; 2009. pp. 91–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Cue dependency of nicotine self-administration and smoking. Pharmacology, Biochemistry, and Behavior. 2001;70:515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, et al. Operant responding for conditioned and unconditioned reinforcers in rats is differentially enhanced by primary reinforcing and reinforcement-enhancing effects of nicotine. Psychopharmacology. 2006b;189:27–36. doi: 10.1007/s00213-006-0522-0. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, et al. Self-adminstered nicotine and noncontingent nicotine enhance reinforced operant responsing in rats: Impact of nicotine dose and reinforcement schedule. Psychopharmacology. 2007;190:353–362. doi: 10.1007/s00213-006-0454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology. 2006a;184:353–366. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology. 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Jacobs KS, Rose C, Sved AF. Acquisition of nicotine self-administration in rats: the effects of dose, feeding schedule, and drug contingency. Psychopharmacology. 1998;136:83–90. doi: 10.1007/s002130050542. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib M, Clements LA, AF Operant responding for a visual stimulus in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology. 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Frenk H, Dar R. Reward potentiation or behavioral activation? A comment on Donny et al. Psychopharmacology. 2004;171:472–473. doi: 10.1007/s00213-003-1622-8. [DOI] [PubMed] [Google Scholar]
- Gancarz AM, Ashrafioun L, San George MA, Hausknecht KA, Hawk LW, Jr, Richards JB. Exploratory studies in sensory reinforcement in male rats: Effects of methamphetamine. Experimental and Clinical Psychopharmacology. 2012;20:16–27. doi: 10.1037/a0025701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrnstein RJ. Relative and absolute strength of response as a function of frequency of reinforcement. Journal of the Experimental Analysis of Behavior. 1961;4:264–272. doi: 10.1901/jeab.1961.4-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrnstein RJ. On the law of effect. Journal of the Experimental Analysis of Behavior. 1970;13:243–266. doi: 10.1901/jeab.1970.13-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR. Economic concepts for the analysis of behavior. Journal of the Experimental Analysis of Behavior. 1980;34:219–238. doi: 10.1901/jeab.1980.34-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A. Economic demand and essential value. Psychological Review. 2008;115:186–198. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Killeen PR, Hall SS. The principal components of response strength. Journal of the Experimental Analysis of Behavior. 2001;75:111–134. doi: 10.1901/jeab.2001.75-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Caggiula AR, Yee SK, Nobuta H, Poland RE, Pechnick RN. Reinstatement of nicotine-seeking behavior by drug-associated stimuli after extinction in rats. Psychopharmacology. 2006;184:417–425. doi: 10.1007/s00213-005-0134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin JA. Response strength in multiple schedules. Journal of the Experimental Analysis of Behavior. 1974;21:389–408. doi: 10.1901/jeab.1974.21-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin JA. Resistance to extinction and behavioral momentum. Behavioral Processes. 2012;90:89–97. doi: 10.1016/j.beproc.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin JA, Mandell C, Atak JR. The analysis of behavioral momentum. Journal of the Experimental Analysis of Behavior. 1983;39:49–59. doi: 10.1901/jeab.1983.39-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin JA, Tota ME, Torquato RD, Shull RL. Alternative reinforcement increases resistance to change: Pavlovian or operant contingencies? Journal of the Experimental Analysis of Behavior. 1990;53:359–379. doi: 10.1901/jeab.1990.53-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, et al. Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology. 2006;184:391–400. doi: 10.1007/s00213-005-0183-4. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Matteson GL, Black JJ, Liu X, Caggiula AR, Craven L, et al. The reinforcement enhancing-effects of nicotine depend on the incentive value of non-drug reinforcers and increase with repeated injections. Psychopharmacology. 2007;89:52–59. doi: 10.1016/j.drugalcdep.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, O’Brien LC, Hall MJ. The role of conditioning history and reinforcer strength in the reinforcement enhancing effects of nicotine in rats. Psychopharmacology. 2012;219:1119–1131. doi: 10.1007/s00213-011-2439-5. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Balfour DJK, Markou A. Chronic bupropion differentially alters the reinforcing, reward-enhancing and conditioned motivational properties of nicotine in rats. Nicotine and Tobacco Research. 2008;10:995–1008. doi: 10.1080/14622200802097571. [DOI] [PubMed] [Google Scholar]
- Raiff BR, Dallery J. Effects of acute and chronic nicotine on responses maintained by primary and conditioned reinforcers in rats. Experimental and Clinical Psychopharmacology. 2006;14:296–305. doi: 10.1037/1064-1297.14.3.296. [DOI] [PubMed] [Google Scholar]
- Raiff BR, Dallery J. The generality of nicotine as a reinforcer enhancer in rats: Effects on responding maintained by primary and conditioned reinforcers and resistance to extinction. Psychopharmacology. 2008;201:305–314. doi: 10.1007/s00213-008-1282-9. [DOI] [PubMed] [Google Scholar]
- Raiff BR, Dallery J. Responding maintained by primary reinforcing visual stimuli is increased by nicotine administration in rats. Behavioural Processes. 2009;82:95–99. doi: 10.1016/j.beproc.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Johnson M. Dissociating nicotine and nonnicotine components of cigarette smoking. Pharmacology, Biochemistry, and Behavior. 2000;67:71–81. doi: 10.1016/s0091-3057(00)00301-4. [DOI] [PubMed] [Google Scholar]
- Shull RL, Gaynor ST, Grimes JA. Response rate viewed as engagement bouts: resistance to extinction. Journal of the Experimental Analysis of Behavior. 2002;77:211–231. doi: 10.1901/jeab.2002.77-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Department of Health and Human Services. Nicotine Addiction: A report of the surgeon general. Rockville, MD: US Department of Health and Human Services, Office of the Assistant Secretary for Health, Office on Smoking and Health; 1988. [Google Scholar]
- Wilkinson JL, Bevins RA. Intravenous nicotine conditions a place preference in rats using an unbiased design. Pharmacology, Biochemistry and Behavior. 2008;88:256–264. doi: 10.1016/j.pbb.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Neuroscience of Psychoactive Substance Use and Dependence. Geneva, Switzerland: World Health Organization; 2004. [Google Scholar]
- Wyckoff LB., Jr The role of observing responses in discrimination learning. Psychological Review. 1952;59:431–442. doi: 10.1037/h0053932. [DOI] [PubMed] [Google Scholar]