Abstract
Objective
We investigated how the number of follow-up visits affects response rates and drop-out among patients in antidepressant trials for Major Depressive Disorder (MDD).
Data Sources
Medline, PsycINFO, and PubMed were searched to identify trials contrasting antidepressants to placebo or active comparator in adults with depression. The index terms “depression—drug therapy,” “depressive disorder—drug therapy,” and “antidepressant agents,” in addition to the class and individual generic name of all antidepressants were combined using the ‘or’ operator. Results were limited to 1) English language articles, 2) publication year 1985 or later, 3) age group ≥ 18, and 4) publication types including clinical trials, controlled clinical trials, meta-analysis, multi-center study, randomized controlled trial, or review.
Study Selection
Included articles reported trials of approved antidepressant medications for MDD in outpatients aged 18–65, were 6–12 weeks in duration, and had response rates specified using a standardized measure. Trials were excluded for enrolling inpatients, pregnant women, psychotic subjects, or those with treatment-resistant depression. These criteria allowed 9,189 articles identified in the literature review to be narrowed to 111 reports.
Data extraction
Demographic characteristics, the number of study visits planned in each treatment cell, duration of active treatment, attrition rates, and response rates to medication and placebo were entered into a database.
Results
In a multilevel meta-analysis, active medication vs. placebo (OR 1.96, p < 0.001), active comparator vs. placebo-controlled study design (OR 1.82, p < 0.001), and longer vs. shorter duration (OR 1.87, p < 0.001) were associated with significantly increased odds of treatment response. After controlling for these variables, the number of study visits did not significantly influence response rates (OR 0.97, p = 0.877). The odds of drop-out were significantly decreased for active comparator vs. placebo-controlled trials (OR 0.67, p = 0.002) and longer vs. shorter duration trials (OR 0.54, p = 0.035), while increasing numbers of study visits significantly increased the odds of participant drop-out (OR 2.77, p < 0.001).
Conclusion
Visit schedules that are much more frequent than are commonly practiced in the community treatment of depression may increase the expense of clinical trials and make them less generalizable to standard clinical treatment.
INTRODUCTION
The aim of an antidepressant clinical trial is to test the specific efficacy of a medication to treat Major Depressive Disorder (MDD), but many non-pharmacologic components of antidepressant treatment also influence treatment response.1 For example, participants in clinical trials receive lengthy screening evaluations and subsequently are followed via visits to a research clinic, where they meet extensively with physicians, nurses, social workers and research assistants. These treatment relationships are thought to be instrumental in helping patients comply with research procedures and may also have significant therapeutic effects.2
The high frequency of follow-up visits specified in most antidepressant clinical trials contrasts with antidepressant treatment practices in the community, where 73.6% of patients are treated exclusively by their general medical provider as opposed to a psychiatrist.3 Less than 20% of patients have a mental health care visit in the first 4 weeks after starting an antidepressant,4 and fewer than 5% of adults beginning treatment with antidepressant medications have as many as 7 physician visits in their first 12 weeks on the medication.5 Thus, the administration of antidepressants in clinical trials, which form the evidence base for antidepressant treatments, bears little resemblance to clinical management of depression in the community.
In the single available study investigating the influence of clinic visits on antidepressant and placebo response, Posternak and Zimmerman (2007) calculated the change in depression severity scores over the first 6 weeks of treatment in 41 RCTs of antidepressants for MDD.6 Studies having 6 weekly assessments (weeks 1–6) were compared to those having 5 (weeks 1–4 and 6) and 4 (weeks 1–2, 4, and 6) assessments. A cumulative therapeutic effect of additional follow-up visits on placebo response was found: between weeks 2 and 6, patients with weekly visits improved 4.24 HRSD points, while those with 1 fewer visit improved 3.33 points and those with 2 fewer visits improved 2.49 points. Participants receiving active medication also experienced more symptom change with increased numbers of follow-up visits, but the relative effect of this increased therapeutic contact was approximately 50% less than that observed in the placebo group. This study was limited by not testing the statistical significance of the differences found and by the restricted data set analyzed (only 41 studies), but the results suggest that visit frequency in an antidepressant trial may influence treatment response.
To better understand the effects of visit frequency, we conducted this multilevel meta-analysis to determine whether visit frequency significantly affects therapeutic response and drop-out rates in antidepressant clinical trials. We improve upon previous investigations of visit frequency by collecting a much larger study sample, utilizing statistical methods that permit significance testing of the results obtained, and by analyzing drop-out rates in addition to treatment response. We hypothesized that after controlling for the effects of treatment assignment (medication vs. placebo), study type (placebo-controlled vs. active comparator), and study duration, an increasing number of study visits would significantly increase the odds of treatment response and decrease the odds of drop-out for a given study patient.
METHOD
Search strategy and selection criteria
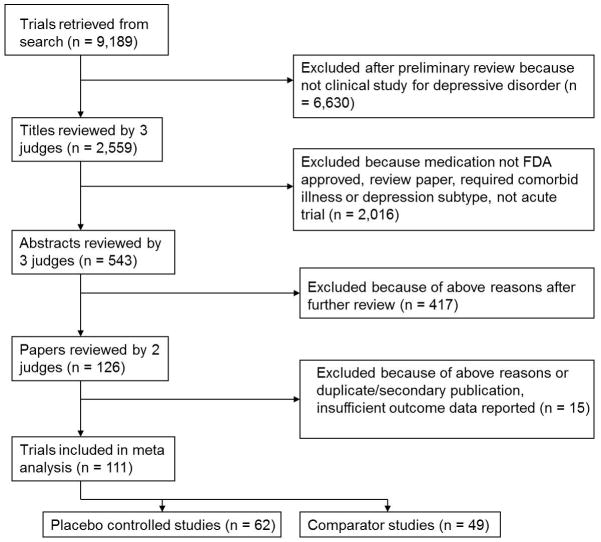
A search of Medline, PsycINFO, and PubMed was conducted to identify RCTs contrasting antidepressants to placebo or active comparator in adults with depression. The index terms “depression—drug therapy,” “depressive disorder—drug therapy,” and “antidepressant agents,” in addition to the class and individual generic name of all antidepressants were combined using the ‘or’ operator. Limiting these results to 1) English language articles, 2) publication year 1985 or later, 3) age group ≥ 18 (to be inclusive), and 4) publication types including clinical trials, controlled clinical trials, meta-analysis, multi-center study, randomized controlled trial, or review, which yielded 9,189 journal articles. The year 1985 was chosen to select trials utilizing more rigorous methods. Two authors (BRR and TMC) conducted a review of these titles to rule out those which were not clinical trials of antidepressants for depression, resulting in 2,559 titles.
Three judges (BRR, TMC, and SPR) reviewed the 2,559 titles, sequentially proceeding from article title to abstract and finally paper text, to determine whether they met inclusion or exclusion criteria (see Figure 1). These evaluations were pooled, and any differences between judges were resolved by discussion. To further ensure all relevant papers were reviewed, the references of all meta-analyses and review articles published since 2000 among the 9,189 journal articles were searched for pertinent references. In addition, the Cochrane Database of Systematic Reviews was electronically searched using the topic ‘antidepressant.’ This yielded 136 protocols and completed reviews, each of whose references was reviewed to ensure they were among the reviewed trials.
Figure 1.
Literature review and selection of studies.
Inclusion criteria stipulated that articles report RCTs of a Food and Drug Administration (FDA) approved antidepressant medication for Major Depressive Disorder (MDD) in outpatient subjects aged 18–65. While meta-analyses were reviewed to identify studies, only data from individual RCTs were included in the analysis. Further criteria required trials to last between 6 and 12 weeks (inclusive), have comparison group of placebo or another FDA-approved antidepressant medication, be written in English, be published 1985 or later, and have response or remission rates specified using a standardized outcome measurement (e.g., Hamilton Rating Scale for Depression (HRSD)7, Beck Depression Inventory (BDI)8, Montgomery-Asberg Depression Rating Scale (MADRS)9, Clinical Global Impression (CGI)10). Trials were excluded for enrolling inpatients, pregnant women, subjects who were psychotic, or those defined to have treatment-resistant depression. Also excluded were antidepressant augmentation studies and trials requiring as inclusion criteria a specific subtype of Major Depression, a specific medical illness, or an Axis I disorder other than depression.
Data extraction
For each included study, demographic characteristics of the participants, details of the treatment condition, duration of active treatment in each study, and response rates to medication and placebo were entered into a database. We started counting the number of visits proscribed in each study with the initiation of treatment (i.e., we began with the week 1 visit and did not count evaluation or screening appointments). In most cases the visit schedule was stated in the methods section of the publication reporting each study. If this was not explicitly reported, we inferred the visit schedule from the number of data points in figures depicting the trajectory of symptom change over the course of the study. Since there was variability in the criteria different studies used to judge depression response, we standardized the response rate data to the extent that was possible. If studies reported multiple response rates based upon different outcome measures, we selected one response rate for extraction according to the following priority list: HRSD ≥ 50% decrease from baseline, MADRS ≥ 50% decrease from baseline, and CGI Improvement score of 1 or 2. Two judges (BRR and TMC) extracted the data, and any differences were resolved by consensus.
Data analyses
Data analyses followed those successfully implemented in four prior manuscripts, where the procedures are described in greater detail.11–14 Mixed effects logistic regression models were used, similar to the approach taken by Bryk and Raudenbush,15 Hox,16 and Haddock, Rindskopf, and Shadish.17 The multilevel logistic regression model is described by two equations: a within-studies equation and a between-studies equation, which accommodates the hierarchical structure of patients nested within medication conditions nested within studies. In the first set of models described below, the outcome variable was the reported response rate for each treatment cell (medication and placebo) in the studies comprising the sample.
The initial step was to determine whether there is significant variability in response rates across studies. To do this, we ignored the nesting within study and fit an unconditional model (Model 1). The within-studies equation for Model 1 is
where ln (p/[1-p]) is the log odds of response and B0 is a constant that is assumed initially to be the same for all groups within a study. At the between-studies level, the equation is
which describes the true response rates as varying around a grand mean (G00) with error (U0). To determine whether there were genuine differences between the studies (heterogeneity) or whether the variation in findings was compatible with chance alone (homogeneity), we examined the Birge ratio, which is calculated by dividing a chi-square by its degrees of freedom.18 The value of the Birge ratio is near 1 when there is only random variation between studies, and as the value exceeds 1, the results of a set of studies lack homogeneity (i.e., they are more varied than expected based on sampling error alone).19
If there is significant variability in response rates across studies (i.e., Birge ratio ≫ 1), it is possible to test whether the hypothesized predictors of treatment response explain a significant portion of this variability. First, we examined whether receiving active medication vs. placebo significantly influenced the odds of treatment response by including treatment assignment as a fixed effect in the within-studies equation (Model 2):
‘Active’ is a dummy variable coded one for antidepressant medication and zero otherwise. Using this method, odds ratios and estimated probabilities of response to treatment for patients receiving medication as opposed to placebo were computed.
Next, we proceeded to the between-studies level, where we added study type and study duration as fixed effects in the between-studies equation (Model 3):
‘Comparator’ is a dummy variable coded one for comparator trials and zero otherwise, and ‘duration’ is the duration of treatment in each study, centered on the overall mean for duration in the sample. Using this method, odds ratios and estimated probabilities of response to treatment in the different study types and durations were computed. We wished to control for the effects of these variables prior to undertaking our primary analysis of interest given the findings of previous meta-analyses that study type and duration are significant predictors of antidepressant medication and placebo response.11–12
Finally, the analysis proceeded to test whether the number of study visits in which patients met with research staff influenced treatment response (Model 4). We added this variable to the between-studies equation, centered on the overall grand mean for number of study visits in our sample:
We anticipated that the number of visits proscribed in an antidepressant clinical trial might be significantly correlated with the duration of treatment. However, we wished to disentangle the effects of study duration (which presumably influences treatment response via true medication effects, true placebo effects, and allowing time for spontaneous improvement) from the frequency of study visits.
Following our analysis of response rates, we conducted an analysis of drop-out rates in the studies comprising our sample. The drop-out analysis followed an identical structure to the response rate analysis, proceeding from an unconditional model (Model 1) to examine the influence of active treatment (Model 2), study type and duration (Model 3), and finally the frequency of follow-up visits (Model 4). All of the regression models were estimated using HLM 6.08. Differences in study characteristics, patient demographics, and clinical features across the different study types were investigated using two-tailed independent samples t-tests for continuous variables and chi-square (χ2) tests for categorical variables (SPSS version 18).
RESULTS
Characteristics of included studies and participants
One hundred eleven studies comprising 62 placebo-controlled and 49 comparator trials met the inclusion and exclusion criteria (Table 1). As shown in Table 2, these included 126 medication conditions enrolling 13,676 participants in the placebo-controlled studies, 62 placebo conditions enrolling 6,750 participants in the placebo-controlled studies, and 99 medication conditions enrolling 8,734 participants in the comparator studies. Mean response rates to medication ranged from 25–74% in the placebo-controlled trials and 29–95% in the comparator studies. For the purpose of comparison, mean response rates to placebo in the placebo-controlled trials ranged from 13–56%. Among the comparator trials, 6 out of 49 studies (12.2%) demonstrated significant differences in depression response rates between active treatment groups. Among the placebo-controlled trials, 51 out of 62 studies (82.3%) demonstrated significant differences in depression response rates between medication and placebo. Although we originally intended to analyze remission rates in addition to response rates, there was not sufficient information provided in the publications examined to permit this analysis.
Table 1.
Summary of included studies and participants.
| Study | Treatment | N (ITT) | Duration | Outcome measure | Response Rate |
|---|---|---|---|---|---|
| Alves et al 199920 | venlafaxine fluoxetine |
40 47 |
12 | HRSD | .85 .75 |
| Amsterdam et al 200321 | selegiline placebo |
145 144 |
8 | MADRS | .33* .21 |
| Baldwin et al 199622 | nefazodone paroxetine |
100 95 |
8 | CGI | .55 .61 |
| Beasley et al 199123 | fluoxetine trazodone |
63 57 |
6 | HRSD | .62 .69 |
| Behnke et al 200324 | mirtazipine sertraline |
171 168 |
8 | HRSD | .68 .68 |
| Benkert et al 200025 | mirtazipine paroxetine |
127 123 |
6 | HRSD | .58 .54 |
| Bielski et al 200426 | escitalopram venlafaxine XR |
97 98 |
8 | HRSD | .61 .48 |
| Bignamini et al 199227 | paroxetine amitriptyline |
151 152 |
6 | HRSD | .60 .65 |
| Bodkin et al 200228 | selegiline TD placebo |
88 88 |
6 | HRSD | .33* .20 |
| Bouchard et al 198729 | citalopram maprotiline |
46 44 |
6 | MADRS | .78 .73 |
| Boyer et al 200830 | desvenlafaxine 50 desvenlafaxine 100 placebo |
164 158 161 |
8 | HRSD | .65* .63* .50 |
| Burke et al 200231 | escitalopram 10 escitalopram 20 citalopram placebo |
118 123 125 119 |
MADRS | .50* .51* .46* .28 |
|
| Byerly et al 198832 | fluoxetine imipramine placebo |
32 34 29 |
6 | CGI | .43* .41* .13 |
| Chouinard et al 199933 | paroxetine fluoxetine |
100 98 |
12 | HRSD | .67 .68 |
| Christiansen et al 199634 | paroxetine amitriptyline |
71 73 |
8 | CGI | .65 .66 |
| Claghorn et al 199635 | fluvoxamine imipramine placebo |
44 44 45 |
6 | CGI | .48* .45* .27 |
| Claghorn et al 199236 | paroxetine placebo |
163 162 |
6 | CGI | .42* .27 |
| Cohn et al 199637 | nefazodone imipramine placebo |
39 38 42 |
8 | HRSD | .64* .64* .36 |
| Cohn et al 198538 | fluoxetine imipramine placebo |
54 54 58 |
6 | HRSD | .72* .42 .30 |
| Coleman et al 199939 | buproprion sertraline placebo |
118 109 117 |
8 | HRSD | .66 .61 .56 |
| Coleman et al 200140 | buproprion fluoxetine placebo |
136 146 145 |
8 | HRSD | .56 .57 .50 |
| Cosa e Silva et al 199841 | venlafaxine fluoxetine |
196 186 |
8 | CGI | .81 .84 |
| Croft et al 199942 | buproprion sertaline placebo |
116 116 116 |
8 | HRSD | .66* .68* .47 |
| Cunningham et al 199743 | venlafaxine venlafaxine XR placebo |
92 87 99 |
12 | CGI | .70* .52* .28 |
| Cunningham et al 199444 | venlafaxine trazodone placebo |
65 73 75 |
6 | HRSD | .72* .60 .55 |
| Dalery et al 200345 | fluvoxamine fluoxetine |
86 91 |
6 | HRSD | .60 .58 |
| Davey et al 198846 | trazodone qd trazodone tid |
95 87 |
6 | CGI | .58 .60 |
| DeMartinis et al 200747 | desvenlafaxine 100 desvenlafaxine 200 desvenlafaxine 400 placebo |
114 116 113 118 |
8 | HRSD | .51* .45 .48* .35 |
| Detke et al 200248 | duloxetine placebo |
121 115 |
9 | HRSD | .45* .23 |
| Detke et al 200249 | duloxetine placebo |
128 139 |
9 | HRSD | .50* .35 |
| Detke et al 200450 | duloxetine 80 duloxetine 120 paroxetine placebo |
95 93 86 93 |
8 | HRSD | .65* .71* .74* .44 |
| De Wilde et al 199351 | paroxetine fluoxetine |
37 41 |
6 | HRSD | .68 .63 |
| Debus et al 198852 | fluoxetine trazodone |
18 17 |
6 | HRSD | .50 .53 |
| Dierick et al 199653 | venlafaxine fluoxetine |
153 161 |
8 | HRSD | .72* .60 |
| Dunbar et al 199354 | paroxetine placebo |
138 135 |
6 | HRSD | .52* .22 |
| Dunlop et al 199055 | fluoxetine 20 fluoxetine 40 fluoxetine 60 placebo |
103 99 97 56 |
6 | HRSD | .40 .40 .35* .26 |
| Dunlop et al 201156 | desvenlafaxine placebo |
285 142 |
12 | HRSD | .61* .46 |
| Fabre et al 199257 | fluvoxamine imipramine placebo |
46 48 44 |
6 | HRSD | .52 .52 .33 |
| Fava et al 199858 | fluoxetine paroxetine placebo |
54 55 19 |
12 | HRSD | .57 .58 .53 |
| Fawcett et al 198959 | fluoxetine amitriptyline |
19 19 |
6 | HRSD | .75 .78 |
| Feighner et al 199160 | buproprion fluoxetine |
59 60 |
6 | HRSD | .63 .58 |
| Feighner et al 199361 | paroxetine imipramine placebo |
240 240 237 |
6 | HRSD | .39* .38* .21 |
| Feighner et al 199962 | citalopram 10 citalopram 20 citalopram 40 citalopram 60 placebo |
131 130 131 129 129 |
6 | MADRS | .48* .46* .61* .58* .32 |
| Feiger et al 199663 | nefazodone sertraline |
71 72 |
6 | HRSD | .59 .57 |
| Feiger et al 200964 | desvenlafaxine placebo |
117 118 |
8 | HRSD | .39 .31 |
| Fontaine et al 199465 | nefazodone low nefazodone high imipramine placebo |
46 44 45 |
6 | HRSD | .35 .57* .49* .31 |
| Fournier et al 199766 | sertraline imipramine |
43 45 |
8 | HRSD | .71 .74 |
| Gentil et al 200267 | venlafaxine amitriptyline |
57 58 |
8 | HRSD | .75 .76 |
| Golden et al 200268 | paroxetine CR paroxetine placebo |
206 211 205 |
12 | HRSD | .60* .56 .48 |
| Goldstein et al 200069 | duloxetine fluoxetine placebo |
66 33 68 |
8 | HRSD | .64 .52 .48 |
| Goldstein et al 200470 | duloxetine 40 duloxetine 80 paroxetine placebo |
86 91 87 89 |
8 | HRSD | .44 .51* .40 .31 |
| Hewett et al 200971 | bupropion XR venlafaxine XR placebo |
187 182 197 |
8 | MADRS | .57* .65* .46 |
| Hewett et al 201072 | bupropion venlafaxine placebo |
202 193 186 |
8 | MADRS | .57 .66* .49 |
| Hicks et al 200273 | nefazodone paroxetine |
20 20 |
8 | HRSD | .55 .80 |
| Higuchi et al 201174 | paroxetine CR paroxetine IR placebo |
158 83 171 |
8 | HRSD | .63* .57 .46 |
| Hong et al 200375 | mirtazipine fluoxetine |
66 66 |
6 | HRSD | .58 .51 |
| Hsu et al 201176 | citalopram sertraline |
21 21 |
6 | MADRS | .41 .29 |
| Hunter et al 201177 | fluoxetine placebo |
12 11 |
8 | HRSD | .50 .54 |
| Kasper et al 200578 | trazodone paroxetine |
50 53 |
6 | HRSD | .87 .91 |
| Keegan et al 199179 | fluoxetine amitriptyline |
18 19 |
6 | HRSD | .63 .69 |
| Khan et al 199880 | venlafaxine 75 venlafaxine 150 venlafaxine 200 placebo |
83 89 81 93 |
12 | HRSD | .52* .52* .60* .33 |
| Khan et al 200781 | escitalopram duloxetine |
136 126 |
8 | HRSD | .61 .52 |
| Khan et al 201182 | vilazodone placebo |
231 232 |
8 | HRSD | .44* .33 |
| Lee et al 200783 | duloxetine paroxetine |
238 240 |
8 | HRSD | .61 .65 |
| Leinonen et al 199984 | mirtazipine citalopram |
136 133 |
8 | MADRS | .85 .88 |
| Lepola et al 200385 | citalopram escitalopram placebo |
159 155 154 |
8 | MADRS | .53 .64* .48 |
| Liebowitz et al 200886 | desvenlafaxine 50 desvenlafaxine 100 placebo |
150 147 150 |
8 | HRSD | .54* .52 .45 |
| Lineberry et al 199087 | buproprion placebo |
110 106 |
6 | HRSD | .51* .34 |
| Lydiard et al 198988 | fluvoxamine imipramine placebo |
17 18 17 |
6 | HRSD | .53 .67* .30 |
| Lydiard et al 199789 | sertraline amityptiline placebo |
132 131 129 |
8 | HRSD | .55* .53* .37 |
| McPartlin et al 199890 | venlafaxine XR paroxetine |
175 161 |
12 | HRSD | .75 .70 |
| Mehtonen et al 200091 | venlafaxine sertraline |
75 72 |
8 | HRSD | .73* .59 |
| Mendels et al 199392 | venlafaxine low venlafaxine med venlafaxine high placebo |
79 76 79 78 |
6 | CGI | .60 .65 .68 .50 |
| Moller et al 200093 | sertraline amitriptyline |
100 105 |
6 | HRSD | .51 .68 |
| Montgomery et al 200494 | escitalopram venlafaxine XR |
146 142 |
8 | MADRS | .77 .80 |
| Moore et al 200595 | escitalopram citalopram |
138 142 |
8 | MADRS | .76* .61 |
| Nemeroff et al 200796 | venlafaxine fluoxetine placebo |
96 100 101 |
6 | HRSD | .53* .45 .37 |
| Nierenberg et al 200797 | duloxetine escitalopram placebo |
273 274 137 |
8 | HRSD | .43* .41 .32 |
| Noguera et al 199198 | fluoxetine imipramine |
60 60 |
6 | CGI | .83* .50 |
| Ohrberg et al 199299 | paroxetine imipramine |
65 65 |
6 | HRSD | .46 .39 |
| Ontiveros et al 1997100 | paroxetine fluoxetine |
60 61 |
6 | HRSD | .71 .67 |
| Ou et al 2011101 | escitalopram citalopram |
115 117 |
6 | HRSD | .72 .74 |
| Owens et al 2008102 | paroxetine CR venlafaxine XR |
40 41 |
8 | MADRS | .65 .71 |
| Patris et al 1996103 | citalopram fluoxetine |
153 161 |
8 | MADRS | .78 .76 |
| Perry et al 1989104 | fluoxetine trazodone |
21 19 |
6 | HRSD | .71 .82 |
| Peselow et al 1989105 | paroxetine imipramine placebo |
40 36 42 |
6 | HRSD | .48* .64* .33 |
| Reimherr et al 1990106 | setraline amitryptiline placebo |
142 144 141 |
8 | HRSD | .54* .60* .35 |
| Rickels et al 1985107 | fluvoxamine qd fluvoxamine bid |
90 84 |
6 | HRSD | .52 .52 |
| Rickels et al 1994108 | nefazodone imipramine placebo |
86 86 86 |
8 | HRSD | .52 .36 .31 |
| Rickels et al 2009109 | vilazodone placebo |
198 199 |
8 | HRSD | .44* .33 |
| Roth et al 1990110 | fluvoxamine desipramine placebo |
27 24 29 |
6 | CGI | .63 .63 .38 |
| Rudolph et al 1998111 | venlafaxine 75 venlafaxine 225 venlafaxine 375 placebo |
77 79 75 92 |
6 | HRSD | .42 .50* .52* .30 |
| Rudolph et al 1999112 | venlafaxine fluoxetine placebo |
95 103 97 |
8 | HRSD | .57 .50 .42 |
| Samuelian et al 1998113 | venlafaxine clomipramine |
52 46 |
7 | HRSD | .59 .43 |
| Sauer et al 2003114 | venlafaxine amitriptyline |
76 75 |
6 | HRSD | .40 .47 |
| Schweizer et al 1994115 | venlafaxine imipramine placebo |
64 71 78 |
6 | HRSD | .60* .37 .35 |
| Septien-Velez et al 2007116 | desvenlafaxine 200 desvenlafaxine 400 placebo |
121 124 124 |
8 | HRSD | .60* .56* .38 |
| Sheehan et al 2009117 | trazodone placebo |
202 204 |
8 | HRSD | .54* .41 |
| Shrivastava et al 1992118 | paroxetine imipramine placebo |
33 36 38 |
6 | HRSD | .42* .25 .26 |
| Smith et al 1992119 | paroxetine placebo |
33 33 |
6 | HRSD | .45* .24 |
| Swann et al 1997120 | phenelzine desipramine |
23 16 |
6 | HRSD | .57 .57 |
| Thase et al 1997121 | venlafaxine placebo |
91 100 |
8 | HRSD | .58* .29 |
| Tourian et al 2009122 | desvenlafaxine 50 desvenlafaxine 100 duloxetine placebo |
148 150 157 160 |
8 | HRSD | .39 .49 .47 .38 |
| Tylee et al 1997123 | venlafaxine fluoxetine |
147 156 |
12 | HRSD | .65 .70 |
| Wade et al 2002124 | escitalopram placebo |
188 189 |
8 | MADRS | .55* .42 |
| Wade et al 2007125 | escitalopram duloxetine |
141 146 |
8 | MADRS | .69* .58 |
| Walczak et al 1996126 | fluvoxamine 25 fluvoxamine 50 fluvoxamine 100 fluvoxamine 150 placebo |
144 144 144 144 144 |
8 | HRSD | .42 .50 .59* .58* .38 |
| Weisler et al 1994127 | buproprion trazodone |
59 52 |
6 | HRSD | .56 .42 |
| Wernicke et al 1988128 | fluoxetine 5 fluoxetine 20 fluoxetine 40 placebo |
94 91 92 77 |
6 | HRSD | .46* .50* .48* .23 |
| Wernicke et al 1987129 | fluoxetine 20 fluoxetine 40 fluoxetine 60 placebo |
97 97 103 48 |
6 | HRSD | .39* .44* .30* .19 |
| Yevtushenko et al 2007130 | escitalopram citalopram 10 citalopram 20 |
108 106 108 |
6 | MADRS | .95* .44 .83* |
p < 0.05 vs. comparison group
Table 2.
Clinical characteristics of included patients and methodological features of studies included in the multilevel meta-analysis.
| Characteristic | Placebo-Controlled Studies | Comparator Studies |
|---|---|---|
| N studies | 62 | 49 |
| N medication treatment groups | 126 | 99 |
| N patients in medication treatment groups | 13,676 | 8,734 |
| N placebo treatment groups | 62 | 0 |
| N patients in placebo treatment groups | 6,750 | 0 |
| Mean age | 41.1 ± 2.5 | 42.1 ± 3.5 |
| Mean drop-out rate | 31.8 ± 14.1 | 24.0 ± 10.2 |
| Mean N ITTa | 108.9 ± 56.7 | 88.2 ± 52.3 |
| Mean pre-treatment HRSDb | 24.6 ± 3.6 | 26.1 ± 4.8 |
| N treatment conditions | N patients | N treatment conditions | N patients | |
|---|---|---|---|---|
|
| ||||
| Study duration | ||||
| 6 wks | 77 | 5,999 | 55 | 3,592 |
| 8 wks | 92 | 12,169 | 36 | 4,218 |
| 12 wks | 4 | 503 | 8 | 924 |
|
| ||||
| Study visits | ||||
| Weekly | 66 | 4,750 | 20 | 1,148 |
| Skip 1 visit | 29 | 3,146 | 4 | 589 |
| Skip 2 visits | 55 | 8,088 | 32 | 2,611 |
| Skip ≥3 visits | 45 | 4,369 | 35 | 3,748 |
|
| ||||
| Meds used | ||||
| SSRIc | 53 | 5,812 | 54 | 4,986 |
| SNRId | 40 | 4,700 | 15 | 1,762 |
| TCAe | 16 | 1,096 | 12 | 733 |
| Atypical ADf | 15 | 1,835 | 17 | 1,230 |
| MAOIg | 2 | 233 | 1 | 23 |
ITT = Intent to treat
HRSD = Hamilton Rating Scale for Depression
SSRI = Selective Serotonin Reuptake Inhibitor
SNRI = Serotonin Norepinephrine Reuptake Inhibitor
TCA = Tricyclic antidepressant
Atypical AD = Atypical antidepressant (e.g., bupropion, nefazodone, mirtazipine, trazodone)
MAOI = Monoamine Oxidase Inhibitor
As shown in Table 2, placebo-controlled studies in our sample had more patients per treatment arm (t = 3.013, df 285, p = 0.003), younger participants (t = −2.646, df 246, p = 0.009), and higher drop-out rates (t = 4.468, df 235 p < 0.001) relative to comparator studies, while the mean baseline depression severity score was significantly higher in comparator vs. placebo-controlled studies (t = −2.646, df 272, p = 0.004). Study duration ranged from 6–12 weeks in both placebo-controlled and comparator studies, and mean study duration was not significantly different between the study types (t = 1.395, df 285, p = 0.164). The number of study visits ranged from 3–12 in both placebo-controlled and comparator studies and was on average greater in placebo-controlled trials (t = 6.137, df 274, p < 0.001).
Analysis of response rates
Coefficients and odds ratios for the predictor variables in the models describing treatment response are tabulated in Table 3. In Model 1, the unconditional model of treatment response rates, variability between studies was over 16 times that expected by chance alone (Birge ratio: χ2/df = 1772.6/106 = 16.7). Therefore, the null hypothesis that response rates are homogeneous across studies was rejected, and the analysis proceeded with the conditional models.
Table 3.
Coefficients and odds ratios for predictor variables at each step of the multilevel meta-analysis of response rates.
| Variable | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Coefficient (SE) | Odds Ratio (CI) | Coefficient (SE) | Odds Ratio (CI) | Coefficient (SE) | Odds Ratio (CI) | Coefficient (SE) | Odds Ratio (CI) | |
| Intercept | −0.018 (0.12) | 0.98 (0.77–1.23) | −0.36 (0.70) | 0.70 (0.55–0.90) | −0.55 (0.13) | 0.57 (0.44–0.75) | −0.61 (0.054) | 0.54 (0.49–0.60) |
| Active | -- | -- | 0.43 (0.066) | 1.54* (1.35–1.76) | 0.42 (0.066) | 1.52* (1.33–1.73) | 0.65 (0.035) | 1.92* (1.79–2.06) |
| Comparator | -- | -- | -- | -- | 0.53 (0.19) | 1.69* (1.12–2.55) | 0.60 (0.085) | 1.81* (1.53–2.15) |
| Duration | -- | -- | -- | -- | 0.46 (0.36) | 1.57 (0.73–3.39) | 0.64 (0.14) | 1.89* (1.43–2.50) |
| Visits | -- | -- | -- | -- | -- | -- | −0.031 (0.20) | 0.97 (0.65–1.44) |
| Variance component | 0.235 | 0.188 | 0.136 | 0.142 | ||||
| X2 | 299.9 | 237.7 | 147.3 | 859.7 | ||||
| df | 18 | 18 | 16 | 103 | ||||
p < 0.05
Including treatment assignment (medication vs. placebo) in Model 2 accounted for 24.8% of the variability observed in response rates. The odds of responding to treatment for patients receiving antidepressant medication were 1.96 times higher compared to patients receiving placebo (95% CI 1.82 – 2.10, p < 0.001). The average medication response rate derived from Model 2 was 57.6%, compared to an average placebo response rate of 36.7%. In Model 3, including study type (placebo-controlled vs. comparator) and duration reduced the variability in response rates by an additional 40.7%. Across treatment assignments and durations, the odds of responding to treatment in comparator studies were 1.82 times greater vs. placebo-controlled studies (95% CI = 1.54 – 2.15, p < 0.001). Controlling for treatment assignment and study type, the odds of treatment response increased 1.87 times for each 1 week increase in study duration above the grand mean of the sample (95% CI = 1.42 – 2.46, p < 0.001). No significant interactions between study type and duration were found.
Adding the data on the number of study visits to create the full model (Model 4) did not explain additional variability in response rates over Model 3. Once treatment assignment, study type, and study duration were accounted for, the number of study visits did not significantly influence response rates in our sample (OR 0.97, 95% CI = 0.65 – 1.44, p = 0.877). We were interested in determining whether the effect of visit frequency might differ for patients receiving medication compared to placebo (i.e., visit frequency x treatment assignment interaction), but it is not possible to examine interactions between within-study variables (Active) and between-study variables (Visits) using this hierarchical modeling approach. As an alternative, we divided the data set into medication treatment cells and placebo treatment cells, then repeated the above analysis separately for each subset of the data. We found that the same pattern of results obtained for the medication and placebo data sets as was found in the combined sample. Treatment response was higher in comparator vs. placebo-controlled studies and increased with study duration, but the number of study visits did not significantly influence response.
An additional subgroup analysis performed to assess the robustness of the results obtained was to limit the analyses to Selective Serotonin Reuptake Inhibitors (SSRIs). No change in the pattern of results obtained was found. Based on the rationale that the effect of study visits should be greatest for subjects completing the study (i.e., patients who drop-out are presumably unaffected by more or less visits later in the study), we repeated the analysis using response rate data for study completers rather than the ITT data set. For the 39/112 studies (35.1%) in the sample providing completer data, the duration of the study (OR 4.93, 95% CI = 1.26 – 19.3, p = 0.023) but not the number of visits (OR 0.42, 95% CI = 0.14 – 1.31, p = 0.133) significantly influenced the odds of treatment response.
Analysis of drop-out rates
Coefficients and odds ratios for the predictor variables in the models describing drop-out rates are tabulated in Table 4. In Model 1, the unconditional model of drop-out rates, variability between studies was over 19 times that expected by chance alone (Birge ratio: χ2/df = 1938.2/98 = 19.7). Therefore, the null hypothesis that drop-out rates are homogeneous across studies was rejected, and the analysis proceeded with the conditional models.
Table 4.
Coefficients and odds ratios for predictor variables at each step of the multilevel meta-analysis of drop-out rates.
| Variable | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Coefficient (SE) | Odds Ratio (CI) | Coefficient (SE) | Odds Ratio (CI) | Coefficient (SE) | Odds Ratio (CI) | Coefficient (SE) | Odds Ratio (CI) | |
| Intercept | −0.99 (0.065) | 0.37 (0.33–0.42) | −0.96 (0.075) | 0.38 (0.33–0.44) | −0.81 (0.089) | 0.45 (0.38–0.53) | −0.85 (0.088) | 0.42 (0.36–0.51) |
| Active | -- | -- | −0.036 (0.041) | 0.96 (0.89–1.05) | −0.026 (0.97) | 0.97 (0.90–1.06) | −0.026 (0.041) | 0.97 (0.90–1.06) |
| Comparator | -- | -- | -- | -- | −0.40 (0.12) | 0.67* (0.53–0.85) | −0.28 (0.11) | 0.76* (0.61–0.95) |
| Duration | -- | -- | -- | -- | −0.62 (0.29) | 0.54* (0.30–0.96) | −1.11 (0.33) | 0.33* (0.17–0.63) |
| Visits | -- | -- | -- | -- | -- | -- | 1.02 (0.26) | 2.77 (1.66–4.63) |
| Variance component | 0.391 | 0.389 | 0.340 | 0.305 | ||||
| X2 | 1938.2 | 1930.65 | 1620.2 | 1516.0 | ||||
| df | 98 | 98 | 96 | 95 | ||||
p < 0.05
Including treatment assignment (medication vs. placebo) in Model 2 did not account for substantial variability in drop-out rates. The odds of drop-out for patients receiving antidepressant medication were not significantly different from the odds of drop-out for patients receiving placebo (OR 0.96, 95% CI 0.89 – 1.05, p = 0.385). In Model 3, including study type and duration reduced the variability in response rates by 13.0%. Across treatment assignments and durations, the odds of drop-out in comparator studies were 0.67 times the odds in placebo-controlled studies (95% CI = 0.53 – 0.85, p = 0.002). Controlling for treatment assignment and study type, the odds of drop-out were reduced by a factor of 0.54 for each 1 week increase in study duration above the grand mean of the sample (95% CI = 0.30 – 0.96, p = 0.035). No significant interactions between study type and duration were found.
In the full model (Model 4), the number of study visits explained an additional 9.0% of the original variability in drop-out rates. Controlling for treatment assignment, study type, and study duration, the odds of drop-out increased 2.77 times for each 1 visit increase in the number of visits above the grand mean of the sample (95% CI = 1.66 – 4.63, p < 0.001). As in the response rate analyses, we investigated whether the effect of visit frequency on drop-out might differ for patients receiving medication compared to placebo. The pattern of results obtained for the medication and placebo data sets was again similar to the combined sample. The odds of drop-out decreased with increasing study duration (medication only: OR 0.35, 95%CI = 0.19 – 0.66, p = 0.002; placebo only: OR 0.19, 95%CI = 0.069 – 0.537, p = 0.003), whereas the odds of drop-out increased with increasing number of study visits (medication only: OR 2.95, 95% CI = 1.60 – 5.42, p = 0.001; placebo only: OR 1.84, 95% CI = 0.48 – 7.10, p = 0.368).
DISCUSSION
This meta-analysis examined the influence of follow-up visit frequency on treatment response and attrition rates in 111 studies of antidepressant medication for adult outpatients with MDD. Consistent with prior results reported by our group and others, the odds of treatment response in the studies we examined were significantly increased by receiving active medication as opposed to placebo, being in a comparator vs. placebo-controlled study, and being in a longer vs. shorter duration study. Taken together, these predictor variables explained 65.5% of the variability observed in response rates among the treatment cells in our sample. Contrary to our hypotheses, visit frequency did not significantly influence the odds of response after accounting for treatment assignment, study type, and duration. We were also surprised to find that greater numbers of study visits significantly increased drop-out rates for participants in these antidepressant trials. Thus, for a given type of study and duration of treatment, greater numbers of study visits conferred no advantage in terms of response rates and actually posed a disadvantage to retaining patients in the study.
It has previously been argued that the intensive visit schedules found in antidepressant trials are necessary in order to maintain compliance with the study procedures, prevent dropout, and monitor the safety of participants randomized to placebo.2 However, our findings suggest that more intense follow-up regimens are actually counterproductive when the goal is to maintain participants within a clinical trial, and this was true for both medication and placebo treatment. It may be the case that some subjects find the weekly visit schedule of many clinical trials to be onerous rather than supportive, making them more rather than less likely to drop-out over the course of the study. Visit schedules that are much more frequent than are commonly practiced in the community treatment of depression also contribute to the ballooning expense of Phase III clinical trials and make them less generalizable to standard clinical treatment. Therefore, decreasing the visit frequency of clinical trials has the potential to decrease the cost of new drug development, improve the retention of patients within studies, and facilitate the practice of evidence-based medicine.
In prior meta-analyses, we have shown that study duration significantly influences response to antidepressant medication,11–12 but the result that increasing study duration is associated with decreased odds of drop-out was unexpected. This finding contradicts the commonly held view that longer studies typically have higher attrition rates and is consistent with recent reports of low drop-out rates in longer duration studies.131 One possible explanation is that longer duration studies generally have lower frequencies of follow-up visits than shorter duration studies (e.g., 8-week duration trials in our sample skipped an average of 2.0 ± 1.1 visits, while 12-week duration trials skipped an average of 4.7 ± 1.7 visits). Since increased visit frequency is associated with higher drop-out rates, decreased visit frequency may explain the lower drop-out rates in longer duration studies. There may also be less investigator-initiated drop-out of participants who miss study visits in longer duration studies (i.e., investigators might be more flexible with visit non-compliance when there are greater numbers of study visits). Alternatively, participants may themselves feel reassured by having longer periods of follow-up and be willing to give study medication more time to work if they are not experiencing a positive response early in the study.
The findings that active comparator study designs (relative to placebo-controlled trials) have higher response rates to antidepressant medication and lower drop-out rates were also consistent with previous meta-analyses we have conducted of antidepressant clinical trials.11–12,14 However, these results were even more striking in the present sample given that patients in the comparator trials had significantly higher baseline depression severity relative to patients in placebo-controlled trials. It may be the case that more severely ill individuals are unwilling to risk the possibility of receiving placebo and prefer to enroll in comparator-type studies. Subjects in comparator trials know they are receiving medications demonstrated to be effective for depression, while participants in placebo-controlled trials are aware they may be taking placebo. Higher expectations of improvement among these individuals in comparator trials may directly increase observed medication response via an enhanced placebo effect and may also lead subjects to form stronger therapeutic alliances, continue treatment during periods of clinical worsening or increased side effects, and report less severe symptoms. Alternatively, lower expectations for therapeutic gain in placebo-controlled trials may decrease medication response rates in those trials and make enrolled subjects more likely to drop-out in the event of symptom worsening or non-improvement.
Finally, a number of limitations should be considered when interpreting the findings of this study. The use of trial-level summary data limited the data available for analysis in this study, as not all authors reported complete information about patient and trial characteristics in their published article. We were unable to test for associations between patient characteristics and the effects of visit frequency, which are potentially of great clinical interest if different types of patients may respond differently to follow-up visits. Additionally, publication bias may have affected which studies were included in these analyses, since RCTs failing to demonstrate significant differences between medication and placebo may not have been published. In our sample 82% of placebo-controlled trials showed a significant difference between at least one medication cell and placebo, which is higher than would be expected if all clinical trial data were published. However, it is not the efficacy of medication compared to placebo that was investigated in this analysis, so publication bias seems unlikely to have affected the overall patterns of response observed across trials.
A more significant limitation of this study is that we determined the number of visits based upon the designed visit schedule for each study rather than upon the actual number of visits that each participant attended. Missed study visits as well as participant drop-out likely resulted in alterations from the proscribed visit schedule in many cases. We performed analyses of completer data in order to explore for effects of drop-out, but not having access to patient-level data from each study made it the case that we were unable to determine the frequency of protocol violations. Finally, the number of study visits proscribed for a given study duration varied over a relatively modest range (i.e., from 3–8 visits in 8 week duration studies), which limits our ability to extrapolate these results to community settings in which visit frequency may vary even more widely. It is also possible that larger differences in visit frequency may have had a measurable effect on response rates. We believe that these limitations inherent to any retrospective review of visit frequency highlights the need to prospectively evaluate the influence of this variable on therapeutic response and medication/visit compliance in antidepressant clinical trials. Prospectively randomizing patients to different visit schedules would not only allow a more valid assessment of the effects of visit frequency but also may permit determination of patient characteristics moderating these effects.
In summary, results from this meta-analysis indicate that a weekly follow-up visit schedule in antidepressant clinical trials does not appreciably influence response to antidepressant medication or placebo but does significantly increase drop-out rates. Investigators should consider a less frequent visit schedule when designing future clinical trials, which may have the advantages of limiting expense and improving participant retention.
CLINICAL POINTS.
Clinicians may be advised to initiate a discussion of follow-up visit frequency with depressed patients at the beginning of treatment in order to integrate their recommendations with patients’ expectations and preferences.
In the treatment of stable patients, clinicians may opt to evaluate patients every two weeks during the initiation of antidepressant medication and then taper visit frequency to monthly when clinically appropriate and in keeping with a given patient’s preferences.
Acknowledgments
Sources of support: This work was supported by a National Institutes of Health Ruth L. Kirschstein National Research Service Award Short-Term Institutional Research Training Grant (T35), National Institute of Mental Health grants K23 MH085236 (BRR), K23 MH075006 (JRS), R21 MH087774 (JRS), a Hope for Depression Research Foundation grant (BRR), and a NARSAD Young Investigator Award (BRR).
Footnotes
Supplementary material: None
Disclosures: Dr. Rutherford, Ms. Persaud, Mr. Cooper, Dr. Brown, and Dr. Sneed have no conflicts of interest to report. Dr. Roose has served as a consultant to Pfizer and Forest Laboratories. This paper has not been previously presented.
Contributor Information
Bret R Rutherford, New York State Psychiatric Institute, Columbia University College of Physicians and Surgeons, New York, NY.
Timothy M. Cooper, Columbia University College of Physicians and Surgeons, New York, NY.
Amanda Persaud, Queens College of the City University of New York, New York, NY.
Patrick J. Brown, New York State Psychiatric Institute, New York, NY.
Joel R. Sneed, Queens College of the City University of New York, New York, NY.
Steven P. Roose, Columbia University College of Physicians and Surgeons, New York State Psychiatric Institute, New York, NY.
References
- 1.Rutherford BR, Roose SP. A Model of Placebo Effects in Antidepressant Clinical Trials. Am J Psychiatry. doi: 10.1176/appi.ajp.2012.12040474. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller MD, Frank E, Reynolds CF. The Art of Clinical Management in Pharmacologic Trials with Depressed Elderly Patients: Lessons from the Pittsburgh Study of Maintenance Therapies in Late-Life Depression. Am J Geriatr Psychiatry. 1999;7:228–234. doi: 10.1097/00019442-199908000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Mojtabai R, Olfson M. National Patterns in Antidepressant Treatment by Psychiatrists and General Medical Providers: Results from the National Comorbidity Survey Replication. J Clin Psychiatry. 2008;69:1064–1074. doi: 10.4088/jcp.v69n0704. [DOI] [PubMed] [Google Scholar]
- 4.Stettin GD, Yao J, Verbrugge RR, et al. Frequency of follow-up care for adult and pediatric patients during initiation of antidepressant therapy. Am J Manag Care. 2006;12:453–461. [PubMed] [Google Scholar]
- 5.Morrato EH, Libby AM, Orton HD, et al. Frequency of Provider Contact after FDA Advisory on Risk of Pediatric Suicidality with SSRIs. Am J Psychiatry. 2008;165:42–50. doi: 10.1176/appi.ajp.2007.07010205. [DOI] [PubMed] [Google Scholar]
- 6.Posternak MA, Zimmerman M. Therapeutic effect of follow-up assessments on antidepressant and placebo response rates in antidepressant efficacy trials. Br J Psychiatry. 2007;190:287–292. doi: 10.1192/bjp.bp.106.028555. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck AT, Ward CH, Mendelson M, et al. An inventory of measuring depression. Arch Gen Psychiatry. 1961;4:53–63. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 9.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 10.Guy W. New Clinical Drug Evaluation Unit (ECDEU) Assessment Manual for Psychopharmacology. Vol. 1976. Rockville, MD: National Institute of Mental Health; 1976. Clinical Global Impressions; pp. 218–222. [Google Scholar]
- 11.Sneed JR, Rutherford BR, Rindskopf D, et al. Design Makes a Difference: A Meta-Analysis of Antidepressant Response Rates in Placebo-Controlled versus Comparator Trials in Late-Life Depression. Am J Geri Psychiatry. 2008;16:65–73. doi: 10.1097/JGP.0b013e3181256b1d. [DOI] [PubMed] [Google Scholar]
- 12.Rutherford BR, Sneed JR, Roose SP. Does Study Design Affect Outcome? The Effects of Placebo Control and Treatment Duration in Antidepressant Trials. Psychother Psychosom. 2009;78:172–181. doi: 10.1159/000209348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rutherford BR, Sneed JR, Tandler J, et al. Deconstructing Pediatric Depression Trials: An Analysis of the Effects of Expectancy and Therapeutic Contact. J Am Acad Child Adolesc Psychiatry. 2011;50:782–795. doi: 10.1016/j.jaac.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rutherford BR, Sneed JR, Roose SP. Does Differential Drop-Out Explain the Influence of Study Design on Antidepressant Response? A Meta-Analysis. J Aff Disord. 2012;140:57–65. doi: 10.1016/j.jad.2012.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bryk AS, Raudenbush SW. Hierarchical linear models. Newbury Park, CA: Sage Publications; 1992. [Google Scholar]
- 16.Hox J. Multilevel Analysis: Techniques and applications. Mahwah, NJ: Lawrence Erlbaum Publishers; 2002. [Google Scholar]
- 17.Haddock CK, Rindskopf D, Shadish WR. Using odds ratios as effect sizes for meta-analysis of dichotomous data: A primer on methods and issues. Psychological Methods. 1998;3:339–353. [Google Scholar]
- 18.Birge RT. The calculation of errors by the method of least squares. Rev Mod Physics. 1932;40:207–227. [Google Scholar]
- 19.Higgins JPT, Thompson SG. Quantifying heterogeneity in a metaanalysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 20.Alves C, Cachola I, Brandao J. Efficacy and tolerability of venlafaxine and fluoxetine in outpatients with major depression. Primary Care Psychiatry. 1999;5:57–63. [Google Scholar]
- 21.Amsterdam JD. A double-blind, placebo-controlled trial of the safety and efficacy of selegiline transdermal system without dietary restrictions in patients with major depressive disorder. J Clin Psychiatry. 2003;64:208–214. doi: 10.4088/jcp.v64n0216. [DOI] [PubMed] [Google Scholar]
- 22.Baldwin DS, Hawley CJ, Abed RT, et al. A multicenter double-blind comparison of nefazodone and paroxetine in the treatment of outpatients with moderate-to-severe depression. J Clin Psychiatry. 1996;57(Suppl 2):46–52. [PubMed] [Google Scholar]
- 23.Beasley CM, Dornseif BE, Pultz JA, et al. Fluoxetine versus trazodone: efficacy and activating-sedating effects. J Clin Psychiatry. 1991;52:294–299. [PubMed] [Google Scholar]
- 24.Behnke K, Sogaard J, Martin S, et al. Mirtazapine orally disintegrating tablet versus sertraline: a prospective onset of action study. J Clin Psychopharm. 2003;23:358–364. doi: 10.1097/01.jcp.0000085408.08426.05. [DOI] [PubMed] [Google Scholar]
- 25.Benkert O, Szegedi A, Kohnen R. Mirtazapine compared with paroxetine in major depression. J Clin Psychiatry. 2000;61:656–663. doi: 10.4088/jcp.v61n0911. [DOI] [PubMed] [Google Scholar]
- 26.Bielski RJ, Ventura D, Chang CC. A double-blind comparison of escitalopram and venlafaxine extended release in the treatment of major depressive disorder. J Clin Psychiatry. 2004;65:1190–1196. doi: 10.4088/jcp.v65n0906. [DOI] [PubMed] [Google Scholar]
- 27.Bignamini A, Rapisarda V Italian Paroxetine Study Group. A double-blind multicentre study of paroxetine and amitriptyline in depressed outpatients. Int Clin Psychopharm. 1992;6(Suppl 4):37–41. doi: 10.1097/00004850-199206004-00008. [DOI] [PubMed] [Google Scholar]
- 28.Bodkin JA, Amsterdam JD. Transdermal selegiline in major depression: a double-blind, placebo-controlled, parallel-group study in outpatients. Am J Psychiatry. 2002;159:1869–1875. doi: 10.1176/appi.ajp.159.11.1869. [DOI] [PubMed] [Google Scholar]
- 29.Bouchard JM, Delaunay J, Delisle JP, et al. Citalopram versus maprotiline: a controlled, clinical multicentre trial in depressed patients. Acta Psychiatrica Scandinavica. 1987;76:583–592. doi: 10.1111/j.1600-0447.1987.tb02923.x. [DOI] [PubMed] [Google Scholar]
- 30.Boyer P, Montgomery S, Lepola U, et al. Efficacy, safety, and tolerability of fixed-dose desvenlafaxine 50 and 100 mg/day for major depressive disorder in a placebo-controlled trial. Int Clin Psychopharmacol. 2008;23:243–53. doi: 10.1097/YIC.0b013e32830cebed. [DOI] [PubMed] [Google Scholar]
- 31.Burke WJ, Gergel I, Bose A. Fixed-dose trial of the single isomer SSRI escitalopram in depressed outpatients. J Clin Psychiatry. 2002;63:331–336. doi: 10.4088/jcp.v63n0410. [DOI] [PubMed] [Google Scholar]
- 32.Byerley WF, Reimherr FW, Wood DR, et al. Fluoxetine, a selective serotonin uptake inhibitor, for the treatment of outpatients with major depression. J Clin Psychopharm. 1988;8:112–115. [PubMed] [Google Scholar]
- 33.Chouinard G, Saxena B, Belanger MC, et al. A Canadian multicenter, double-blind study of paroxetine and fluoxetine in major depressive disorder. J Aff Disorders. 1999;54:39–48. doi: 10.1016/s0165-0327(98)00188-8. [DOI] [PubMed] [Google Scholar]
- 34.Christiansen PE, Behnke K, Clack CH, et al. Paroxetine and amitriptyline in the treatment of depression in general practice. Acta Psychiatrica Scandinavica. 1996;93:158–163. doi: 10.1111/j.1600-0447.1996.tb10623.x. [DOI] [PubMed] [Google Scholar]
- 35.Claghorn JL, Earl CQ, Walczak DD, et al. Fluvoxamine maleate in the treatment of depression: a single-center, double-blind, placebo-controlled comparison with imipramine in outpatients. J Clin Psychopharm. 1996;16:113–120. doi: 10.1097/00004714-199604000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Claghorn JL, Kiev A, Rickels K, et al. Paroxetine versus placebo: a double-blind comparison in depressed patients. J Clin Psychiatry. 1992;53:434–438. [PubMed] [Google Scholar]
- 37.Cohn CK, Robinson DS, Roberts DL, et al. Responders to antidepressant drug treatment: a study comparing nefazodone, imipramine, and placebo in patients with major depression. J Clin Psychiatry. 1996;57(Suppl 2):15–18. [PubMed] [Google Scholar]
- 38.Cohn JB, Wilcox C. A comparison of fluoxetine, imipramine, and placebo in patients with major depressive disorder. J Clin Psychiatry. 1985;46:26–31. [PubMed] [Google Scholar]
- 39.Coleman CC, Cunningham LA, Foster VJ, et al. Sexual dysfunction associated with the treatment of depression: a placebo-controlled comparison of bupropion sustained release and sertraline treatment. Ann Clin Psychiatry. 1999;11:205–215. doi: 10.1023/a:1022309428886. [DOI] [PubMed] [Google Scholar]
- 40.Coleman CC, King BR, Bolden-Watson C, et al. A placebo-controlled comparison of the effects on sexual functioning of bupropion sustained release and fluoxetine. Clin Ther. 2001;23:1040–1058. doi: 10.1016/s0149-2918(01)80090-4. [DOI] [PubMed] [Google Scholar]
- 41.Costa e Silva J. Randomized, double-blind comparison of venlafaxine and fluoxetine in outpatients with major depression. J Clin Psychiatry. 1998;59:352–357. doi: 10.4088/jcp.v59n0703. [DOI] [PubMed] [Google Scholar]
- 42.Croft H, Settle E, Houser T, et al. A placebo-controlled comparison of the antidepressant efficacy and effects on sexual functioning of sustained-release bupropion and sertraline. Clin Ther. 1999;21:643–658. doi: 10.1016/S0149-2918(00)88317-4. [DOI] [PubMed] [Google Scholar]
- 43.Cunningham LA Venlafaxine XR 208 Study Group. Once-daily venlafaxine extended release XR and venlafaxine immediate release IR in outpatients with major depression. Ann Clin Psychiatry. 1997;9:157–164. doi: 10.1023/a:1026277907818. [DOI] [PubMed] [Google Scholar]
- 44.Cunningham LA, Borison RL, Carman JS, et al. A comparison of venlafaxine, trazodone, and placebo in major depression. J Clin Psychopharm. 1994;14:99–106. [PubMed] [Google Scholar]
- 45.Dalery J, Honig A. Fluvoxamine versus fluoxetine in major depressive episode: a double-blind randomised comparison. Hum Psychopharm. 2003;18:379–384. doi: 10.1002/hup.490. [DOI] [PubMed] [Google Scholar]
- 46.Davey A. A comparison of two oral dosage regimens of 150 mg trazodone in the treatment of depression in general practice. Psychopharmacology. 1988;95(Suppl):S25–30. doi: 10.1007/BF00172626. [DOI] [PubMed] [Google Scholar]
- 47.DeMartinis NA, Yeung PP, Entsuah R, et al. A double-blind, placebo-controlled study of the efficacy and safety of desvenlafaxine succinate in the treatment of major depressive disorder. J Clin Psychiatry. 2007;68:677–688. doi: 10.4088/jcp.v68n0504. [DOI] [PubMed] [Google Scholar]
- 48.Detke MJ, Lu Y, Goldstein DJ, et al. Duloxetine, 60 mg once daily, for major depressive disorder: a randomized double-blind placebo-controlled trial. J Clin Psychiatry. 2002;63:308–315. doi: 10.4088/jcp.v63n0407. [DOI] [PubMed] [Google Scholar]
- 49.Detke MJ, Lu Y, Goldstein DJ, et al. Duloxetine 60 mg once daily dosing versus placebo in the acute treatment of major depression. J Psychiatr Res. 2002;36:383–390. doi: 10.1016/s0022-3956(02)00060-2. [DOI] [PubMed] [Google Scholar]
- 50.Detke MJ, Wiltse CG, Mallincrodt CH, et al. Duloxetine in the acute and long-term treatment of major depressive disorder: a placebo- and paroxetine-controlled trial. Eur Neuropsychopharm. 2004;14:457–470. doi: 10.1016/j.euroneuro.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 51.De Wilde J, Spiers R, Mertens C, et al. A double-blind, comparative, multicentre study comparing paroxetine with fluoxetine in depressed patients. Acta Psychiatrica Scandinavica. 1993;87:141–145. doi: 10.1111/j.1600-0447.1993.tb03345.x. [DOI] [PubMed] [Google Scholar]
- 52.Debus JR, Rush AJ, Himmel C, et al. Fluoxetine versus trazodone in the treatment of outpatients with major depression. J Clin Psychiatry. 1988;49:422–426. [PubMed] [Google Scholar]
- 53.Dierick M, Ravizza L, Realini R, et al. A double-blind comparison of venlafaxine and fluoxetine for treatment of major depression in outpatients. Progr Neuro-Psychopharm & Biol Psych. 1996;20:57–71. doi: 10.1016/0278-5846(95)00292-8. [DOI] [PubMed] [Google Scholar]
- 54.Dunbar GC, Claghorn JL, Kiev A, et al. A comparison of paroxetine and placebo in depressed outpatients. Acta Psychiatrica Scandinavica. 1993;87:302–305. doi: 10.1111/j.1600-0447.1993.tb03376.x. [DOI] [PubMed] [Google Scholar]
- 55.Dunlop SR, Dornseif BE, Wernicke JF, et al. Pattern analysis shows beneficial effect of fluoxetine treatment in mild depression. Psychopharm Bull. 1990;26:173–180. [PubMed] [Google Scholar]
- 56.Dunlop BW, Reddy S, Yang L, et al. Symptomatic and functional improvement in employed depressed patients: a double-blind clinical trial of desvenlafaxine versus placebo. J Clin Psychopharmacol. 2011;31:569–576. doi: 10.1097/JCP.0b013e31822c0a68. [DOI] [PubMed] [Google Scholar]
- 57.Fabre LF. A 6-week, double-blind trial of paroxetine, imipramine, and placebo in depressed outpatients. J Clin Psychiatry. 1992;53(Suppl):40–43. [PubMed] [Google Scholar]
- 58.Fava M, Amsterdam JD, Delilto JA, et al. A double-blind study of paroxetine, fluoxetine, and placebo in outpatients with major depression. Ann Clin Psychiatry. 1998;10:145–150. doi: 10.1023/a:1022337927842. [DOI] [PubMed] [Google Scholar]
- 59.Fawcett J. Fluoxetine versus amitriptyline in adult outpatients with major depression. Curr Ther Res. 1989;45:821–832. [Google Scholar]
- 60.Feighner JP, Gardner EA, Johnston JA, et al. Double-blind comparison of bupropion and fluoxetine in depressed outpatients. J Clin Psychiatry. 1991;52:329–335. [PubMed] [Google Scholar]
- 61.Feighner JP, Cohn JB, Fabre LF, et al. A study comparing paroxetine placebo and imipramine in depressed patients. J Aff Disorders. 1993;28:71–79. doi: 10.1016/0165-0327(93)90035-i. [DOI] [PubMed] [Google Scholar]
- 62.Feighner JP, Overo K. Multicenter, placebo-controlled, fixed-dose study of citalopram in moderate-to-severe depression. J Clin Psychiatry. 1999;60:824–830. doi: 10.4088/jcp.v60n1204. [DOI] [PubMed] [Google Scholar]
- 63.Feiger A, Kiev A, Shrivastava RK, et al. Nefazodone versus sertraline in outpatients with major depression: focus on efficacy, tolerability, and effects on sexual function and satisfaction. J Clin Psychiatry. 1996;57(Suppl 2):53–62. [PubMed] [Google Scholar]
- 64.Feiger AD, Tourian KA, Rosas GR, et al. A placebo-controlled study evaluating the efficacy and safety of flexible-dose desvenlafaxine treatment in outpatients with major depressive disorder. CNS Spectr. 2009;14:41–50. doi: 10.1017/s1092852900020046. [DOI] [PubMed] [Google Scholar]
- 65.Fontaine R, Ontiveros A, Elie R, et al. A double-blind comparison of nefazodone, imipramine, and placebo in major depression. J Clin Psychiatry. 1994;55:234–241. [PubMed] [Google Scholar]
- 66.Fournier J. A double-blind comparison of sertraline and imipramine in outpatients with major depression: acute 8 weeks and continuation 16 weeks treatment. Hum Psychopharm. 1997;12:203–215. [Google Scholar]
- 67.Gentil V, Kerr-Correa F, Moreno R. Double-blind comparison of venlafaxine and amitriptyline in outpatients with major depression with or without melancholia. J Psychopharm. 2000;14:61–66. doi: 10.1177/026988110001400108. [DOI] [PubMed] [Google Scholar]
- 68.Golden RN, Nemeroff CB, McSorley P, et al. Efficacy and tolerability of controlled-release and immediate-release paroxetine in the treatment of depression. J Clin Psychiatry. 2002;63:577–584. doi: 10.4088/jcp.v63n0707. [DOI] [PubMed] [Google Scholar]
- 69.Goldstein DJ, Mallinckrodt C, Lu Y, et al. Duloxetine in the treatment of major depressive disorder: a double-blind clinical trial. J Clin Psychiatry. 2002;63:225–231. doi: 10.4088/jcp.v63n0309. [DOI] [PubMed] [Google Scholar]
- 70.Goldstein DJ, Lu Y, Detke MJ, et al. Duloxetine in the treatment of depression: a double-blind placebo-controlled comparison with paroxetine. J Clin Psychopharm. 2004;24:389–399. doi: 10.1097/01.jcp.0000132448.65972.d9. [DOI] [PubMed] [Google Scholar]
- 71.Hewett K, Chrzanowski W, Schmitz M, et al. Eight-week, placebo-controlled, double-blind comparison of the antidepressant efficacy and tolerability of bupropion XR and venlafaxine XR. J Psychopharmacol. 2009;23:531–538. doi: 10.1177/0269881108089602. [DOI] [PubMed] [Google Scholar]
- 72.Hewett K, Gee MD, Krishen A, et al. Double-blind, placebo-controlled comparison of the antidepressant efficacy and tolerability of bupropion XR and venlafaxine XR. J Psychopharmacol. 2010;24:1209–1216. doi: 10.1177/0269881109106953. [DOI] [PubMed] [Google Scholar]
- 73.Hicks JA, Argyropoulos SV, Rich AS, et al. Randomised controlled study of sleep after nefazodone or paroxetine treatment in out-patients with depression. Br J Psychiary. 2002;180:528–535. doi: 10.1192/bjp.180.6.528. [DOI] [PubMed] [Google Scholar]
- 74.Higuchi T, Hong JP, Jung HY, et al. Paroxetine controlled-release formulation in the treatment of major depressive disorder: a randomized, double-blind, placebo-controlled study in Japan and Korea. Psychiatry Clin Neurosci. 2011;65:655–663. doi: 10.1111/j.1440-1819.2011.02243.x. [DOI] [PubMed] [Google Scholar]
- 75.Hong CJ, Hu WH, Chen CC, et al. A double-blind, randomized, group-comparative study of the tolerability and efficacy of 6 weeks’ treatment with mirtazapine or fluoxetine in depressed Chinese patients. J Clin Psychiatry. 2003;64:921–926. doi: 10.4088/jcp.v64n0810. [DOI] [PubMed] [Google Scholar]
- 76.Hsu JW, Su TP, Huang CY, et al. Faster onset of antidepressant effects of citalopram compared with sertraline in drug-naïve first-episode major depressive disorder in a Chinese population: a 6-week double-blind, randomized comparative study. J Clin Psychopharmacol. 2011;31:577–581. doi: 10.1097/JCP.0b013e31822c091a. [DOI] [PubMed] [Google Scholar]
- 77.Hunter AM, Cook IA, Greenwald SD, et al. The antidepressant treatment response index and treatment outcomes in a placebo-controlled trial of fluoxetine. J Clin Neurophysiol. 2011;28:478–482. doi: 10.1097/WNP.0b013e318230da8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kasper S, Olivieri L, Di Loreto G, et al. A comparative, randomised, double-blind study of trazodone prolonged-release and paroxetine in the treatment of patients with major depressive disorder. Curr Med Res & Opinion. 2005;21:1139–1146. doi: 10.1185/030079905X53243. [DOI] [PubMed] [Google Scholar]
- 79.Keegan D, Bowen RC, Blackshaw S, et al. A comparison of fluoxetine and amitriptyline in the treatment of major depression. Int Clin Psychopharm. 1991;6:117–124. doi: 10.1097/00004850-199100620-00007. [DOI] [PubMed] [Google Scholar]
- 80.Khan A, Upton GV Venlafaxine Investigator Study Group. The use of venlafaxine in the treatment of major depression and major depression associated with anxiety: a dose-response study. J Clin Psychopharm. 1998;18:19–25. doi: 10.1097/00004714-199802000-00004. [DOI] [PubMed] [Google Scholar]
- 81.Khan A, Bose A, Alexopoulos GS, et al. Double-blind comparison of escitalopram and duloxetine in the acute treatment of major depressive disorder. Clin Drug Investig. 2007;27:481–492. doi: 10.2165/00044011-200727070-00005. [DOI] [PubMed] [Google Scholar]
- 82.Khan A, Cutler AJ, Kajdasz DK, et al. A randomized, double-blind, placebo-controlled, 8-week study of vilazodone, a serotonergic agent for the treatment of major depressive disorder. J Clin Psychiatry. 2011;72:441–447. doi: 10.4088/JCP.10m06596. [DOI] [PubMed] [Google Scholar]
- 83.Lee P, Shu L, Xu X, et al. Once-daily duloxetine 60 mg in the treatment of major depressive disorder: multicenter, double-blind, randomized, paroxetine-controlled, non-inferiority trial in China, Korea, Taiwan and Brazil. Psychiatry Clin Neurosci. 2007;61:295–307. doi: 10.1111/j.1440-1819.2007.01666.x. [DOI] [PubMed] [Google Scholar]
- 84.Leinonen E, Skarstein J Nordic Antidepressant Study Group. Efficacy and tolerability of mirtazapine versus citalopram: a double-blind, randomized study in patients with major depressive disorder. Int Clin Psychopharm. 1999;14:329–337. doi: 10.1097/00004850-199911000-00002. [DOI] [PubMed] [Google Scholar]
- 85.Lepola UM, Loft H, Reines EH. Escitalopram 10–20 mg/day is effective and well tolerated in a placebo-controlled study in depression in primary care. Int Clin Psychopharm. 2003;18:211–217. doi: 10.1097/00004850-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 86.Liebowitz MR, Manley AL, Padmanabhan SK, et al. Efficacy, safety, and tolerability of desvenlafaxine 50 mg/day and 100 mg/day in outpatients with major depressive disorder. Curr Med Res Opin. 2008;24:1877–1890. doi: 10.1185/03007990802161923. [DOI] [PubMed] [Google Scholar]
- 87.Lineberry CG, Johnston JA, Raymond RN, et al. A fixed-dose 300 mg efficacy study of bupropion and placebo in depressed outpatients. J Clin Psychiatry. 1990;51:194–199. [PubMed] [Google Scholar]
- 88.Lydiard RB, Laird LK, Morton WA, et al. Fluvoxamine, imipramine, and placebo in the treatment of depressed outpatients: effects on depression. Psychopharm Bull. 1989;25:68–70. [PubMed] [Google Scholar]
- 89.Lydiard RB, Stahl SM, Hertzman M, et al. A double-blind, placebo-controlled study comparing the effects of sertraline versus amitriptyline in the treatment of major depression. J Clin Psychiatry. 1997;58:484–491. doi: 10.4088/jcp.v58n1104. [DOI] [PubMed] [Google Scholar]
- 90.McPartlin G, Reynolds A, Anderson C. A comparison of once-daily venlafaxine XR and paroxetine in depressed outpatients treated in general practice. Primary Care Psychiatry. 1998;693:127–132. [Google Scholar]
- 91.Mehtonen OP, Sogaard J Venlafaxine 631 Study Group. Randomized, double-blind comparison of venlafaxine and sertraline in outpatients with major depressive disorder. J Clin Psychiatry. 2000;61:95–100. doi: 10.4088/jcp.v61n0204. [DOI] [PubMed] [Google Scholar]
- 92.Mendels J, Johnston R, Mattes J, et al. Efficacy and safety of bid doses of venlafaxine in a dose-response study. Psychopharm Bull. 1993;29:169–174. [PubMed] [Google Scholar]
- 93.Moller HJ, Glaser K, Leverkus F, et al. Double-blind, multicenter comparative study of sertraline versus amitriptyline in outpatients with major depression. Pharmacopsychiatry. 2000;33:206–212. doi: 10.1055/s-2000-8357. [DOI] [PubMed] [Google Scholar]
- 94.Montgomery SA, Huusom AK, Bothmer J. A randomised study comparing escitalopram with venlafaxine XR in primary care patients with major depressive disorder. Neuropsychobiology. 2004;50:57–64. doi: 10.1159/000078225. [DOI] [PubMed] [Google Scholar]
- 95.Moore N, Verdoux H, Fantino B. Prospective, multicentre, randomized, double-blind study of the efficacy of escitalopram versus citalopram in outpatient treatment of major depressive disorder. Int Clin Psychopharm. 2005;20:131–137. doi: 10.1097/00004850-200505000-00002. [DOI] [PubMed] [Google Scholar]
- 96.Nemeroff CB, Thase ME EPIC 014 Study Group. A double-blind, placebo-controlled comparison of venlafaxine and fluoxetine treatment in depressed outpatients. J Psychiatr Res. 2007;41:351–359. doi: 10.1016/j.jpsychires.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 97.Nierenberg AA, Greist JH, Mallinckrodt CH, et al. Duloxetine versus escitalopram and placebo in the treatment of patients with major depressive disorder: onset of antidepressant action, a non-inferiority study. Curr Med Res Opin. 2007;23:401–416. doi: 10.1185/030079906X167453. [DOI] [PubMed] [Google Scholar]
- 98.Noguera R, Altuna R, Alvarez E, et al. Fluoxetine vs clomipramine in depressed patients: a controlled multicentre trial. J Aff Disorders. 1991;22:119–124. doi: 10.1016/0165-0327(91)90045-t. [DOI] [PubMed] [Google Scholar]
- 99.Ohrberg S, Christiansen PE, Severin B, et al. Paroxetine and imipramine in the treatment of depressive patients in psychiatric practice. Acta Psychiatrica Scandinavica. 1992;86:437–444. doi: 10.1111/j.1600-0447.1992.tb03294.x. [DOI] [PubMed] [Google Scholar]
- 100.Ontiveros A. A double-blind, comparative study of paroxetine and fluoxetine in out-patients with depression. Br J Clin Res. 1997;8:23–32. [Google Scholar]
- 101.Ou JJ, Xun GL, Wu RR, et al. Efficacy and safety of escitalopram versus citalopram in major depressive disorder: a 6-week, multicenter, randomized, double-blind, flexible-dose study. Psychopharmacology. 2011;213:639–646. doi: 10.1007/s00213-010-1822-y. [DOI] [PubMed] [Google Scholar]
- 102.Owens MJ, Krulewicz S, Simon JS, et al. Estimates of serotonin and norepinephrine transporter inhibition in depressed patients treated with paroxetine or venlafaxine. Neuropsychopharmacology. 2008;33:3201–3212. doi: 10.1038/npp.2008.47. [DOI] [PubMed] [Google Scholar]
- 103.Patris M, Bouchard JM, Bougerol T, et al. Citalopram versus fluoxetine: a double-blind, controlled, multicentre, phase III trial in patients with unipolar major depression treated in general practice. Int Clin Psychopharm. 1996;11:129–136. [PubMed] [Google Scholar]
- 104.Perry PJ, Garvey MJ, Kelly MW, et al. A comparative trial of fluoxetine versus trazodone in outpatients with major depression. J Clin Psychiatry. 1989;50:290–294. [PubMed] [Google Scholar]
- 105.Peselow E, Filippi AM, Goodnick P, et al. The short and long-term efficacy of paroxetine HCl: Data from a 6-week double-blind parallel design trial vs imipramine and placebo. Psychopharm Bull. 1989;25:267–271. [PubMed] [Google Scholar]
- 106.Reimherr FW, Chouinard G, Cohn CK, et al. Antidepressant efficacy of sertraline: a double-blind, placebo- and amitriptyline-controlled, multicenter comparison study in outpatients with major depression. J Clin Psychiatry. 1990;51(Suppl B):18–27. [PubMed] [Google Scholar]
- 107.Rickels K, Smith WT, Glaudin V, et al. Comparison of two dosage regimens of fluoxetine in major depression. J Clin Psychiatry. 1985;46:38–41. [PubMed] [Google Scholar]
- 108.Rickels K, Schweizer E, Clary C, et al. Nefazodone and imipramine in major depression: a placebo-controlled trial. Br J Psychiary. 1994;164:802–805. doi: 10.1192/bjp.164.6.802. [DOI] [PubMed] [Google Scholar]
- 109.Rickels K, Athanasiou M, Robinson DS, et al. Evidence for efficacy and tolerability of vilazodone in the treatment of major depressive disorder: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2009;70:326–333. doi: 10.4088/jcp.08m04637. [DOI] [PubMed] [Google Scholar]
- 110.Roth D, Mattes J, Sheehan KH, et al. A double-blind comparison of fluvoxamine, desipramine and placebo in outpatients with depression. Progr Neuro-Psychopharm & Biol Psychiatry. 1990;14:929–939. doi: 10.1016/0278-5846(90)90078-u. [DOI] [PubMed] [Google Scholar]
- 111.Rudolph RL, Fabre LF, Feighner JP, et al. A randomized, placebo-controlled, dose-response trial of venlafaxine hydrochloride in the treatment of major depression. J Clin Psychiatry. 1998;59:116–122. doi: 10.4088/jcp.v59n0305. [DOI] [PubMed] [Google Scholar]
- 112.Rudolph RL, Feiger AD. A double-blind, randomized, placebo-controlled trial of once-daily venlafaxine extended release XR and fluoxetine for the treatment of depression. J Aff Disorders. 1999;56:171–181. doi: 10.1016/s0165-0327(99)00067-1. [DOI] [PubMed] [Google Scholar]
- 113.Samuelian JC, Hackett D. A randomized, double-blind, parallel-group comparison of venlafaxine and clomipramine in outpatients with major depression. J Psychopharm. 1998;12:273–278. doi: 10.1177/026988119801200307. [DOI] [PubMed] [Google Scholar]
- 114.Sauer H, Huppertz-Helmhold S, Dierkes W. Efficacy and safety of venlafaxine ER vs amitriptyline ER in patients with major depression of moderate severity. Pharmacopsychiatry. 2003;36:169–175. doi: 10.1055/s-2003-43052. [DOI] [PubMed] [Google Scholar]
- 115.Schweizer E, Feighner J, Mandos LA, et al. Comparison of venlafaxine and imipramine in the acute treatment of major depression in outpatients. J Clin Psychiatry. 1994;55:104–108. [PubMed] [Google Scholar]
- 116.Septien-Velez L, Pitrosky B, Padmanabhan SK, et al. A randomized, double-blind, placebo-controlled trial of desvenlafaxine succinate in the treatment of major depressive disorder. Int Clin Psychopharmacol. 2007;22:338–347. doi: 10.1097/YIC.0b013e3281e2c84b. [DOI] [PubMed] [Google Scholar]
- 117.Sheehan DV, Croft HA, Gossen ER, et al. Extended-release Trazodone in Major Depressive Disorder: A Randomized, Double-blind, Placebo-controlled Study. Psychiatry. 2009;6:20–33. [PMC free article] [PubMed] [Google Scholar]
- 118.Shrivastava RK, Shrivastava SH, Overweg N, et al. A double-blind comparison of paroxetine, imipramine, and placebo in major depression. J Clin Psychiatry. 1992;53(Suppl):48–51. [PubMed] [Google Scholar]
- 119.Smith WT, Glaudin V. A placebo-controlled trial of paroxetine in the treatment of major depression. J Clin Psychiatry. 1992;53(Suppl):36–39. [PubMed] [Google Scholar]
- 120.Swann AC, Bowden CL, Rush AJ, et al. Desipramine versus phenelzine in recurrent unipolar depression: clinical characteristics and treatment response. J Clin Psychopharm. 1997;17:78–83. doi: 10.1097/00004714-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 121.Thase ME The Venlafaxine XR, 209 Study Group. Efficacy and tolerability of once-daily venlafaxine extended release XR in outpatients with major depression. J Clin Psychiatry. 1997;58:393–398. doi: 10.4088/jcp.v58n0904. [DOI] [PubMed] [Google Scholar]
- 122.Tourian KA, Padmanabhan SK, Groark J, et al. Desvenlafaxine 50 and 100 mg/d in the treatment of major depressive disorder: an 8-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group trial and a post hoc pooled analysis of three studies. Clin Ther. 2009;31:1405–1423. doi: 10.1016/j.clinthera.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 123.Tylee A, Beaumont G, Bowden MW. A double-blind, randomized, 12-week comparison study of the safety and efficacy of venlafaxine and fluoxetine in moderate to severe depression in general practice. Primary Care Psychiatry. 1997;3:51–58. [Google Scholar]
- 124.Wade A, Lemming OM, Bang Hedegaard K. Escitalopram 10 mg/day is effective and well tolerated in a placebo-controlled study in depression in primary care. Int Clin Psychopharm. 2002;17:95–102. doi: 10.1097/00004850-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 125.Wade A, Gembert K, Florea I. A comparative study of the efficacy of acute and continuation treatment with escitalopram versus duloxetine in patients with major depressive disorder. Curr Med Res Opin. 2007 Jul;23(7):1605–14. doi: 10.1185/030079907x210732. [DOI] [PubMed] [Google Scholar]
- 126.Walczak DD, Apter JT, Halikas JA, et al. The oral dose-effect relationship for fluvoxamine: a fixed-dose comparison against placebo in depressed outpatients. Ann Clin Psychiatry. 1996;8:139–151. doi: 10.3109/10401239609147751. [DOI] [PubMed] [Google Scholar]
- 127.Weisler RH, Johnston JA, Lineberry CG, et al. Comparison of bupropion and trazodone for the treatment of major depression. J Clin Psychopharm. 1994;14:170–179. [PubMed] [Google Scholar]
- 128.Wernicke JF, Dunlop SR, Dornseif BE, et al. Low-dose fluoxetine therapy for depression. Psychopharm Bull. 1988;24:183–188. [PubMed] [Google Scholar]
- 129.Wernicke JF, Dunlop SR, Dornseif BE, et al. Fixed-dose fluoxetine therapy for depression. Psychopharm Bull. 1987;23:164–168. [PubMed] [Google Scholar]
- 130.Yevtushenko VY, Belous AI, Yevtushenko YG, et al. Efficacy and tolerability of escitalopram versus citalopram in major depressive disorder: a 6-week, multicenter, prospective, randomized, double-blind, active-controlled study in adult outpatients. Clin Ther. 2007;29:2319–2332. doi: 10.1016/j.clinthera.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 131.Fava M, Mischoulon D, Iosifescu D, et al. A double-blind, placebo-controlled study of aripiprazole adjunctive to antidepressant therapy among depressed outpatients with inadequate response to prior antidepressant therapy )ADAPT-A Study) Psychother Psychosom. 2012;81:87–97. doi: 10.1159/000332050. [DOI] [PubMed] [Google Scholar]