Abstract
Objective
Transdifferentiation of fibroblasts to endothelial cells (ECs) may provide a novel therapeutic avenue for diseases including ischemia and fibrosis. Here we demonstrate that human fibroblasts can be transdifferentiated into functional ECs by using only two factors, Oct4 and Klf4, under inductive signaling conditions.
Approach and Results
To determine if human fibroblasts could be converted into ECs by transient expression of pluripotency factors, human neonatal fibroblasts were transduced with lentiviruses encoding Oct4 and Klf4 in the presence of soluble factors that promote the induction of an endothelial program. After 28 days, clusters of induced endothelial (iEnd) cells appeared and were isolated for further propagation and subsequent characterization. The iEnd cells resembled primary human ECs in their transcriptional signature by expressing endothelial phenotypic markers such as CD31, VE-cadherin, and von Willebrand Factor. Furthermore, the iEnd cells could incorporate acetylated low density lipoprotein, and form vascular structures in vitro and in vivo. When injected into the ischemic limb of mice, the iEnd cells engrafted, increased capillary density, and enhanced tissue perfusion. During the transdifferentiation process, the endogenous pluripotency network was not activated, suggesting that this process bypassed a pluripotent intermediate step.
Conclusions
Pluripotent factor–induced transdifferentiation can be successfully applied for generating functional autologous ECs for therapeutic applications.
Keywords: angiogenesis, endothelium, peripheral vascular disease, stem cells, transdifferentiation, direct reprogramming
INTRODUCTION
Ischemic vascular disease (IVD), where blood flow to tissues is significantly reduced, is a major cause of mortality and morbidity. IVD-related diseases include peripheral arterial disease (PAD), coronary artery disease, and cerebrovascular disease, which in their later stages can result in gangrene, myocardial infarction or stroke, respectively. Pre-clinical studies of adult stem cell therapies using bone marrow mononuclear cells, mesenchymal stem cells, adipose stem cells, and endothelial progenitor cells have generated promising results, with evidence for enhanced neovascularization, reduced ischemic injury and/or improved organ functions.1–3 Clinical trials using adult stem cells, such as bone marrow mononuclear cells, also showed a modest therapeutic benefit in small clinical studies, although the results need confirmation in larger randomized clinical trials.4, 5 However, adult stem cell therapy is limited by the adverse effects of age and disease on the number and quality of adult stem and progenitor cells.6, 7 Pluripotent stem cells, such as embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs), which can be generated from a patient’s somatic cells, such as skin fibroblasts, provide another potential source of therapeutic endothelial cells. We previously demonstrated that human pluripotent stem cell–derived endothelial cells can enhance limb perfusion and angiogenesis in murine models of PAD.8, 9 However, generating human iPSCs is inefficient, laborious and time consuming. Furthermore, concerns about the incomplete differentiation and tumorigenicity of these cells are a significant obstacle to their therapeutic application. In searching for an alternative strategy for production of induced endothelial cells, we sought to generate functional endothelial cells from somatic cells directly without transiting through a pluripotent intermediate.
Nuclear reprogramming to pluripotency using current methods is inefficient. Furthermore, our understanding of the determinants of nuclear reprogramming is incomplete. However, it is clear that only a small percentage of induced cells eventually become iPSCs. Furthermore, it is also apparent that pluripotency is achieved only after extended overexpression of iPSC factors and appropriate signaling inputs. We reasoned that, at earlier timepoints, we could guide the epigenetically activated cells (induced by the iPSC factors) into lineage-specific cell types under other conditions without traversing pluripotency. We previously found that temporally controlled, transient expression of iPSC factors in mouse fibroblasts, followed by administration of lineage-specific signals, induced generation of nearly homogenous cardiac cells or neural progenitor cells rapidly without activation of pluripotency.10, 11 Here we demonstrated that this transient pluripotency-factor-based signaling-directed (TPS) transdifferentiation approach could be further applied to generate functional induced endothelial (iEnd) cells from human fibroblasts with only two factors: Oct4 and Klf4 (OK). The iEnd cells exhibit characteristic endothelial cell phenotype in vitro and in vivo and are capable of functionally promoting vascular regeneration and blood perfusion in a murine model of PAD.
In our previous TPS transdifferentation studies, we showed that three or four iPSC-factors (Oct4, Klf4, Sox2, with or without c-Myc) could initially destabilize the epigenetic state of murine fibroblasts, enabling lineage-specific cell fate by soluble factor induction.10, 11 For clinical applications, developing such strategy in human cells with no or reduced use of genetic manipulation would be highly desirable. Our recent efforts on iPSC generation showed that reprogramming conditions could be enhanced with small molecules to allow generation of iPSCs with fewer exogenously delivered transcription factors.12, 13 Given that the required ectopic expression of iPSC factors is substantially reduced in the context of TPS transdifferentiation, we hypothesized that it may be feasible to develop a condition with fewer factors for reprogramming human fibroblasts into iEnd cells.
Materials and Methods
Materials and Methods are available in the online-only Supplement.
RESULTS
OK Expression and Inductive Signaling Directs Endothelial Transdifferentiation of Human Fibroblasts
To determine if human fibroblasts could be converted into endothelial cells by this TPS transdifferentiation strategy, human neonatal fibroblasts (CRL-2097 and BJ) were transduced with lentiviruses encoding Oct4 and Klf4, and cultured in the 1:1 mixture of fibroblast medium and chemically defined endothelial cell growth medium (Figure 1A). After culturing for 6–7 days in this condition, the medium was changed to endothelial induction medium I, supplemented with basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF) and bone morphogenetic protein-4 (BMP4), which promote induction of an endothelial program.14 Extended BMP4 treatment failed to produce any cells positive for the endothelial cell marker, CD31. However, when BMP4 was withdrawn from the medium at day 14, CD31+ cells that organized into proliferative clusters became detectable by day 18. The average frequency of CD31+ cells was relatively low with only ~1% of the total cells expressing this marker at day 28. Previous studies reported that activation of cyclic AMP-dependent protein kinase (PKA) enhances endothelial specification.15 Therefore, we examined the effect of adding 8-Br-cAMP to the culture medium during days 14–28 on endothelial cell induction, and found that 8-Br-cAMP could increase endothelial transdifferentiation from human fibroblasts by nearly fourfold (3.85% efficiency) as measured by the abundance of CD31+ cells on day 28 based on fluorescence activated cell sorting (FACS; Figure I in the online-only Data Supplement).
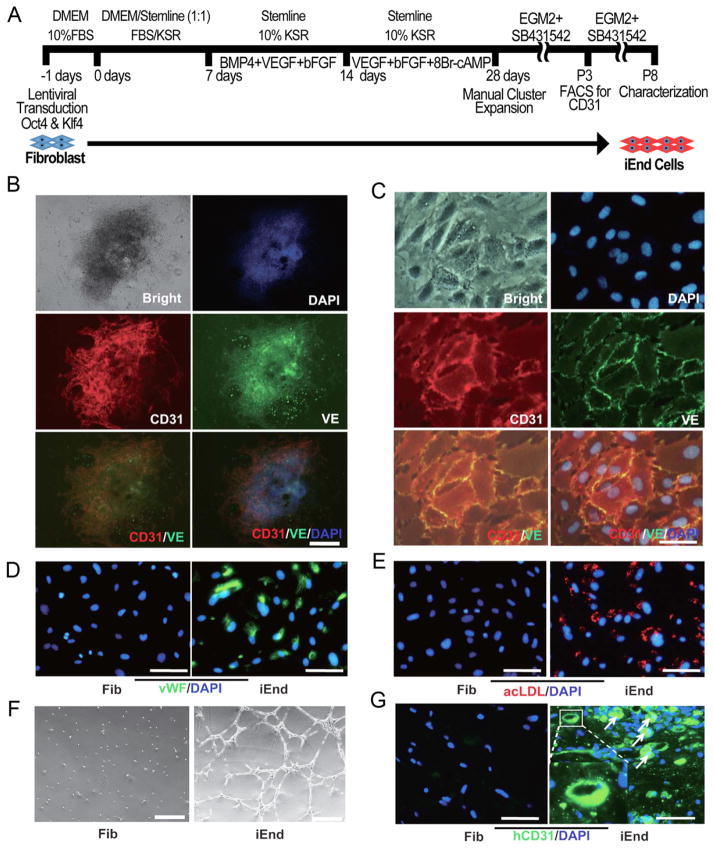
Figure 1. Reprogramming of human fibroblasts (CRL2097) to functional endothelial cells by expression of Oct4/Klf4 and sequential induction signals.
(A) Reprogramming of human fibroblasts (CRL2097) to endothelial cells. The diagram is a schematic overview of the time course and the sequential treatments with different induction media. (B) CD31 and VE-cadherin (VE) double positive cell clusters were detected on day 28. (C) Immunostaining of endothelial cell markers CD31 and VE after FACS purification showing the iEnd cells were double positive for CD31 and VE. (D) Immunostaining of endothelial cell marker vWF in iEnd cells. (E) Uptake of acetylated LDL by iEnd cells. (F) Formation of a capillary-like network on matrigel by iEnd cells, but not fibroblasts, after 24 hours. (G) Formation of capillary structures in matrigel plugs in vivo after subcutaneous transplantation in SCID mice for 2 weeks. Sections were stained using an antibody specific for human CD31. All the cell samples in (C–G) were characterized after FACS isolation and expansion of iEnd cells in EGM2 medium in the presence of 10 μM SB431542 for 5 passages. Scale bars: 200 μm (B); 20 μm (C); 50 μm (D–E); 100 μm (F); 50 μm (G).
At day 28 after induction, cells were immunostained with antibodies for the endothelial cell specific markers, CD31 and VE-Cadherin (VE). At this point, many small clusters of cells with cobblestone morphology had emerged, and nearly all clusters stained positive for these two markers (Figure 1B and Figure IIA in the online-only Data Supplement). To enrich for the iEnd cells, these clusters were manually isolated and cultured in chemically defined EGM2 endothelial expansion medium. To further enhance their expansion, we added to the EGM2 growth media SB431542, a specific TGFβ receptor inhibitor that was reported to promote ESC-derived endothelial cell growth and sheet formation,16 and observed more effective expansion of the iEnd cells.
By manually picking the endothelial-like clusters for expansion, we could enrich the purity of the iEnd cells to 61% after expansion for 3 passages based on the expression of CD31 by FACS (Figure III in the online-only Data Supplement). After FACS purification, the iEnd cells were 97% pure based on CD31+ expression. The purified iEnd cells showed the typical endothelial cobblestone morphology and were further molecularly and functionally characterized after five passages. Based on immunofluorescence staining, the iEnd cells showed co-localized expression of the endothelial markers CD31 and VE (Figure 1C and Figure IIB in the online-only Data Supplement). The iEnd cells also expressed von Willebrand factor (vWF) whereas the expression was absent in the parental fibroblasts (Figure 1D and Figure IIC in the online-only Data Supplement).
Functionally, the iEnd cells could efficiently take up fluorescently labeled acetylated low density lipoprotein (ac-LDL), a characteristic of endothelial cells (Figure 1E and Figure IID in the online-only Data Supplement). These iEnd cells also efficiently formed capillary tube-like networks when seeded onto matrigel in vitro, whereas the parental fibroblast remained rounded and failed to organize into networks (Figure 1F and Figure IIE in the online-only Data Supplement).
Furthermore, although various cell types can form networks in matrigel, only endothelial cells can form capillaries with lumens in matrigel.17 Therefore, we characterized the endothelial identity of the iEnd cells based on their functional capacity to form capillary lumens when embedded subcutaneously within matrigel plugs in SCID mice. At two weeks post implantation, histological analysis demonstrated that the iEnd cells within plugs maintained expression of human-specific CD31 and could organize to form capillaries (Figure 1G), whereas the parental fibroblasts neither expressed CD31 nor formed tubular structures in vivo. These results collectively suggested that the iEnd cells exhibited the molecular and functional hallmarks of endothelial cells in vitro and in vivo.
iEnd Cells Share Similar Transcriptome to Primary Endothelial Cells
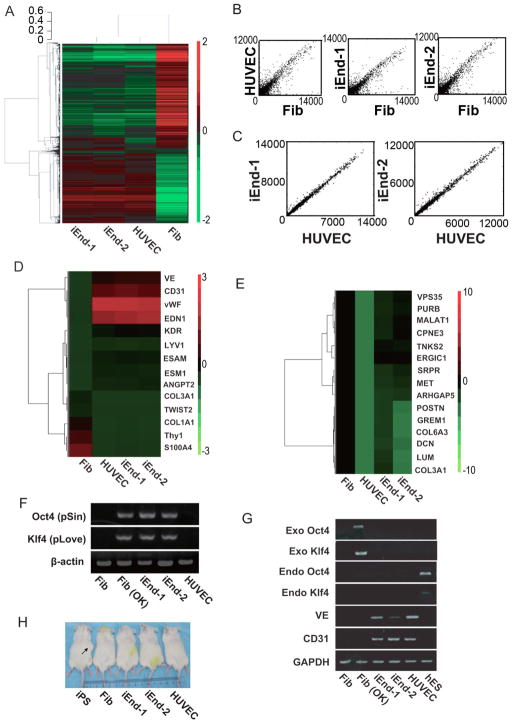
To further characterize the iEnd cells, global gene expression analysis of iEnd-1 (derived from CRL-2097), iEnd-2 (derived from BJ), human fibroblasts, and human umbilical vein endothelial cells (HUVECs) showed that iEnd cells are distinct from fibroblasts and most similar to HUVECs at the transcriptome level (Figure 2A–C). Many genes known to be involved in endothelial cell specification, such as CD31, VE, Tie-1, and vWF, were significantly up-regulated in the iEnd cells as compared to fibroblasts (Figure 2D). Concomitantly, fibroblast-specific genes such as Col1A1, Thy1, S100A4 and Twist were significantly down-regulated in the iEnd cells (Figure 2D). Consistent with what has been reported in other reprogramming studies, we also found that a small subset of fibroblast-related transcripts in the iEnd cells were not down-regulated to the level in HUVECs (Figure 2E). Although the functional significance of those few genes is unknown, this may indicate an epigenetic memory derived from the parental fibroblasts.
Figure 2. Transcriptome profiling of iEnd cells and analysis of transgene silencing.
(A) Hierarchical clustering analysis of global gene expression pattern in fibroblast, HUVECs, iEnd-1 (CRL-2097-derived iEnd cells) and iEnd-2 (BJ-derived iEnd) cells. The color bar at the right side indicates gene expression in scale. Red and green colors represent higher and lower gene expression levels, respectively. (B) Homogeneity of gene expression visualized by scatter plot. Scatter plots were drawn with the averaged intensities of gene expression in HUVECs, iEnd-1 and iEnd-2 cells against fibroblasts. (C) Homogeneity of gene expression between iEnd-1 and iEnd-2 cells with respect to HUVECs. Scatter plots were drawn with the averaged intensities of gene expression in iEnd-1 and iEnd-2 cells against HUVECs. (D) Heat map of endothelial and fibroblast markers. The expression of the marker genes is shown. (E) Heat map is shown of a subset of genes having residual expression of fibroblast-related genes in iEnd-1 and iEnd-2 cells. The heat map shows genes that have a comparable expression level in iEnd-1, iEnd-2 and fibroblast cells, but a lower expression level in HUVECs. (F) Genomic PCR to verify integration of lentiviral constructs used for direct endothelial reprogramming. β-actin was used as a loading control. Fib (OK) indicates fibroblasts transduced with OK factors. (G) An RT-PCR analysis of exogenous (exo) Oct4, exo Klf4, endogenous (endo) Oct4, endo Klf4, CD31 and VE in the indicated samples. The glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control. (H) Teratoma formation assay using 2×106 positive control iPSC, fibroblast, iEnd-1, iEnd-2 and HUVECs were injected into SCID mice. The arrow indicates teratoma formation by iPSCs at 4 weeks after injection. No teratomas could be seen in mice injected with fibroblasts, iEnd-1 cells, iEnd-2 cells or HUVECs.
Furthermore, RT-PCR analysis revealed that the integrated Oct4-pSin and Klf4-pLove transgenes (Figure 2F) were completely silenced in iEnd cells (Figure 2G). It was further confirmed by RT-PCR that the iEnd cells expressed high level of endogenous CD31 and VE, but no endogenous Oct4 and Klf4 (Figure 2G). Importantly, the iEnd cells did not generate teratomas after injection into SCID mice (Figure 2H). Taken together, our data demonstrated that the direct reprogramming of fibroblasts into endothelial cells by OK factors did not depend on sustained overexpression of the exogenous reprogramming factors.
Direct Conversion of Fibroblasts into iEnd Cells Is a Gradual Process That Bypasses iPSC Generation
To further characterize the epigenetic changes at three loci (Oct4, CD31 and VE) during the transdifferentiation, we performed chromatin immunoprecipitation (ChIP) assays on manually dissected iEnd cell clusters on days 18 and 28, in comparison to parental fibroblasts and HUVECs (Figure 3A). Relative levels of histone H3K4me3 and H3K27me3 (markers closely tied to transcriptional activation and repression respectively) in the promoter regions of these genes were examined. As expected, we observed an increase of H3K4me3 and a concomitant decrease of H3K27me3 at the Oct4 promoter at day 18 after reprogramming induction, followed by a return to the H3K27me3-dominant repressed state at day 28. In contrast, our analysis showed that both CD31 and VE promoter regions underwent a burst of activating H3K4me3 at day 18, compared with the starting fibroblasts at day 0, the level of which activation approached those of control HUVECs by day 28. Concurrently, levels of repressive H3K27me3 at CD31 and VE promoter regions significantly decreased in comparison with those of the starting fibroblasts and approached those of HUVECs at day 28. These results indicated that epigenetic transition to endothelial fate starts as early as day 18 without a parallel commitment to pluripotency.
Figure 3. Direct conversion of fibroblasts into iEnd cells is a gradual process that bypasses iPSC generation.
(A) Histone modifications during direct reprogramming. ChIP assays examining epigenetic modifications at Oct4, CD31 and VE promoter loci during reprogramming were performed in HUVECS and in the transdifferentiating cells at day 0, day 18, day 28 after TPS was initiated. Cell samples at days 18 and 28 were collected by manual selection of clusters having cobblestone-like morphology and assayed without further expansion. Quantitative PCR was used to assess changes in histone H3K4 (top) and K27 (bottom) trimethylation levels. Data is represented as percentage of input (*p<0.05, n = 3). (B) Direct endothelial reprogramming does not involve iPSC generation. Time course analysis is shown for pluripotency and endothelial markers by quantitative RT-PCR. Cell samples at days 18 and 28 were collected by manual selection of clusters based on cobblestone-like morphology. Human ESCs and HUVECs were used as positive controls for pluripotency and endothelial markers, respectively. Gene expression levels were normalized to GADPH and shown as relative fold change (*p<0.05, n=3). (C) Immunostaining of Nanog during transdifferentiation on days 18 and 28. Human iPSCs were used as a positive control for Nanog staining.
To confirm whether the iEnd cells were directly generated from human fibroblasts without passing through a pluripotent stage, we examined the expression of pluripotency genes Oct4, Sox2, and Nanog by qPCR. As expected, owing to the exogenous expression of Oct4, the total expression of Oct4 at day 18 was higher than at day 0, but returned to the level seen in HUVECs at day 28. Endogenous Nanog and Sox2 transcripts were essentially unchanged compared with intact fibroblasts (day 0) during the entire transdifferentiation process (Figure 3B). We further collected iEnd cell clusters on days 18 and 28 to immunostain for the expression of Nanog. Unsurprisingly, there was no Nanog expression in the CD31+ clusters (Figure 3C). These results indicate that formation of CD31+ clusters does not require activation of the endogenous pluripotency network.
Similarly, we also quantified the gene expression of endothelial progenitors and more mature endothelial cells in the time course samples that had undergone the transdifferentiation. Quantitative PCR analyses showed that the endothelial program was already under way at day 18, as indicated by the upregulated expression of CD31 (Figure 3B). To determine whether the endothelial transdifferentiation program encompassed the formation of mesodermal progenitors, we accessed the temporal expression kinetics of Flk1,18 a typical marker of mesodermal progenitors.19, 20 The expression of Flk1 reached a high level at day 18 and then decreased by day 28, indicating that the iEnd cells passed through a progenitor-like state at day 18 before reaching a more mature endothelial fate by day 28. The expression of endothelial markers CD31 and VE at day 28 approached levels observed in HUVECs. These results collectively indicated that our induced endothelial transdifferentiation bypassed iPSC generation but may still have followed the normal endothelial specification process.
Three Factor (OKS) and Four Factor (OKSM) Are Also Permissive to Endothelial Transdifferentiation
Our results suggest that two reprogramming factors (OK) are sufficient to initiate transdifferentiation of human fibroblasts into endothelial cells with the proper signaling inputs. However, the addition of other reprogramming factors is permissive to initiating such transdifferentiation as well. Induction by ectopic expression of Oct4, Klf4 and Sox2 (OKS) for the first 7 days and cultured under the same conditions for 28 days also resulted in the generation of iEnd cells (Figure IVA–E in the online-only Data Supplement) from human fibroblasts. The efficiency of 3-factor-induced transdifferentiation was 11.8% on day 28 (Figure IV-E in the online-only Data Supplement), which was notably higher than the efficiency of transdifferentiation with only 2 factors. In fact, this transdifferentiation approach could also be similarly extended to murine fibroblast cells, where iEnd cells were generated from secondary murine embryonic fibroblasts (MEF) harboring doxycycline-inducible Oct4, Klf4, Sox2 and cMyc (OKSM) (Figure V in the online-only Data Supplement), corroborating previous reports that 4 factors can be used to generate iEnd cells.21 In the doxycycline-inducible MEF system we could observe formation of iEnd clusters as early as day 12. Taken together, these data suggests that transient expression of the OK reprogramming factors are required for endothelial transdifferentiation, although OKS and OKSM factors are also permissive to transdifferentiation in both human as well as murine fibroblast sources.
iEnd Cells Show Therapeutic Benefit In a Mouse Model of Peripheral Arterial Disease
A current limitation of many in vitro transdifferentiated cell types from fibroblasts is the absence or lack of in vivo rescue characterization for disease models.11, 22–25 To further functionally characterize our iEnd cells and provide a proof-of-concept demonstration of their potential therapeutic utility, we determined if the iEnd cells after transplantation could functionally enhance limb perfusion and angiogenesis in a murine hind limb ischemia model, which is a well-established model of PAD. We genetically labeled the iEnd cells and parental fibroblast with a lentivirus encoding firefly luciferase and GFP to enable non-invasive tracking of the cells using bioluminescence imaging (BLI) and FACS sorting of transduced cells. After verifying the endothelial identity of the purified iEnd cells by molecular and functional assays, the cells were stably transduced with the double fusion reporter construct with 86% efficiency for double positive expression of GFP and CD31 (Figure VI in the online-only Data Supplement). These highly pure iEnd cells were used for intramuscular injection into the ischemic limb at days 0 and 7 after induction of hindlimb ischemia by unilateral ligation of the femoral artery. To verify that transduction of the bioluminescence and fluorescence reporter genes did not affect iEnd cell phenotype, we immunostained for the colocalized expression of endothelial cell markers CD31 and VE by immunostaining (Figure 4A).
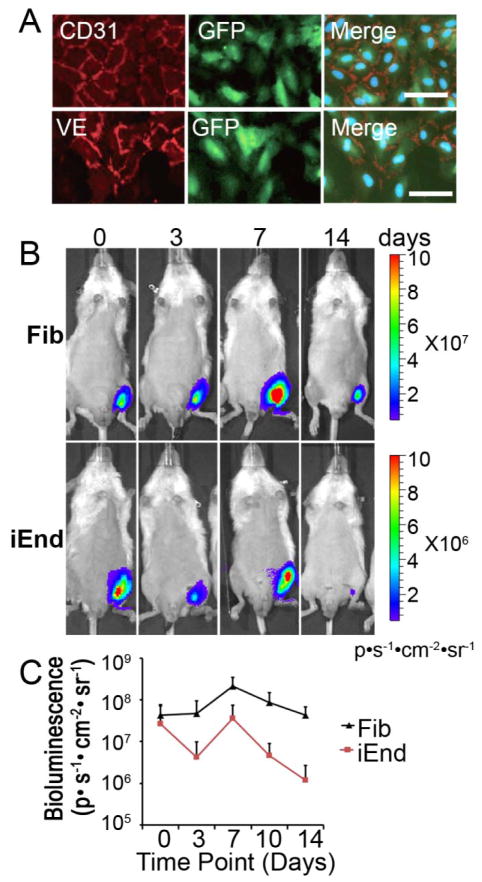
Figure 4. iEnd cells persist after transplantation into the ischemic hindlimb.
(A) Immunostaining of endothelial cell markers CD31 and VE of iEnd cells after transduction with a lentiviral construct encoding luciferase and GFP and subsequent purification. (B) Fibroblasts or iEnd cells (5×105) were transplanted on days 0 and 7 into the ischemic hindlimb of mice (n=5). Localization and viability of iEnd cells or parental fibroblasts in the ischemic hindlimb by BLI. (C) Quantification of bioluminescence signal. Scale bar: 50μm.
Using BLI, we non-invasively monitored cell survival and localization of the iEnd cells in the ischemic hindlimb. The cells survived and remained localized to the ischemic limb, although a typical decrease in cell viability after cell transplantation into a site of ischemia was observed (Figure 4B), as was also observed in the parental fibroblasts. Quantification of BLI demonstrated a positive bioluminescence signal (ie. above the background level of 104 p/s/cm2/sr), confirming the persistence of cells even after 14 days post injection (Figure 4C). To further verify engraftment of transplanted cells, we performed a histological analysis for the tissues at day 14 after delivery of viable iEnd cells. Immunostaining with the human-specific CD31 antibody demonstrated that the iEnd cells appeared to form both small capillary structures and larger vessels with distinctive lumens (Figure 5). Using a CD31 antibody that was cross-reactive to human and mouse origin, we observed that human-derived vessels were interspersed within the murine vessels.
Figure 5. Histological analysis of iEnd cell localization in the ischemic hindlimb.
Immunofluorescence staining of human and mouse capillaries in the ischemic hindlimb. CD31 antibodies that are human specific (hCD31) or reactive to both human and mouse CD31 (m/hCD31) were used to distinguish the origin of the vessels. The iEnd cells formed small capillaries and larger vascular structures in the ischemic limb. Scale bar: 50 μm.
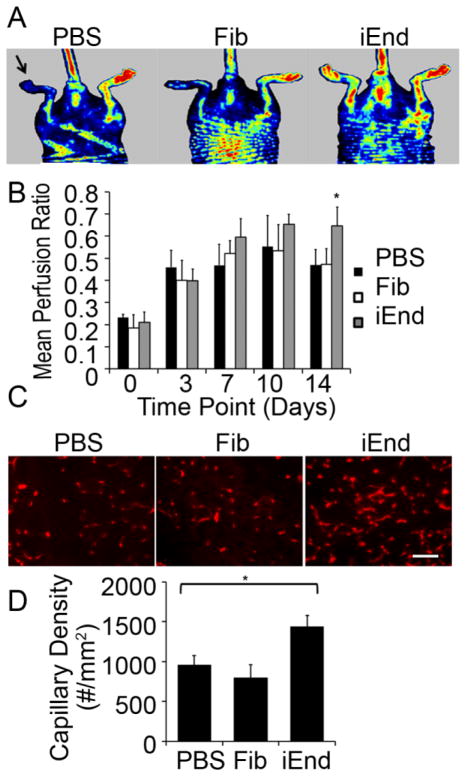
To assess the therapeutic benefit of iEnd cell transplantation, we examined the functional recovery of limb perfusion by laser Doppler spectroscopy (Figure 6A–B & Figure VII in the online-only Data Supplement). At day 14 after delivery, mice injected with the iEnd cells showed a significant improvement in the mean perfusion ratio (0.64 ± 0.09) when compared to both the control mice injected with saline (0.47 ± 0.07) and fibroblasts (0.47 ± 0.07) (p<0.01). We further confirmed the improvement in limb perfusion with immunofluorescence quantification of total vascular density with a CD31 antibody that cross reacts with both human and mouse endothelial cells (Figure 6C). The results showed that mice transplanted with iEnd cells had a 50% greater total capillary density than those treated with either saline (1437± 140 capillaries/mm2 iEnd vs 960 ±114 capillaries/mm2 PBS, p<0.0025) or fibroblasts (800 ± 156 capillaries/mm2, p<0.0025), as shown in Figure 6D. These in vivo results suggested that the iEnd cells are functionally capable of improving limb perfusion by supporting neovascularization of the ischemic hind limb tissue. These results are consistent with the findings from our previous reports demonstrating the functionality of pluripotent stem cell-derived endothelial cells in the ischemic hind limb.8, 9
Figure 6. iEnd cells increased capillary density and improved perfusion in a mouse model of peripheral arterial disease.
(A) Laser Doppler blood spectroscopy images demonstrate improved blood perfusion on day 14 after iEnd cell transplantation. The arrow denotes the ischemic limb. (B) Perfusion ratio of ischemic limbs at day 14 after cell treatment. The perfusion ratio (ischemic/control limb) was significantly greater in mice that received iEnd cell transplantation, compared to those receiving the parental fibroblasts or PBS (*p<0.01, n=5). (C) Improved neovascularization in the ischemic limbs of mice receiving iEnd cells after 14 days. Immunofluorescence showed CD31 staining of ischemic tissues from mice treated with PBS, Fib or iEnd cells. (D) Bar graph showed quantification of the total capillary density (bottom) (* p<0.0025). Scale bar: 50μm.
DISCUSSION
In this study, we demonstrated the conversion of fibroblasts to functional endothelial cells in the human system with only two factors, Oct4 and Klf4, by the TPS transdifferentiation strategy. This strategy can also be extended towards generating iEnd cells using 3 (OKS) or 4 (OKSM) factors using human or murine fibroblasts. This approach validates the broad applicability of the TPS transdifferentiation strategy for generating desired lineage-specific cell types from somatic cells. Once the cells have been sufficiently epigenetically activated, they may be directed toward many different lineages and may in part follow “natural” development.
This method represents an alternative attractive strategy to the derivation of autologous endothelial cells without the generation of iPSCs. Although the transdifferentiation of human fibroblasts requires 28 days, it is still a quicker process than iPSC line establishment followed by endothelial differentiation. In the MEF system we could observe formation of iEnd clusters as early as day 12. Molecular characterization confirmed cell transition from fibroblasts toward endothelial cells without activating the endogenous pluripotency network. We also demonstrated that iEnd cells resembled primary endothelial cells in phenotype as well as function both in vitro and in vivo. In a disease setting of hindlimb ischemia the iEnd cells also engrafted and exhibited therapeutic potential. Treatment with these cells increased the capillary density and blood perfusion, and they were able to integrate into the blood vessels and survive. Collectively, those results suggested that these cells could be further developed as a potential therapy for IVD.
Future studies will be necessary to determine whether the iEnd cells represent a novel method to generate patient-specific endothelial cells that traverse through a vascular progenitor state. Although current transdifferentiation paradigm aims to obtain a particular type of cells that are typically isolated from the direct reprogramming culture, characterizing other cell types generated during the reprogramming process would provide new insights and facilitate improving reprogramming conditions. In particular, teratoma formation of OK-transduced fibroblasts at intermediate time points will determine whether transiently overexpressing OK-transduced fibroblasts can avoid generating cells with tumorigenic potential. In addition, further characterization of the genetic profile of iEnd cells at a progenitor-like stage on day 18 will provide new insights into the molecular pathways mediating the transdifferentiation process.
Recently, OKSM factors were shown to induce endothelial transdifferentiation of human fibroblasts.21, 26 Our observations using doxycycline-inducible MEF support the finding that transient activation of OSKM together with the appropriate inductive signals (TPS), can generate murine iEnd cells (Figure V in the online-only Data Supplement), Moreover, we demonstrate that that only the OK factors are necessary for endothelial transdifferentiation of human fibroblasts, which may be therapeutically more relevant since it obviates the use of oncogene c-myc. This finding is consistent with the reported roles of these two factors in maintaining endothelial function or promoting multilineage blood progenitor generation of fibroblasts. Previously, Klf4 was shown to bind to the VE promoter and regulate endothelial cell phenotype and function by inducing VE-cadherin gene expression and endothelial barrier function.27 In addition, ectopic Oct4 expression was shown to activate the adult hematopoiesis program in human fibroblasts to generate multilineage blood progenitors.23 Since endothelial cells and hematopoietic lineages are thought to originate from a common hemangioblast precursor, it is possible that Oct4 may also exert influence on endothelial specification. Together, these results highlight the role of Oct4 and Klf4 in regulating endothelial-related phenotype and signaling pathways.
It is widely accepted that endothelial cells are heterogeneous in morphology, function and transcriptome, depending on the local vascular bed. 28–30 Specification into arterial, venous, and lymphatic subtypes are, in part, regulated by signaling pathways such as Notch1 and well as microenvironmental factors such as shear stress. 31, 32 It is likely that the iEnd cells represent a heterogeneous mixture of all three subtypes, since the non-subtype-specific endothelial marker, CD31, was used for purification. It remains to be determined whether subtype-specific iEnd cells would be superior to heterogeneous iEnd cells for therapeutic angiogenesis. These experiments are interesting and warranted, but beyond the scope of this study.
In summary, we showed that therapeutically functional endothelial cells can be derived from human fibroblasts with only two factors, Oct4 and Klf4, when combined with the appropriate inductive signals, in a process we term transient pluripotency-factor-based signaling-directed (TPS) transdifferentiation. Since the reprogrammed cells did not go through a pluripotent intermediate state, the risk of tumorigenicity is minimized. Furthermore, this process obviates the use of c-myc, so it may be a safer approach that obviates oncogenesis. TPS transdifferentiation can be successfully applied for transdifferentiation of both human and murine cells. This process might be further optimized, with more small-molecular enhancers, for instance, to generate endothelial cells at a higher efficiency and with faster kinetics. Furthermore, the use of non-integrating genetic delivery, such as mRNA or episomal vectors, could avoid potential issues associated with viral integration. This approach could be an attractive strategy to derive autologous therapeutic endothelial cells and can serve as a useful model to study lineage specification and conversion.
Supplementary Material
Significance.
This study demonstrates that human fibroblasts can be transdifferentiated into functional ECs by using only two factors (Oct4 and Klf4) under inductive signaling conditions, while obviating the use of oncogenes such as c-myc. The iEnd cells resemble primary ECs in their phenotype, transcriptome, and functional capacity to enhance vascularization in the setting of hindlimb ischemia. TPS transdifferentiation can be applied for both human and murine cells, suggesting the universality of this reprogramming approach. We further demonstrate that the transdifferentiation process bypassed a pluripotency intermediate step, since the endogenous pluripotency network was not activated. The epigenetic changes at three loci (Oct4, CD31 and VE) demonstrated that that epigenetic transition to endothelial fate started as early as day 18. This work highlights the important role of transient nuclear reprogramming in directing cell fate into endothelial lineage, and has important implications in the development of safe cell therapies for treatment of vascular diseases.
Acknowledgments
We thank Drs. Fuqin Su and Zhijie Chang at Tsinghua University for providing animal assistance and immunostaining. We also thank other members in the Ding lab for helpful discussions and technical assistance.
FUNDING SOURCES
This study is supported by funding to S.D. from NICHD, NHLBI, NEI, and NIMH/NIH, California Institute for Regenerative Medicine, Prostate Cancer Foundation, and the Gladstone Institute. Part of this research was also supported by the “Strategic Priority Research Program” of the Chinese Academy of Sciences (XDA01040302) to S.D and Natural Science Foundation of China (NSFC 30900859) to J. L. This study was also supported by grants from National Institutes of Health to J.P.C. (U01HL100397, RC2HL103400) and N.F.H. (K99HL098688).
Footnotes
DISCLOSURES
None
References
- 1.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and Cytokine-Induced Mobilization of Bone Marrow-Derived Endothelial Progenitor Cells for Neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 2.Heeschen C, Lehmann R, Honold J, Assmus B, Aicher A, Walter DH, Martin H, Zeiher AM, Dimmeler S. Profoundly Reduced Neovascularization Capacity of Bone Marrow Mononuclear Cells Derived from Patients with Chronic Ischemic Heart Disease. Circulation. 2004;109:1615–1622. doi: 10.1161/01.CIR.0000124476.32871.E3. [DOI] [PubMed] [Google Scholar]
- 3.Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H, Ishino K, Ishida H, Shimizu T, Kangawa K, Sano S, Okano T, Kitamura S, Mori H. Monolayered Mesenchymal Stem Cells Repair Scarred Myocardium after Myocardial Infarction. Nat Med. 2006;12:459–465. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 4.Strauer BE, Steinhoff G. 10 Years of Intracoronary and Intramyocardial Bone Marrow Stem Cell Therapy of the Heart: From the Methodological Origin to Clinical Practice. J Am Coll Cardiol. 2011;58:1095–1104. doi: 10.1016/j.jacc.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 5.Dimmeler S, Burchfield J, Zeiher AM. Cell-Based Therapy of Myocardial Infarction. Arterioscler Thromb Vasc Biol. 2008;28:208–216. doi: 10.1161/ATVBAHA.107.155317. [DOI] [PubMed] [Google Scholar]
- 6.Landmesser U, Hornig B, Drexler H. Endothelial Function: A Critical Determinant in Atherosclerosis? Circulation. 2004;109:II27–33. doi: 10.1161/01.CIR.0000129501.88485.1f. [DOI] [PubMed] [Google Scholar]
- 7.Davignon J, Ganz P. Role of Endothelial Dysfunction in Atherosclerosis. Circulation. 2004;109:III27–32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 8.Rufaihah AJ, Huang NF, Jame S, Lee JC, Nguyen HN, Byers B, De A, Okogbaa J, Rollins M, Reijo-Pera R, Gambhir SS, Cooke JP. Endothelial Cells Derived from Human Ipscs Increase Capillary Density and Improve Perfusion in a Mouse Model of Peripheral Arterial Disease. Arterioscler Thromb Vasc Biol. 2011;31:e72–79. doi: 10.1161/ATVBAHA.111.230938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang NF, Niiyama H, Peter C, De A, Natkunam Y, Fleissner F, Li Z, Rollins MD, Wu JC, Gambhir SS, Cooke JP. Embryonic Stem Cell-Derived Endothelial Cells Engraft into the Ischemic Hindlimb and Restore Perfusion. Arterioscler Thromb Vasc Biol. 2010;30:984–991. doi: 10.1161/ATVBAHA.110.202796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Efe JA, Hilcove S, Kim J, Zhou H, Ouyang K, Wang G, Chen J, Ding S. Conversion of Mouse Fibroblasts into Cardiomyocytes Using a Direct Reprogramming Strategy. Nat Cell Biol. 2011;13:215–222. doi: 10.1038/ncb2164. [DOI] [PubMed] [Google Scholar]
- 11.Kim J, Efe JA, Zhu S, Talantova M, Yuan X, Wang S, Lipton SA, Zhang K, Ding S. Direct Reprogramming of Mouse Fibroblasts to Neural Progenitors. Proc Natl Acad Sci U S A. 2011;108:7838–7843. doi: 10.1073/pnas.1103113108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi Y, Desponts C, Do JT, Hahm HS, Scholer HR, Ding S. Induction of Pluripotent Stem Cells from Mouse Embryonic Fibroblasts by Oct4 and Klf4 with Small-Molecule Compounds. Cell Stem Cell. 2008;3:568–574. doi: 10.1016/j.stem.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Zhu S, Li W, Zhou H, Wei W, Ambasudhan R, Lin T, Kim J, Zhang K, Ding S. Reprogramming of Human Primary Somatic Cells by Oct4 and Chemical Compounds. Cell Stem Cell. 2010;7:651–655. doi: 10.1016/j.stem.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu SJ, Feng Q, Caballero S, Chen Y, Moore MA, Grant MB, Lanza R. Generation of Functional Hemangioblasts from Human Embryonic Stem Cells. Nat Methods. 2007;4:501–509. doi: 10.1038/nmeth1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamizu K, Kawasaki K, Katayama S, Watabe T, Yamashita JK. Enhancement of Vascular Progenitor Potential by Protein Kinase a through Dual Induction of Flk-1 and Neuropilin-1. Blood. 2009;114:3707–3716. doi: 10.1182/blood-2008-12-195750. [DOI] [PubMed] [Google Scholar]
- 16.Watabe T, Nishihara A, Mishima K, Yamashita J, Shimizu K, Miyazawa K, Nishikawa S, Miyazono K. Tgf-Beta Receptor Kinase Inhibitor Enhances Growth and Integrity of Embryonic Stem Cell-Derived Endothelial Cells. J Cell Biol. 2003;163:1303–1311. doi: 10.1083/jcb.200305147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong WT, Huang NF, Botham CM, Sayed N, Cooke JP. Endothelial Cells Derived from Nuclear Reprogramming. Circ Res. 2012;111:1363–1375. doi: 10.1161/CIRCRESAHA.111.247213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal Blood Vessel Development and Lethality in Embryos Lacking a Single Vegf Allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 19.Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, Field LJ, Keller GM. Human Cardiovascular Progenitor Cells Develop from a Kdr+ Embryonic-Stem-Cell-Derived Population. Nature. 2008;453:524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 20.Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, Naito M, Nakao K. Flk1-Positive Cells Derived from Embryonic Stem Cells Serve as Vascular Progenitors. Nature. 2000;408:92–96. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- 21.Margariti A, Winkler B, Karamariti E, Zampetaki A, Tsai TN, Baban D, Ragoussis J, Huang Y, Han JD, Zeng L, Hu Y, Xu Q. Direct Reprogramming of Fibroblasts into Endothelial Cells Capable of Angiogenesis and Reendothelialization in Tissue-Engineered Vessels. Proc Natl Acad Sci U S A. 2012;109:13793–13798. doi: 10.1073/pnas.1205526109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct Reprogramming of Fibroblasts into Functional Cardiomyocytes by Defined Factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szabo E, Rampalli S, Risueno RM, Schnerch A, Mitchell R, Fiebig-Comyn A, Levadoux-Martin M, Bhatia M. Direct Conversion of Human Fibroblasts to Multilineage Blood Progenitors. Nature. 2010;468:521–526. doi: 10.1038/nature09591. [DOI] [PubMed] [Google Scholar]
- 24.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct Conversion of Fibroblasts to Functional Neurons by Defined Factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoo AS, Sun AX, Li L, Shcheglovitov A, Portmann T, Li Y, Lee-Messer C, Dolmetsch RE, Tsien RW, Crabtree GR. Microrna-Mediated Conversion of Human Fibroblasts to Neurons. Nature. 2011;476:228–231. doi: 10.1038/nature10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurian L, Sancho-Martinez I, Nivet E, Aguirre A, Moon K, Pendaries C, Volle-Challier C, Bono F, Herbert JM, Pulecio J, Xia Y, Li M, Montserrat N, Ruiz S, Dubova I, Rodriguez C, Denli AM, Boscolo FS, Thiagarajan RD, Gage FH, Loring JF, Laurent LC, Izpisua Belmonte JC. Conversion of Human Fibroblasts to Angioblast-Like Progenitor Cells. Nat Methods. 2012 doi: 10.1038/nmeth.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cowan CE, Kohler EE, Dugan TA, Mirza MK, Malik AB, Wary KK. Kruppel-Like Factor-4 Transcriptionally Regulates Ve-Cadherin Expression and Endothelial Barrier Function. Circ Res. 2010;107:959–966. doi: 10.1161/CIRCRESAHA.110.219592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dyer LA, Patterson C. Development of the Endothelium: An Emphasis on Heterogeneity. Semin Thromb Hemost. 2010;36:227–235. doi: 10.1055/s-0030-1253446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aird WC. Phenotypic Heterogeneity of the Endothelium: Ii. Representative Vascular Beds. Circ Res. 2007;100:174–190. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- 30.Aird WC. Phenotypic Heterogeneity of the Endothelium: I. Structure, Function, and Mechanisms. Circ Res. 2007;100:158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 31.Hellstrom M, Phng LK, Gerhardt H. Vegf and Notch Signaling: The Yin and Yang of Angiogenic Sprouting. Cell Adh Migr. 2007;1:133–136. doi: 10.4161/cam.1.3.4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masumura T, Yamamoto K, Shimizu N, Obi S, Ando J. Shear Stress Increases Expression of the Arterial Endothelial Marker Ephrinb2 in Murine Es Cells Via the Vegf-Notch Signaling Pathways. Arterioscler Thromb Vasc Biol. 2009;29:2125–2131. doi: 10.1161/ATVBAHA.109.193185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.