Abstract
Because retinoic acid (RA) exerts a stimulatory effect on macrophages and tubercle bacilli target alveolar macrophages, the therapeutic potential of RA was examined in rats with tuberculosis. In the main study, 15 rats were randomized to treatment with oil (control) or RA,100 µg/100 g body weight per dose, given 3 times weekly for 3 and 5 wk after infection with Mycobacterium tuberculosis strain H37Rv. There was a significant difference in the severity of tuberculosis histopathology between control and RA-treated rats, and oral administration of RA decreased the number of colony-forming units (CFU) in both lung and spleen at 3 and 5 wk after H37Rv infection (P < 0.005). CD4-positive and CD8-positive T cells, natural killer cells, and CD163-positive macrophages increased (P < 0.05) in the infected lung tissues of RA-treated rats. Expression of IFNγ and inducible nitric oxide synthetase messenger RNA (mRNA) was higher in the infected lung tissues of RA-treated rats than in control rats. Alveolar macrophages from rats treated in vivo with RA and infected in vitro with M. tuberculosis showed significantly higher expression of TNFα and IL-1β mRNA than macrophages in control rats. To our knowledge, this is the first reported study to demonstrate that orally administered RA significantly inhibits the in vivo growth of M. tuberculosis and the development of tuberculosis.
Introduction
All-trans-retinoic acid (RA)6, an active form of vitamin A, has the ability to reduce human mortality through effects that are considered to be related to the immune system (1). Vitamin A deficiency results in multiple abnormalities of innate and adaptive immunity involving cell differentiation, hematopoiesis, and blood and lymphoid organ cell populations, and the organism’s ability to respond to challenges by pathogens, antigens, and mitogens. In normal animals, RA has been shown to stimulate innate and adaptive immune responses (2–5). For example, aging rats chronically fed a marginal vitamin A diet had decreased numbers of peripheral blood mononuclear cells and the cell lytic efficacy of natural killer (NK) cells was reduced, as well as changes in the distribution and function of T cells, B cells, and NKT cells (6–8). RA regulates IFNγ-induced IFN regulatory factor (IRF)-1 transcription factor by affecting multiple components of the IFNγ-signaling pathways (9). RA is also used for the treatment of severe cystic acne, severe psoriasis, and acute promyelocytic leukemia, for which it induces differentiation (10).
Tuberculosis has been recognized as a major infectious disease worldwide; one-third of the world’s population is infected with tubercle bacilli and is thus at risk for reactivation disease when immunity fails. Tubercle bacilli harbor and replicate in alveolar macrophages whose functions are affected by tubercle bacilli. RA also influences macrophage functions (8). RA is protective when added after infection at a pharmacologic concentration of 10−5 mol/L and when added before infection at a near-physiologic concentration of 10−7 mol/L (11). Synergistic actions of vitamin D and RA are able to downregulate tryptophan-aspartate-containing coat protein (TACO) gene transcription in human macrophages (12). It has also been reported that the entry and intracellular survival of Mycobacterium tuberculosis is significantly restricted in THP-1 macrophages exposed to chenodeoxycholic acid/RA (13).
Taken together, these findings suggest that RA may have therapeutic potential for tuberculosis. In this study, we examined the therapeutic efficacy of orally administered RA on development of rat tuberculosis after aerosol infection with M. tuberculosis.
Materials and Methods
Animals
Specific pathogen-free and viral antibody-free Wistar-Lewis rats (LEW/CrlCrlj), 7–10 wk old, were purchased from Charles River Laboratories, Japan. The rats were housed in a biohazard level 3 facility and consumed nonpurified diet (Rat chow II, Charles River Japan) and water ad libitum throughout the entire experimental period. All animal procedures were approved by the Ethical Committee of The Research Institute of Tuberculosis.
Experimental infections
M. tuberculosis H37Rv (ATCC27294) was grown in Middlebrook 7H9 broth with 0.05% Tween 80 for 2 wk. Then, the broth was filtered with an Acrodisk filter no. 4650 (pore size 5.0 µm, Pall) to disperse bacillary clumps. The filtered bacillary solution was then stored frozen at −80°C until use. The rats were infected via the aerosol route using a Glas-Col aerosol generator, in which the nebulizer compartment was filled with 5 mL of a suspension containing 107 colony-forming units (CFU) of H37Rv bacilli under conditions that would allow ~500 bacilli to be inhaled by each rat. Similar experiments were performed twice with similar results and 1 experiment is shown for the 2 studies. The number of viable bacteria in the lungs was determined at specific time points by plating 10-fold serial dilutions of individual partial organ homogenates on 1% Ogawa’s medium and counting bacterial colonies after 4 wk of incubation at 37°C, as previously described (14).
Oral administration of vitamin A as RA
After aerosol infection with tubercle bacilli, all-trans-RA, the most bioactive form of vitamin A, was administered orally. The RA solution, ~5 g/L, was prepared by dissolving 12 mg RA in 120 µL ethanol and mixing with 120 µL of Tween 80 and 2.2 g corn oil, similar to the preparation described elsewhere (5). The RA stock solution and control solution without RA were stored at 4°C for as long as 2 wk. During the preparation of the RA dose, care was taken not to expose the pure compound or the dose preparation to bright light or air.
Oral administration was started from the day after infection (d1) and continued for 5 consecutive days (d5). From the week after infection until the end of wk 4, oral administration was performed 3 times per week on alternate days. The RA dose, which was equivalent to 100 µg RA/100 g body weight per dose, was given to rats (n = 15). For control rats (n = 15), the vehicle dose was given in the same manner as that for RA-treated rats. Oral administration was performed throughout the experimental period in a safety cabinet placed in a level 3 biosafety facility without illumination inside the cabinet, but with room illumination, to avoid degradation of the RA.
CFU assay
At 1, 3, and 5 wk after aerosol infection, groups of 3 rats were anesthetized with pentobarbital sodium, the abdominal cavities were incised, and exsanguination was carried out by splenectomy and transection of the left renal artery and vein. The lungs, spleens, and livers were excised and weighed. Part of the right lower lobes of the lungs and part of the spleen were weighed separately and used to evaluate the in vivo growth of M. tuberculosis (14). The lung and spleen samples for in vivo CFU assay were each homogenized with a mortar and pestle and then placed in test tubes and 1 mL of sterile saline was added to each sample. After homogenization, 100 µL of the homogenate was plated in 10-fold serial dilutions on 1% Ogawa slant medium. Colonies on the medium were counted after 4 wk of incubation at 37°C (14,15).
RNA extraction and real-time PCR
Another portion of the remaining right lower lobe of the lungs and the spleen were used for RT-PCR analysis to examine the expression levels of several cytokine messenger RNA (mRNA) in these samples during M. tuberculosis infection. These samples were snap-frozen in liquid nitrogen and stored at −85°C until use. RNA extraction was performed as described previously (14). Briefly, the frozen tissues were homogenized in a microcentrifuge tube with an autoclaved disposable 1000-µL tip cooled by dipping in liquid nitrogen. Then the homogenates were treated with 1 mL of TRIzol reagent (Invitrogen Japan, K.K.), as specified by the manufacturer. After RNA isolation, total RNA concentration was measured with a spectrophotometer and the agarose gel electrophoresis pattern of the total RNA was examined. The total RNA were reverse transcribed into cDNA with Moloney murine leukemia virus RT (Invitrogen). ABI Taqman Gene Expression Assay was used for relative quantitative measurement of the mRNA expression of IFNγ (Rn00594078_m1), TNFα (Rn00562055_m1), inducible nitric oxide synthetase (iNOS) (Rn00561646_m1), IL-1β (Rn00580432_m1), IL-2 (Rn00587673_m1), IL-4 (Rn01456866_m1), IL-6 (Rn00561420_m1), IL-10 (Rn00563409_m1), IL-12 p40 (Rn00575112_m1), IP-10 (CXCL-10, Rn00594648_m1), and TGFβ (Rn00572010_m1). A TaqMan Rodent GAPDH Control Reagents set was used for normalization for data analysis. Real-time RT-PCR was performed according to the instructions for the ABI PRISM 7900HT Sequence Detection system (Applied BioSystems). Data were analyzed by the ΔΔCT method using to the ABI PRISM Sequence Detection system software package (version 2.1; Applied BioSystems) working on a Windows 2000 OS. The results obtained from RA-treated and control rats were expressed relatively, with expression in the targets compared with those of uninfected rats that were calibrated with the expression of an internal control gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (16).
Effect of in vitro RA treatment on bacterial burden and cytokine mRNA expression
Alveolar macrophages were prepared from uninfected rats to examine mRNA expression after RA treatment and H37Rv infection. After the rats had been anesthetized with pentobarbital, alveolar macrophages were obtained by bronchoalveolar lavage (17). Briefly, the trachea was cannulated and 2.5 mL of physiological saline was introduced. The saline was recovered using a 5-mL disposable syringe. After bronchoalveolar cells that were collected were cultured at 37°C on petri dishes for 6 h, the cells collected consisted of >99% macrophages, as assessed by cell morphology. The cell suspensions (1 × 107 cells) were plated in 50-cm2 culture flasks and incubated in RPMI1640 medium supplemented with 10% FCS and 10 µL of 2 mmol/L RA for 24 h at 37°C in 5% CO2 in air. For the control experiment, 10 µL of absolute ethanol was added to the culture instead of RA. Then, the cells were stimulated with live M. tuberculosis H37Rv with a multiplicity of infection of 50:1 for 18 h. The cells were transferred to a 50-mL centrifugation tube and the cells were recovered after centrifugation. TRIzol reagent was added to the pellets and total RNA were extracted. cDNA were prepared by RT and real-time PCR analyses were done using the cDNA according to the methods described previously (16). GAPDH mRNA expression of uninfected broncheoalveolar lavage (BAL) cells without RA treatment was used for calibration and GAPDH mRNA expression in each cell group was used for normalization.
Histopathological examination
For light microscopy, the left middle lobe of the lung was excised and fixed with a 20% formalin-buffered methanol solution, Mildform 20NM (containing 8% formaldehyde and 20% methanol) (Wako Pure Chemical), dehydrated with a graded ethanol series, treated with xylene, and embedded in paraffin. Sections 5 µm thick were cut from each paraffin block and stained with either hematoxylin and eosin or Ziehl-Neelsen stain for acid-fast bacilli (14,18).
FACS analysis
Pulmonary mononuclear cells were isolated and stained for fluorescence-activated cell sorter (FACS) using reagents and methods similar to those described previously (14). After blocking with 5% bovine serum albumin, the cells were stained for 20 min at 4°C with monoclonal antibodies specific for various rat monocytic and lymphoid cells. These included phycoerythrin-conjugated OX62 (CD103, dendritic cell); fluorescein isothiocyanate-conjugated ED1 (CD68, dendritic cell/macrophage/monocyte); OX8 (CD8); OX52 (CD6, T lymphocyte), anti-rat W3/25 (CD4 T-cell); 10/78 (CD161, NK cells and T cell), R73 (α/β T-cell), and V65 (γ/δT-cell). Thereafter, the cells were fixed in 2% paraformaldehyde/PBS, examined with a FACS (FACSCAN, BD), and analyzed with Cell Quest software (Pharmingen). For ED1 immunostaining, the mononuclear cells (1 × 109/L) were made permeable with Leukoperm (Serotec) for 15 min before reaction with ED1 (19).
Statistical methods
Values are means ± SE for the number of replicates indicated. Differences between 2 groups were determined by Student’s t test and differences between multiple groups were determined by ANOVA and a paired or unpaired t-test as a post hoc test. Differences of P < 0.05 were considered significant.
Results
Mycobacterial burden in the lungs and spleen
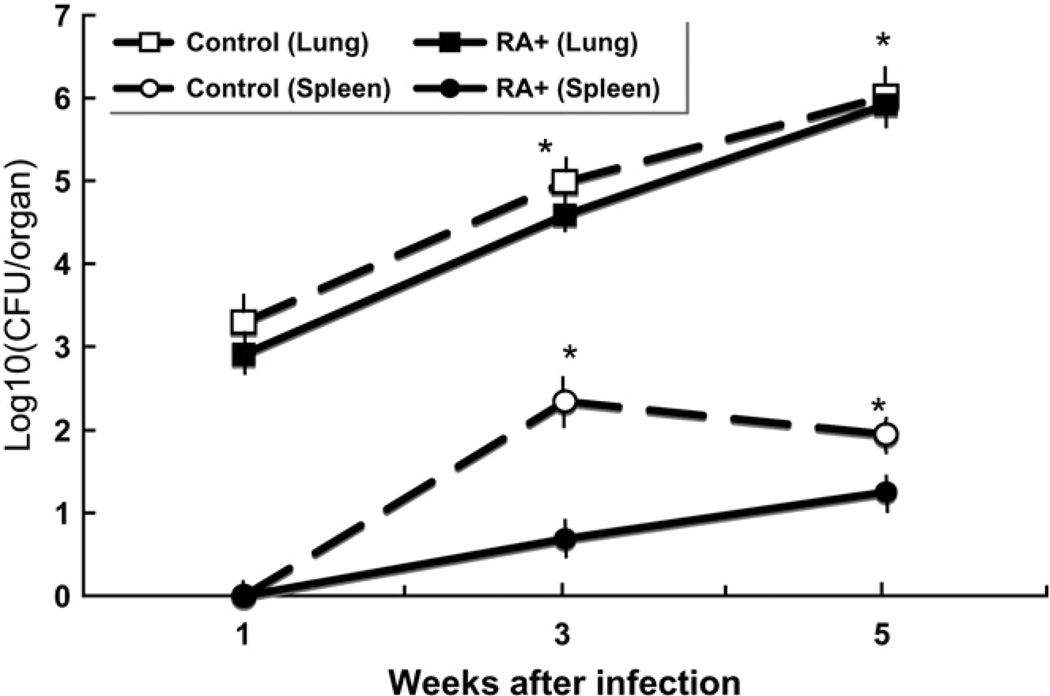
We examined the in vivo mycobacterial burdens of M. tuberculosis infected rats with or without oral administration of RA. After H37Rv infection and 3 and 5 wk of oral administration of RA, the number of CFU in both the lungs and spleen was lower at each time shown (P < 0.05; Fig. 1) compared with the untreated group.
FIGURE 1.
RA administration (RA+) significantly decreased lung and spleen CFU in M. tuberculosis H37Rv-infected rats. Values are means ± SE, n = 3. *Different from RA+ at that time, P < 0.005.
Light microscopic observation of infected lungs
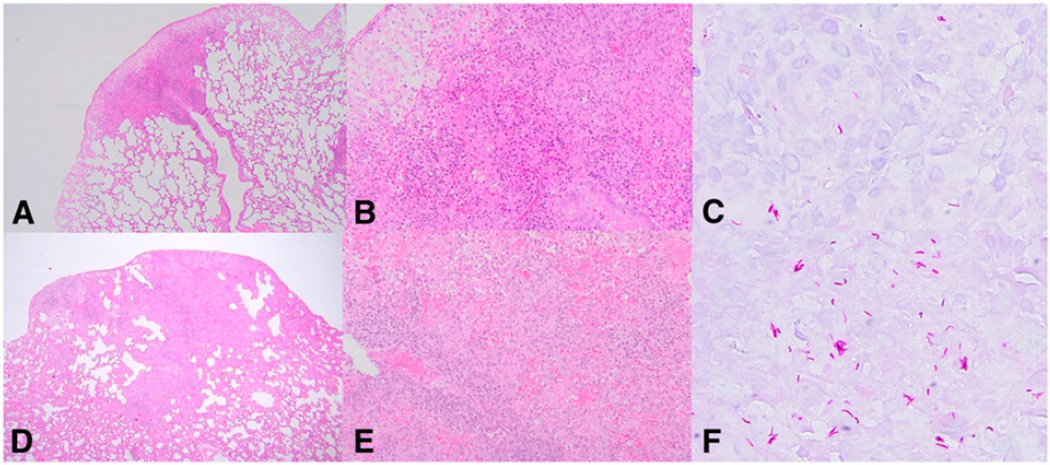
Representative light micrographs of infected lung tissues obtained from RA-treated rats are shown in Fig. 2, A–C and from control rats in Fig. 2, D–F. Lung tissue of RA-treated rats had smaller lesion areas (Fig. 2A vs. 2D) and fewer acid-fast bacilli in the lesions than control rats (Fig. 2C vs. 2F). In control rat lung tissue, typical granulomas were formed, where many lymphocytes surrounded epithelioid cells (Fig. 2D,E). Although typical granulomas were formed similarly in the lungs of RA-treated rats (Fig. 2A,B), the granulomas were smaller and contained fewer cells than those in control rats.
FIGURE 2.
Histopathology of M. tuberculosis H37Rv-infected RA-treated rats (A–C) and H37Rv-infected control rats (D–F). RA-treated rats showed smaller lesion areas (A,B) and fewer acid-fast bacilli in the lesions (C) than control rats (D–F). A, B, D, and E show hematoxylin and eosin staining; magnification ×135 for A and D, ×270 for B and E. C and F show Ziehl-Neelsen staining, magnification ×600.
Cell analysis by flow cytometry
To examine the cell populations comprising the lung lesions, we performed FACS analysis of the cells in lung homogenates. FACS analysis revealed that CD4-positive T cells, CD8-positive T cells, NK cells, α/β T cells, CD25-positive T cells, and CD163-positive monocyte/macrophages were higher (P < 0.05) in RA-treated rats compared with those in control rats 3 wk after infection (Table 1).
TABLE 1.
Flow cytometric analysis of lung BAL cells from M. tuberculosis H37Rv-infected RA-treated (RA+) and control (RA−) rats 3 and 5 wk postinfection1
| Relative intensity | ||||
|---|---|---|---|---|
| 3 wk after infection | 5 wk after infection | |||
| Cell subpopulation | RA+ | RA− | RA+ | RA− |
| CD4 | 4.1 ± 0.2* | 1.5 ± 0.1 | 7.2 ± 0.3 | 7.1 ± 0.3 |
| CD8 | 3.1 ± 0.1* | 2.1 ± 0.1 | 4.4 ± 0.1 | 7.3 ± 0.3 |
| NK | 4.6 ± 0.1* | 1.9 ± 0.1 | 5.0 ± 0.1 | 5.6 ± 0.2 |
| α/β T | 5.1 ± 0.2* | 2.2 ± 0.2 | 13.0 ± 0.7 | 12.2 ± 0.4 |
| γ/δ T | 0.3 ± 0.02 | 0.35 ± 0.03 | 0.4 ± 0.01 | 0.7 ± 0.03 |
| CD25 | 0.9 ± 0.04* | 0.2 ± 0.01 | 0.6 ± 0.03 | 0.7 ± 0.02 |
| CD103 | 4.1 ± 0.2 | 4.2 ± 0.3 | 7.0 ± 0.5 | 10.0 ± 0.09 |
| CD163 | 0.7 ± 0.05* | 0.3 ± 0.01 | 0.8 ± 0.06 | 0.6 ± 0.02 |
Values are means ± SEM, n = 3.
Different from RA at that time, P < 0.05.
Cytokine and enzyme mRNA by real-time PCR
Next, we performed real-time PCR analysis using cDNA reverse transcribed from total RNA extracted from lung and spleen tissues of rats treated in vivo. The data thus obtained were expressed as an intensity relative to GAPDH. In the lung, expression of IFNγ and iNOS mRNA differed with RA treatment at 5 wk, when expression was highest. For TNFα and IL-1β mRNA, expression was affected by RA at both 3 wk and 5 wk. Expression of IL-2, IL-12βR, and IP-10 mRNA differed at either 1 wk or 3 wk, but not at 5 wk. Thus, RA affected several cytokines in the lung in temporally different patterns (Supplemental Table 1).
In contrast, in spleen tissues, expression of mRNA for IFNγ, TNFα, iNOS, IL-1β, and IL-1 in RA-treated rats was highest at 1 wk after infection and was more than double those of the control group, whereas expression of IL-6, IL-10, IL-12 p40, IP-10, and TGFβ mRNA in RA-treated rats peaked later after infection and RA did not alter expression (Supplemental Table 2).
Cytokines in infected alveolar macrophages
The level of mRNA was also determined in BAL cells collected from H37Rv-infected rats and treated with RA in vitro. For most cytokines, the level was low or undetectable in the absence of infection (data not shown). BAL macrophages treated with RA had higher levels of IFNγ, TNFα, iNOS, IL-10, and IP-10 mRNA relative to GAPDH mRNA than BAL cells cultured without RA (Table 2).
TABLE 2.
Cytokine relative mRNA levels 18-h postinfection in control (RA−) and RA-treated (RA+) rat lung BAL cells infected in vitro with M. tuberculosis H37Rv1,2
| Intensity (relative to GAPDH) | ||
|---|---|---|
| Cytokine or enzyme | RA− | RA+ |
| IFNγ | ND | 4.2 ± 0.3 |
| TNFα | 4.2 ± 0.3 | 29 ± 2* |
| iNOS | 505 ± 46 | 2506 ± 225* |
| IL-10 | 105 ± 10 | 4102 ± 230* |
| IP-10 (CXCL10) | 21 ± 2 | 118 ± 10* |
Values are means ± SEM, n = 3.
Different from RA−, P < 0.05.
Uninfected cultures had low or nondetectable (ND) levels of these cytokines and are not shown.
Discussion
In this study, we have shown that orally administered RA can inhibit the growth of M. tuberculosis in vivo. No previous report to our knowledge has described the effect of RA on the development of tuberculosis in vivo.
We utilized a Lewis rat tuberculosis model to study treatment with RA, because we had already established such a model (19) and also because rats have been used previously to examine the biological functions of RA (10). When the CFU were counted in alveolar macrophages infected with H37Rv M. tuberculosis and in RA-treated alveolar macrophages infected with H37Rv M. tuberculosis in vitro, the number decreased significantly in RA-treated macrophages (data not shown). Two previous reports have provided evidence that RA stimulates macrophages to kill tubercle bacilli. First, RA has a protective effect when it is added at a pharmacologic concentration to cultured human macrophages after infection and at a physiologic concentration when it is added before infection (11). Furthermore, entry and intracellular survival of M. tuberculosis are significantly restricted in THP-1 human macrophage-like cells exposed to chenodeoxycholic acid and RA (13). It has been reported that iNOS plays an important role in killing tubercle bacilli (17). As RA itself does not induce iNOS expression in vitro, another mechanism for killing M. tuberculosis may exist. Recently, it was shown that TACO plays a crucial role in the entry/survival of M. tuberculosis within human macrophages (13). Because RA downregulates TACO gene transcription, TACO may be involved in killing M. tuberculosis. Further study will be required to clarify this possibility.
The levels of TNFα, IL-1β, and iNOS mRNA expression are elevated in the lung tissues of rats with tuberculosis treated orally with RA and BAL cells from M. tuberculosis-infected rats treated with RA in vitro showed similar effects. These cytokines play important roles in the defense against tuberculosis (15,17,18). These molecules function as anti-tuberculosis immune factors. There is another possible mechanism related to defense against tuberculosis. IRF-1, a transcription factor and tumor suppressor involved in cell growth regulation and immune responses, has been shown to be induced by RA (9,20). We have also reported that M. tuberculosis-infected mice lacking IRF-1 expression die of disseminated tuberculosis (21). Thus, RA may regulate IFN-induced IRF-1 functions by affecting multiple components of the IFN-signaling pathways. In normal animals, RA has been shown to stimulate innate and adaptive immune responses (2–5). Thus, it is thought that RA stimulates immune cells to secrete cytokines and that its functions are multiple.
Histopathologically, there was a significant difference in the sizes of pulmonary granulomas between RA-treated and control rats in this study. RA has an inherent capacity to increase the constituent cells in granulomas, as evaluated by FACS. As stated above, RA may also be involved in killing tubercle bacilli (13). It has been suggested that RA, like vitamin D, may have some immunoprotective role against tuberculosis, as historically intimated by the regular use of vitamin A- and D-rich cod liver oil for the prevention of tuberculosis before the introduction of modern chemotherapy (11). RA-dependent downregulation of TACO, which has now been shown to be regulated by these nutrient metabolites, may be a promising new avenue for the treatment of tuberculosis.
Supplementary Material
Footnotes
Supported by a research grant from the Ministry of Health, Welfare, and Labor, Japan (I.S.) and NIH grant no. DK-41479 (A.C.R.).
Author disclosures: H. Yamada, S. Mizuno, A. C. Ross, and I. Sugawara, no conflicts of interest.
Supplemental Tables 1 and 2 are available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: BAL, broncheoalveolar lavage; CFU, colony-forming unit; FACS, fluorescence-activated cell sorting; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IRF, IFN regulatory factor; mRNA, messenger RNA; NK, natural killer (cells); iNOS, inducible nitric oxide synthetase; RA, retinoic acid; TACO, tryptophan-aspartate-containing coat protein.
Literature Cited
- 1.Villamor E, Fawzi WW. Effects of vitamin A supplementation on immune responses and correlation with clinical outcomes. Clin Microbiol Rev. 2005;18:446–464. doi: 10.1128/CMR.18.3.446-464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwata M, Eshima Y, Kagechika H. Retinoic acids exert direct effects on T cells to suppress T(h)1 development and enhance T(h)2 development via retinoic acid receptors. Int Immunol. 2003;15:1017–1025. doi: 10.1093/intimm/dxg101. [DOI] [PubMed] [Google Scholar]
- 3.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Ma Y, Ross AC. The anti-tetanus immune response of neonatal mice is augmented by retinoic acid combined with polyriboinosinic:polyribocytidylic acid. Proc Natl Acad Sci USA. 2005;102:13556–13561. doi: 10.1073/pnas.0506438102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma Y, Chen Q, Ross AC. Retinoic acid and polyriboinosinic:polyribocytidylic acid stimulate robust anti-tetanus antibody production while differentially regulating type 1/type 2 cytokines and lymphocyte populations. J Immunol. 2005;174:7961–7969. doi: 10.4049/jimmunol.174.12.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paterson DJ, Jefferies WA, Green JR, Brandon MR, Corthesy P, Puklavec M, Williams AF. Antigens of activated rat T lymphocytes including a molecule of 50,000 Mr detected only on CD4 positive T blasts. Mol Immunol. 1987;24:1281–1290. doi: 10.1016/0161-5890(87)90122-2. [DOI] [PubMed] [Google Scholar]
- 7.Dawson HD, Li NQ, DeCicco KL, Nibert JA, Ross AC. Chronic marginal vitamin A status reduces natural killer cell number and function in aging Lewis rats. J Nutr. 1999;129:1510–1517. doi: 10.1093/jn/129.8.1510. [DOI] [PubMed] [Google Scholar]
- 8.Chen Q, Ross AC. Retinoic acid regulates cell cycle progression and cell differentiation in human monocytic THP-1 cells. Exp Cell Res. 2004;297:68–81. doi: 10.1016/j.yexcr.2004.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo XM, Ross AC. Physiological and receptor-selective retinoids modulate interferon gamma signaling by increasing the expression, nuclear localization, and functional activity of interferon regulatory factor-1. J Biol Chem. 2005;280:36228–36236. doi: 10.1074/jbc.M505749200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross AC, Harrison EH. Vitamin A: nutritional aspects of retinoids and carotenoids. In: Rucker RR, Zempleni J, McCormick DM, Suttie JW, editors. Handbook of vitamins. 4th ed. Boca Raton (FL): CRC Press; 2007. pp. 1–40. [Google Scholar]
- 11.Crowle AJ, Ross EJ. Inhibition by retinoic acid of multiplication of virulent tubercle bacilli in cultured human macrophages. Infect Immun. 1989;57:840–844. doi: 10.1128/iai.57.3.840-844.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anand PK, Kaul D. Vitamin D3-dependent pathway regulates TACO gene transcription. Biochem Biophys Res Commun. 2003;310:876–877. doi: 10.1016/j.bbrc.2003.09.087. [DOI] [PubMed] [Google Scholar]
- 13.Anand PK, Kaul D. Downregulation of TACO gene transcription restricts mycobacterial entry/survival within human macrophages. FEMS Microbiol Lett. 2005;250:137–144. doi: 10.1016/j.femsle.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 14.Sugawara I, Yamada H, Mizuno S. Pathological and immunological profiles of rat tuberculosis. Int J Exp Pathol. 2004;85:125–134. doi: 10.1111/j.0959-9673.2004.00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamada H, Mizumo S, Horai R, Iwakura Y, Sugawara I. Protective role of interleukin-1 in mycobacterial infection in IL-1 alpha/beta double-knockout mice. Lab Invest. 2000;80:759–767. doi: 10.1038/labinvest.3780079. [DOI] [PubMed] [Google Scholar]
- 16.Yamada H, Udagawa T, Mizuno S, Hiramatsu K, Sugawara I. Newly designed primer sets available for evaluating various cytokines and iNOS mRNA expression in guinea pig lung tissues by RT-PCR. Exp Anim. 2005;54:163–172. doi: 10.1538/expanim.54.163. [DOI] [PubMed] [Google Scholar]
- 17.Sugawara I, Yamada H, Kaneko H, Mizuno S, Takeda K, Akira S. Role of interleukin-18 (IL-18) in mycobacterial infection in IL-18-gene-disrupted mice. Infect Immun. 1999;67:2585–2589. doi: 10.1128/iai.67.5.2585-2589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugawara I, Mizuno S, Yamada H, Matsumoto M, Akira S. Disruption of nuclear factor-interleukin-6, a transcription factor, results in severe mycobacterial infection. Am J Pathol. 2001;158:361–366. doi: 10.1016/S0002-9440(10)63977-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugawara I, Yamada H, Mizuno S. Pathological and immunological profiles of rat tuberculosis. Int J Exp Pathol. 2004;85:125–134. doi: 10.1111/j.0959-9673.2004.00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo XM, Ross AC. Retinoic acid exerts dual regulatory actions on the expression and nuclear localization of interferon regulatory factor-1. Exp Biol Med. 2006;231:619–631. doi: 10.1177/153537020623100517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamada H, Mizuno S, Sugawara I. Interferon regulatory factor 1 in mycobacterial infection. Microbiol Immunol. 2002;46:751–760. doi: 10.1111/j.1348-0421.2002.tb02760.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.