Abstract
Renal pacemakers set the origin and frequency of the smooth muscle contractions that propel wastes from the kidney to the bladder. Although congenital defects impairing this peristalsis are a leading cause of pediatric renal failure, the mechanisms underlying renal pacemaker activity remain unknown. Using ratiometric optical mapping and video microscopy, we discovered that hyperpolarization-activated cation (HCN) channel block with the specific anatagonist ZD7288 (30 μm; IC50) abolished the pacemaker depolarizations that initiate murine upper urinary tract peristalsis. Optical mapping and immunohistochemistry indicate that pacemaker potentials are generated by cells expressing HCN isoform-3, and that HCN3+ cells are coupled to definitive smooth muscle via gap junctions. Furthermore, we demonstrate that HCN3+ cells coexpress T-type Ca2+ (TTC) channels and that TTC channel inhibition with R(−)efonidipine or NNC55-0396 decreased contractile frequency in a dose-dependent manner. Collectively, these data demonstrate that HCN3+/TTC+ cells are the pacemakers that set the origin and rate of upper urinary tract peristalsis. These results reveal a conserved mechanism controlling autorhythmicity in 2 distinct muscle types, as HCN and TTC channels also mediate cardiac pacemaker activity. Moreover, these findings have translational applications, including the development of novel diagnostics to detect fetal urinary tract motility defects prior to renal damage.—Hurtado, R., Bub, G., Herzlinger, D. A molecular signature of tissues with pacemaker activity in the heart and upper urinary tract involves coexpressed hyperpolarization-activated cation and T-type Ca2+ channels.
Keywords: smooth muscle, kidney, peristalsis, ion channels, optical mapping
pacemaker cells elicit rhythmic membrane depolarizations that are transmitted to neighboring excitable cells via gap junctions (1–3). Their distribution within a muscular tissue is critical, as it defines the origin of contraction, while their rate of depolarization regulates contraction frequency. Indeed, abnormal pacemaker function results in pathological conditions, such as cardiac arrhythmias (4), abnormal gut motility (5), and aberrant upper urinary tract (UUT) peristalsis leading to urine reflux and persistent urinary tract infections (6).
Due to the high morbidity associated with abnormal pacemaker function, much effort has been made to identify the molecular mechanisms driving autorhythmic pacemaker depolarizations. Cardiac pacemaker potentials are driven, in part, by hyperpolarization-activated cation (HCN) channels (7–10), whereas intestinal pacemaker activity is dependent on the tyrosine kinase receptor c-kit (11, 12) and slow wave currents conducted by the Ca2+-activated Cl− channel ANO1 (13). Despite the advances in elucidating the ion channels of cardiac and gut pacemaker activity, the molecular mechanisms underlying UUT pacemaker depolarizations remain unknown.
Renal pacemaker activity localizes to the junction between the connective tissue core of the kidney and the smooth muscle coat of the UUT. In animals with a unipapillary kidney, such as the mouse (Fig. 1), this site is where the renal pelvis joins the connective tissue core of the kidney, or the pelvis-kidney junction (PKJ; refs. 14–16). Morphological and physiological studies demonstrate that a subset of smooth muscle cells present at the PKJ (17–19) elicit rhythmic membrane depolarizations characteristic of pacemaker cells (20). Moreover, experiments inhibiting excitation-contraction coupling via nifedipine block of L-type calcium channels directly revealed pacemaker activity in isolated tissue strips of the PKJ (20, 21). Consistent with gut (22, 23) and urogenital tract (24, 25) smooth muscle physiology, nifedipine inhibition of L-type calcium channels rendered the smooth muscle electrically quiescent and enabled the detection of spontaneous rhythmic pacemaker depolarizations at the PKJ (20, 21). Although these studies have firmly documented pacemaker activity at the PKJ, the mechanisms driving PKJ pacemaker depolarizations remain unknown.
Figure 1.
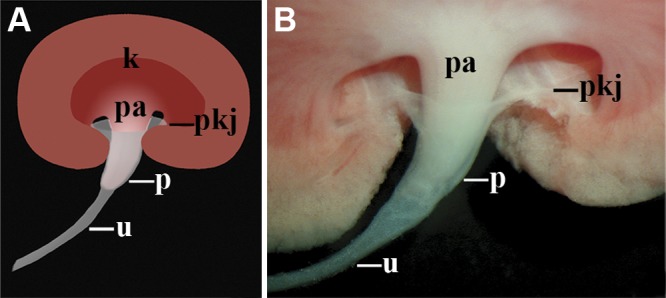
The murine UUT is composed of the renal pelvis and ureter. Schematic (A) and whole-mount image (B) of the murine UUT. Urine produced by the kidney (k) flows from the terminal renal tubules present in the renal papilla (pa) into the UUT, which includes the funnel-shaped renal pelvis (p) and the ureter (u). The flared, proximal end of the renal pelvis joins the connective tissue core of the kidney at the PKJ (pkj). The distal, narrow end of the renal pelvis is continuous with the ureter. The water-impermeable epithelium and smooth muscle coat of the UUT facilitates the movement of urine, in a relatively unmodified form, from the kidney to the bladder.
We have previously shown that cells expressing HCN isoform 3 (HCN3) are present in the murine urinary tract, and that HCN channel block perturbs peristalsis (26). In this study, we discovered that the pacemaker depolarizations that localize the origin, set the frequency, and coordinate UUT contractions are dependent on HCN channel activity. Functional and immunohistochemical studies demonstrate that pacemaker depolarizations are elicited by HCN-expressing tissue localized to the PKJ, that HCN3+ cells are integrated into the UUT smooth muscle at the PKJ, and that HCN3+ cells are coupled to the UUT smooth muscle via gap junctions. Moreover, we demonstrate that HCN3+ UUT pacemakers, like HCN+ pacemakers of the heart, coexpress low-voltage-gated T-type calcium (TTC) channels that play a role in setting contraction frequency (27–29). Collectively, these data functionally denote the renal pacemakers that have eluded detection until now and show that pacemakers of the UUT and heart share an unexpectedly conserved molecular signature.
MATERIALS AND METHODS
Animals
All mice were housed in the Weill Medical College of Cornell University Animal Facility and treated according to the Research Animal Resource Center Guidelines. Adult mice were purchased from Taconic Farms (Germantown, NY, USA).
Kidney explants and PKJ tissue fragments
Kidney explants prepared as described by Hurtado et al. (26) were transferred onto 24-mm Costar Transwell Permeable Supports (0.4-μm polycarbonate membrane; Corning, Corning, NY, USA) and placed into 6-well tissue culture plates (Corning) containing 1.5 ml Tyrode's solution per well. Tyrode's solution (800 μl) was added directly on top of the explants, and samples were rocked on a nutator housed within a 5% CO2 incubator, allowing a bathing solution of Tyrode's to pass over the explant without completely submerging it. ZD7288 (Tocris Bioscience, Ellisville, MI, USA) stock solution of 100 mM in PBS was stored at −20°C, diluted 1:2 with Hybri-Max DMSO (Sigma-Aldrich, St. Louis, MO, USA), and brought to final concentrations, as noted, in Tyrode's (DMSO was maintained below 0.2% at all times). Nifedipine (Sigma) stock solution of 100 mM in DMSO was stored at −20°C and brought to a final concentration of 10 μM in Tyrode's to inactivate the UUT smooth muscle, as has been previously described (20–25). NNC 55–0396 (Sigma) stock solution of 10 mM in DMSO was stored at −20°C, and was brought down to final concentrations in Tyrode's solution as noted. Stock solution of R(−)efonidipine, a generous gift from Nissan Chemical Industries (Minamisaitama, Japan), at 10 mM in DMSO was stored at −20°C and was brought down to final concentration in Tyrode's solution as noted. Inhibitor and control solutions were added directly on top of explants and changed every 0.5 h for a total of 2 h. PKJ tissue fragments were obtained by microdissection, as described previously (20, 21). In short, the UUT was separated from the kidney in Tyrode's saline solution, and the PKJ was excised out. The isolated PKJ was then dissected into representative tissue fragments using tungsten dissecting needles (Roboz Surgical, Gaithersburg, MD, USA) in Tyrode's solution.
Optical mapping
Propagation of cellular excitation and activation sequences of kidney explants were determined by optical mapping techniques, as described previously (26, 30, 31). In short, kidney explants were stained with the voltage-sensitive dye RH237 (Invitrogen, Grand Island, NY, USA; 50 μM in Tyrode's) or di-4-ANEPPS (Invitrogen; 20 μM in Tyrode's) for 10 min within a 5% CO2 incubator. Spread of excitation throughout the urinary tract was monitored by changes in fluorescence signals of RH237. After blocking contractions with cytochalasin D (75 μM, Sigma) to inhibit motion artifact, whole-mount explants were loaded with RH237 and analyzed via excitation at 528 nm (tungsten halogen light source) and detection of long-pass-filtered emission signal (>650 nm; Chroma Technology, Rockingham, VT, USA) using a Cardioplex 80- × 80-pixel CCD mounted on a MacroScope-2a system (Redshirt Imaging, Boston, MA, USA) at an acquisition speed of 125 Hz for 9600 ms. Membrane depolarization detected by dual-wavelength shifts in fluorescent intensity of di-4-ANEPPS was monitored via excitation at 470 ± 20 nm (OptoLED light source; Cairn Research, Faversham, UK) and detection of separated (OptoSplit II; Cairn Research) bandpass-filtered green emission (502–557 nm; Chroma Technology) and red emission (642–708 nm; Chroma Technology) using a Neo-CMOS camera (Andor Technology, South Windsor, CT, USA) mounted on a MVX10 macroscope (Olympus, Center Valley, PA, USA) at an acquisition speed of 50 Hz for 20,000 ms. In studies using di-4-ANEPPS, ratiometric imaging enabled the differentiation of motion artifact and voltage changes (32–35). Motion results in equivalent shifts in pixel intensity at the 2 measured wavelengths, whereas voltage changes resulted in a drop in intensity at the longer wavelengths (red) and a corresponding increase in shorter wavelengths (green). Nifedipine block in pacemaker depolarizations studies further inhibited motion artifact by rendering the smooth muscle quiescent. Data were acquired by the Cardioplex (Redshirt Imaging) or Solis (Andor Technology) software. Data were then processed (background subtraction followed by a 5-frame running average to minimize effects of image noise) and analyzed using the Gview mapping program written by G.B.
Immunohistochemistry
For frozen sections, kidneys were fixed in 4% paraformaldehyde for 6 h, washed in PBS, cryoprotected by 30% sucrose in PBS, and embedded in optimal cutting temperature (OCT) compound (Sakura Finetek USA, Inc., Torrance, CA, USA). Frozen sections of 12 μm thickness were cut, rehydrated with PBS, permeablized with 0.2% TritonX-100 in PBS for 20 min, and blocked with 1% normal donkey serum in PBS for 1 h. Rat anti-HCN3 monoclonal antibody (clone TLL6C5; Millipore, Billerica, MA, USA) was used at a concentration of 1:700, mouse anti-smooth muscle actin (SMA) conjugated to Cy3 (Sigma) was used at 1:2200, sheep polyclonal antibody to Cx40 (Abcam, Cambridge, MA, USA) was used at 1:1000, rabbit polyclonal to Cx43 (Cell Signaling, Danvers, MA, USA) was used at 1:500, and rabbit polyclonal antibody to Cav3.1 (Alomone, Jerusalem, Israel) was used at 1:700, all diluted in 1% normal donkey serum in PBS, at 37°C for 3 h. After 5 washes with PBS, sections were incubated with Alexa Fluor-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA, USA), as noted, at a concentration of 1:1000 diluted in 1% normal donkey serum in PBS, at 37°C for 1.5 h. For vibratome sections, kidneys were embedded in 7% low melting point agarose. Vibratome sections of 80 μm thickness were permeablized and blocked with 0.5% TritonX-100, 1% normal donkey serum in TBS overnight at room temperature, then 37°C for 3 h. Anti-HCN3 and anti-Cav3.1 were used at a concentration of 1:700 in blocking solution for 6 h at room temperature. After washing overnight in 0.5% TritonX-100 in PBS, sections were incubated with alkaline phospatase conjugated secondary antibodies (Jackson ImmunoResearch) at a concentration of 1:1200 in blocking solution for 3 h at room temperature. After washing with 0.5% TritonX-100, chromagenic detection was done by NBT-BCIP staining as described previously (36).
Data documentation
All images of kidney sections were taken using digital photo cameras, bright field with a DKC-5000 (Sony, San Diego, CA, USA), and fluorescent with a Hamamatsu C4742-95 (Hamamatsu Corp., Middlesex, NJ, USA) or a Zeiss LSM Live 5-line scanner confocal microscope (Carl Zeiss Microscopy, Thornwood, NY, USA), as noted. Real-time movies and continuous image capturing was done with a Nikon CoolPix 995 (Nikon, Melville, NY, USA).
RESULTS
UUT pacemaker depolarizations are dependent on HCN channel activity
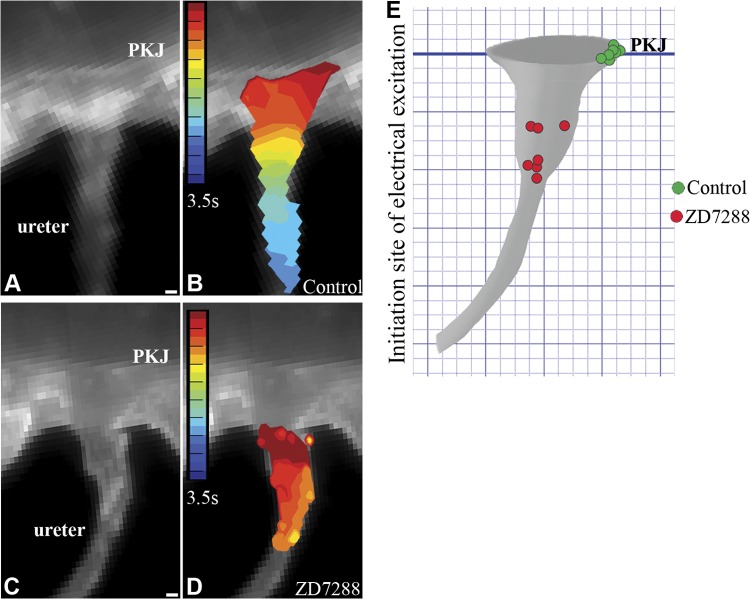
Optical mapping using the voltage-sensitive dye RH237 and real-time video microscopic analyses on intact murine urinary tract explants demonstrate that spontaneous electrical (Fig. 2A, B, E) and contractile activity (Supplemental Movie S1) initiate at the PKJ and then propagate down the renal pelvis and ureter in a coordinated, wave-like manner (n=7). Inhibition of HCN channel activity with the selective HCN channel blocker ZD7288 (9, 37) slows the rate of peristalsis in a dose-dependent manner (26), and at the drug IC50 (30 μM; ref. 38) the origin of electrical activity is shifted distal to the PKJ and peristalsis is perturbed (n=7; Fig. 2C, D, E). In contrast to the coordinated proximal to distal electrical and contractile activity detected in control explants, drug-inhibited explants twitched (Supplemental Movie S2), undergoing desynchronized, near-simultaneous excitation of focal muscle segments distal to the PKJ (Fig. 2D). Since the rate, origin, and coordination of autonomic muscles are dependent on pacemaker activity, these data raise the possibility that HCN channel activity is required for generating UUT pacemaker potentials.
Figure 2.
HCN channel conductance is required for localizing the origin of UUT peristalsis to the PKJ. A–D) Videomicroscopy (A, C) and optical mapping (B, D) were used to analyze peristaltic and electrical activity in whole-mount urinary tract explants with and without HCN channel inhibition (see Supplemental Movies S1 and S2). Explants incubated with Tyrode's saline alone (A, B) or with the HCN channel inhibitor ZD7288 (30 μM; C, D) were loaded with the voltage-sensitive dye RH237. Isochronal maps (B, D) of membrane depolarization over time (red to blue) were recorded, with red demarking the initiation site of electrical excitation. Explants treated with Tyrode's solution alone exhibited unidirectional contractile (Supplemental Movie S1) and electrical waves that initiate at the PKJ (red) and propagate distally down the renal pelvis and ureter (yellow to blue) (A, still image; B, isochronal map). In contrast, explants treated with ZD7288 exhibited twitch-like contractions (Supplemental Movie S2) and desynchronized electrical activity that initiated in random segments of the UUT distal to the PKJ (C, still image; D, isochronal map). E) Quantification of data analyzing the effect of HCN inhibition on the origin of UUT contraction. Origin of contraction in control explants localized to the PKJ (green circles) in 7 of 7 explants. In contrast, the origin of contraction in all 7 explants incubated with ZD7288 was shifted to random sites of the UUT, distal to the PKJ (red circles). Scale bars = 100 μm.
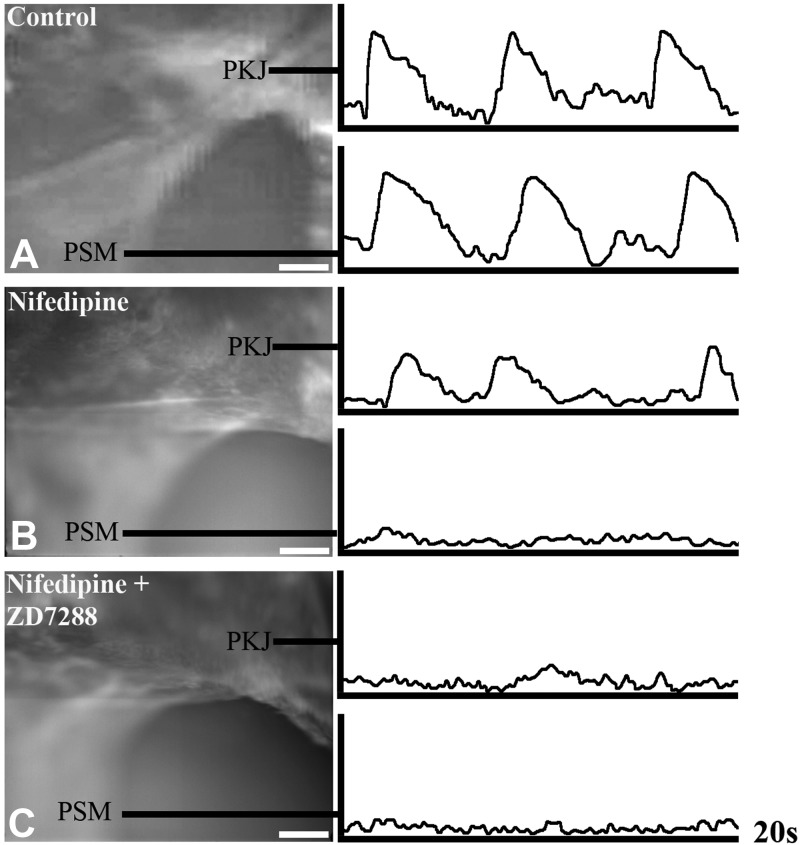
To directly test this hypothesis, we developed ratiometric optical mapping protocols to resolve pacemaker depolarizations at the PKJ at high resolution (Fig. 3). Urinary tract explants were isolated and loaded with the membrane potential sensitive dye di-4-ANEPPS (39), which undergoes depolarizing voltage-dependent shifts in green emission (increased intensity) and red emission (decreased intensity) wavelengths (32). Changes in the ratio of green/red emission wavelengths of di-4-ANEPPS follow action potential contours and have been used to report membrane potentials at both the cellular and tissue levels (33–35). As can be seen in Fig. 3A, rhythmic depolarizations detected at the PKJ precede depolarizations in more distal segments of the ureter. To resolve pacemaker depolarizations from the composite electrical activity detected at the PKJ, we inhibited smooth muscle excitation via nifedipine block of L-type Ca channels. Consistent with previously published reports, nifedipine inhibited the generation and propagation of smooth muscle action potentials (22–25) and revealed small rhythmic pacemaker depolarizations at the PKJ (n=6; Fig. 3B and refs. 20, 21). To determine whether these pacemaker depolarizations are dependent on HCN channel activity, explants inhibited with nifedipine were simultaneously incubated with ZD7288. Strikingly, HCN channel inhibition abolished the pacemaker depolarizations elicited at the PKJ (n=6; Fig. 3C). Thus, our functional studies demonstrate that HCN channel activity is required for the pacemaker depolarizations elicited at the PKJ and for controlling the rate, origin, and coordination of UUT peristalsis. Collectively, these data demonstrate that HCN channel conductance underlies UUT pacemaker activity.
Figure 3.
HCN channel conductance is required for UUT pacemaker activity. To determine whether HCN channel conductance is required for UUT pacemaker activity, we used ratiometeric optical mapping to analyze pacemaker depolarizations with and without HCN channel inhibition. A) UUT explants were loaded with di-4-ANEPPS dye, which undergoes voltage-dependent shifts of dual emission wavelengths. Rhythmic depolarizations detected at the PKJ preceded depolarizations in the more distal pelvic smooth muscle (PSM) in urinary tract explants treated with Tyrode's saline alone (n=6). B) To isolate pacemaker depolarizations from the composite electrical activity at the PKJ, we inhibited smooth muscle excitation via nifedipine (10 μM) block of L-type Ca channels. Nifedipine inhibited the generation and propagation of PSM action potentials, and enabled the detection of spontaneous rhythmic pacemaker depolarizations at the PKJ (n=6). C) To determine whether pacemaker depolarizations elicited at the PKJ are dependent on HCN channel conductance, explants incubated with nifedipine were simultaneously incubated with ZD7288 (30 μM). Pacemaker potentials at the PKJ were abolished by HCN channel block (n=4). Scale bars = 100 μm.
Pacemaker depolarizations are elicited by HCN-expressing tissue localized to the PKJ
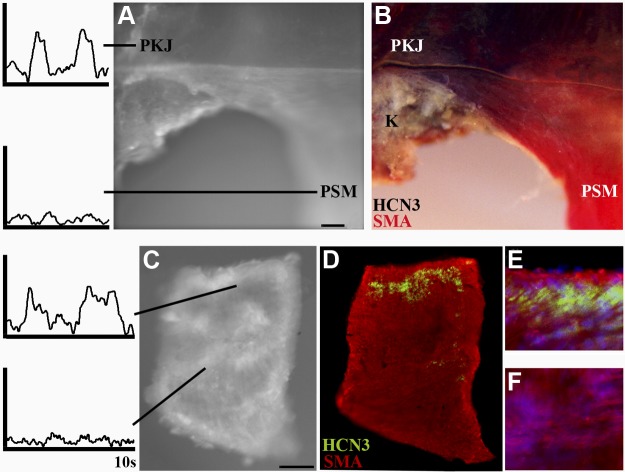
To test whether HCN-dependent depolarizations at the PKJ localize to sites of HCN expression, we performed ratiometric optical mapping and retrospective immunohistochemical staining for HCN3, which is abundantly expressed at the PKJ in comparison to other HCN isoforms (26, 40). Pacemaker depolarizations were detected at the PKJ by ratiometric optical mapping of nifedipine-inhibited, di-4-ANEPPS-loaded UUT explants (n=3; Fig. 4A). Subsequent chromogenic immunohistochemical analyses of mapped UUT explants demonstrate that abundant levels of HCN3 (purple precipitation product) is expressed at the PKJ, where pacemaker depolarizations were recorded (Fig. 4B). In contrast, HCN3 expression was not detected in the kidney parenchyma or the definitive pelvic smooth muscle (Fig. 4B, SMA; red), which lacked pacemaker activity. These data demonstrate that UUT pacemaker depolarizations are elicited by HCN-expressing tissue at the PKJ.
Figure 4.
Pacemaker depolarizations are elicited by HCN-expressing tissue of the PKJ. To determine whether pacemaker depolarizations are elicited by HCN-expressing tissue of the PKJ, we first localized pacemaker activity in UUT preparations by ratiometric optical mapping and then subsequently analyzed these mapped tissues for HCN expression. A) Intact UUT explants were incubated with nifedipine, loaded with di-4-ANEPPS, and analyzed by ratiometric optical mapping. Nifedipine block rendered the pelvic smooth muscle (PSM) electrically quiescent and enabled the detection of pacemaker depolarizations at the PKJ. B) To determine whether pacemaker activity localizes to the site of HCN expression, mapped explants were subsequently processed for the detection of HCN3 and SMA via chromagenic immunohistochemistry. HCN3 (purple reaction product) was abundantly expressed at the PKJ where pacemaker depolarizations were elicited, but not in the pelvic smooth muscle (SMA, red reaction product) or the parenchyma of the kidney (K). C–F) To further define the cell population eliciting pacemaker depolarizations, we analyzed electrical activity and protein expression in small PKJ tissue fragments microdissected from the UUT. C) Ratiometric optical mapping analyses of nifedipine-inhibited, di-4-ANEPPS-loaded tissue fragments revealed spontaneous rhythmic pacemaker depolarizations within localized regions of PKJ tissue fragments. D–F) Subsequent immunofluorescent analyses of mapped tissue fragments (D) localized HCN3-expressing cells (green) to the site of pacemaker depolarizations (E, high magnification), and demonstrate that the adjacent smooth muscle (red, SMA) is electrically quiescent on nifedipine block (F, high magnification). Scale bars = 100 μm.
To further resolve the cell population eliciting pacemaker depolarizations, we analyzed isolated PKJ tissue by ratiometric optical mapping (Fig. 4C) and retrospective immunofluorescence microscopy (Fig. 4D–F). The PKJ was dissected out of the UUT, and small representative fragments were isolated by microdissection, as described previously (20, 21). PKJ fragments were then inhibited with nifedipine, loaded with di-4 ANEPPS, and analyzed by ratiometric optical mapping. Results of these experiments demonstrate that small rhythmic pacemaker depolarizations are elicited within localized regions of isolated PKJ fragments (n=4; Fig. 4C). To determine whether the pacemaker depolarizations detected in isolated PKJ tissues are elicited by focal domains of HCN-expressing tissue, we subsequently analyzed mapped tissue by double immunofluorescence for HCN3 and SMA. As can be seen in Fig. 4D, HCN3+ cells localize to the site of PKJ pacemaker depolarizations (Fig. 4C), whereas the adjacent definitive smooth muscle (Fig. 4E) was electrically quiescent. Collectively, these optical mapping and retrospective immunohistochemical analyses of intact UUT explants and isolated PKJ fragments demonstrate that UUT tissue enriched with HCN+ cells elicits pacemaker depolarizations.
HCN-expressing cells are in direct contact and coupled to UUT smooth muscle via gap junctions
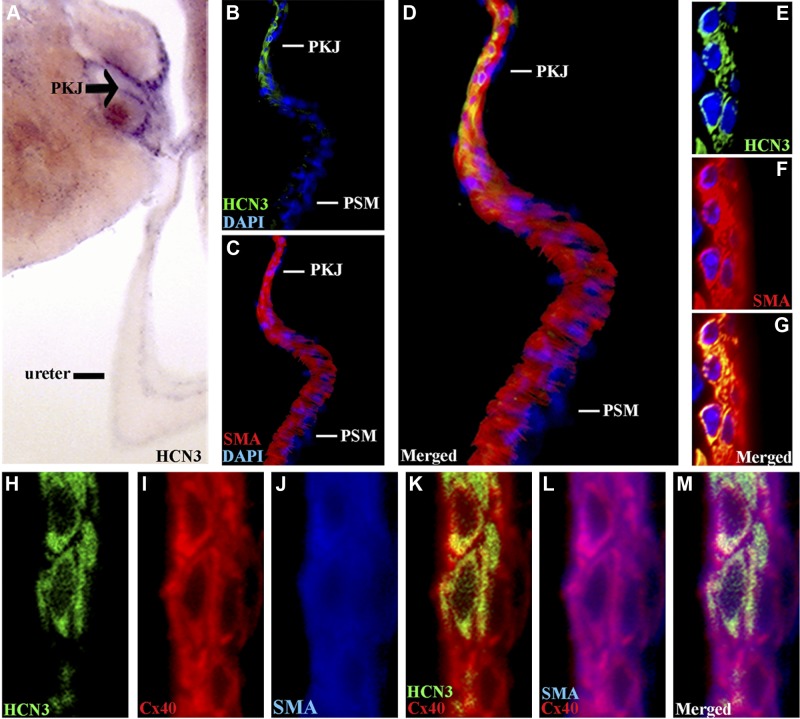
To determine whether HCN-expressing cells of the PKJ are competent to transmit spontaneous pacemaker depolarizations to the UUT smooth muscle, we tested whether HCN+ cells are in direct contact and coupled via gap junctions to smooth muscle. Chromagenic immunohistochemistry of 80-μm UUT sections demonstrated that HCN3 is highly expressed and localized to the PKJ (Fig. 5A). Immunofluorescent staining for HCN3 (green), SMA (red), and DAPI (blue) on 12-μm sections demonstrated a direct association between HCN-expressing cells and the definitive smooth muscle at the PKJ (Fig. 5B–G). SMA staining (Fig. 5C) demarked the UUT smooth muscle coat, which gradually thickens as it transitions from its most proximal end at the PKJ to the more distal segments of the pelvis (41). Costaining for HCN3 (Fig. 5B) demonstrated that HCN+ cells are localized within the smooth muscle coat at the PKJ (Fig. 5D, merged), which was detected at the single-cell level using confocal microscopy (Fig. 5E–G). Thus, HCN-expressing cells in direct contact and integrated within the smooth muscle coat at the PKJ are in a location consistent with a role in triggering UUT peristalsis.
Figure 5.
HCN3-expressing cells are localized to the PKJ and coupled to adjacent smooth muscle by gap junctions. A) Chromagenic immunohistochemistry on 80-μm UUT sections demonstrates that HCN3 (purple reaction product) is highly expressed and localized to the PKJ (arrow). B–D) Triple immunofluorescent staining for SMA (red; C, D), HCN3 (green; B, D), and DAPI (blue) demonstrates that HCN3-expressing cells (B) are integrated within the UUT smooth muscle coat at the PKJ (C), but not the thicker pelvic smooth muscle (PSM) of the more distal UUT (D, merged). E–G) Confocal microscopy of the PKJ demonstrates that HCN3+ cells (green; E) are adjacent to and in direct contact with the smooth muscle (red; F, G; G, merged). H–M) Triple immunofluorescent staining for HCN3 (green; H, K, M), Cx40 gap junction (red; I, K–M) and SMA (blue; J, L, M) demonstrates that HCN3 (H) cells of the PKJ are coupled to adjacent smooth muscle (J) by Cx40 (I), which is coexpressed by both cell populations (K, HCN3/Cx40 merged; L, SMA/Cx40 merged; M, all merged).
To identify the coupling mechanism underlying the propagation of electrical waves from the PKJ through the UUT smooth muscle, as detected in our optical mapping analyses, we analyzed the spatial distribution of gap junctions expressed in the urinary tract (42). Immunofluorescent analysis demonstrated that the gap junction protein connexin 40 (Cx40) is abundantly expressed in the UUT smooth muscle coat at the PKJ (Fig. 5H–M), whereas Cx43 is expressed at lower levels (Supplemental Fig. S1). Triple immunofluorescent staining for HCN3 (Fig. 5H, green), Cx40 (Fig. 5I, red), and SMA (Fig. 5J, blue) demonstrated that Cx40 is expressed by both HCN3+ (Fig. 5K, HCN3/Cx40 merged) and smooth muscle (Fig. 5L, SMA/Cx40 merged) cells, coupling these adjacent cell populations (Fig. 5M, all merged). These data demonstrating that HCN3+ cells are coupled to the definitive smooth muscle at the PKJ, combined with our functional studies demonstrating that PKJ pacemaker depolarization is dependent on HCN channel conductance and is elicited by HCN-expressing tissue, indicate that HCN3-expressing cells localized to the PKJ are UUT pacemakers.
TTC channels are coexpressed by HCN+ cells at the PKJ, and TTC activity regulates the frequency of UUT peristalsis
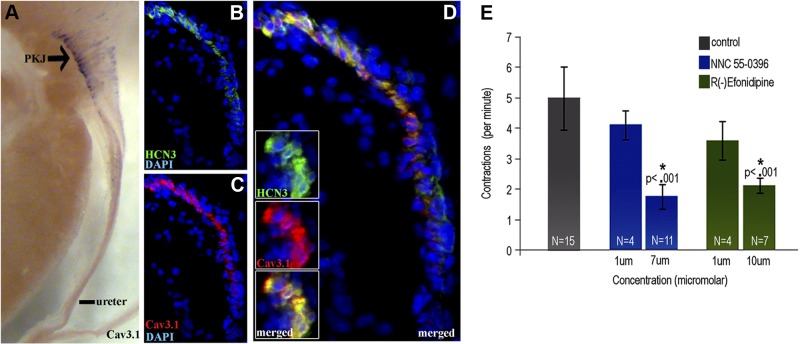
To further support the hypothesis that HCN-expressing cells localized to the PKJ are UUT pacemakers, we assayed this cell population for ion channels that have been shown to mediate HCN+ pacemaker cell activity. Specifically, we investigated whether HCN-expressing cells at the PKJ, like HCN+ cardiac pacemakers at the SA node (43, 44), coexpress low-voltage-gated TTC channels. Chromagenic immunohistochemistry for the TTC channel family members Cav3.1, Cav3.2, and Cav3.3 on 80-μm-thick urinary tract sections showed that Cav3.1 is highly expressed at the PKJ (Fig. 6A). To define the cell population expressing TTC channels at the PKJ, we performed triple immunofluorescent staining for Cav3.1 (red), HCN3 (green), and DAPI (blue) (Fig. 6B–D). Results of these experiments show that Cav3.1 (Fig. 6C) is expressed by HCN3+ cells (Fig. 6B) at the PKJ (Fig. 6D, merged), which was detected at the single-cell level by confocal microscopy (Fig. 6D, inset).
Figure 6.
HCN3+ cells of the PKJ coexpress TTC channels, and TTC channel activity regulates the frequency of UUT contractions. A) Chromagenic immunohistochemistry on 80-μm UUT sections demonstrates that TTC channel isoform Cav3.1 (purple reaction product) is highly expressed and localized to the PKJ. B–D) Triple immunofluorescent staining for HCN3 (green; B, D), Cav3.1 (red; C, D), and DAPI (blue) demonstrates that HCN3+ cells (B) of the PKJ coexpress Cav3.1 (C; D, merged), which was detected at the single-cell level via confocal microscopy (D, inset). E) To determine the role of TTC channel activity on UUT peristalsis, explants were incubated with or without the TTC channel antagonists R(−)efonidipine or NNC 550936. TTC channel inhibition decreased the rate of UUT contraction in a dose-dependent manner, with incubation at the IC50 of R(−)efondipine (10 μM) and NNC 55–0396 (7 μM) significantly reducing contractile frequency by 57% (P<0.001) and 64% (P<0.001), respectively.
The coexpression of TTC channels by HCN+ cells of the SA node and the PKJ suggests that TTC channel activity may play a similar functional role in controlling both UUT and cardiac contractions. Low-voltage-gated TTC channels drive a depolarizing conductance that is activated by small HCN-dependent pacemaker depolarizations, and studies using organic inhibitors of TTC channels show that inhibition of TTC channel activity decreases the heart rate (45, 46). Therefore, we tested whether the frequency of UUT contractions is decreased by either of the two specific organic inhibitors of TTC channels, R(−)efonidipine (47, 48) and NNC 55–0396 (49, 50) (Fig. 6E). Control explants incubated with Tyrode's saline alone contracted 5 ± 1.03/min, consistent with frequencies reported in vivo (51) and ex vivo (26). In contrast, inhibition with R(−)efonidipine or NNC 55–0396 decreased the frequency of contraction in a dose-dependent manner. Incubation at concentrations below the drug IC50 produced a modest decrease in contraction rate, whereas incubation at the IC50 reported for R(−)efondipine (47) and NNC 55–0396 (49) significantly decreased the frequency of contraction by 57% (P<0.001) and 64% (P<0.001), respectively. These protein expression and functional analyses show that HCN+ cells at the PKJ coexpress TTC channels, and that TTC channel activity modulates the frequency of UUT contraction. Thus, HCN3+ cells coexpress at least one other ion channel that is consistent with their role as UUT pacemakers.
DISCUSSION
Although the dependence of coordinated UUT peristalsis on pacemaker cells localized to the PKJ has been appreciated for >140 yr, the molecular mechanisms underlying this pacemaker activity have remained elusive. In this study we have, for the first time, imaged the PKJ pacemaker depolarizations that localize the origin, set the frequency, and coordinate UUT peristalsis and demonstrated that they are dependent on HCN channel activity. Optical mapping and immunhistochemical analyses demonstrate that pacemaker depolarizations are elicited by HCN-expressing tissue that is localized to the PKJ, that HCN+ cells are coupled to the definitive smooth muscle by gap junctions at the PKJ, and that HCN3+ cells coexpress TTC channels, which play a role in mediating contraction frequency. Collectively these functional and immunohistochemical studies indicate that cells coexpressing HCN and TTC channels present at the PKJ are the UUT pacemakers that have eluded detection until now.
Our functional studies demonstrate that coordinated UUT peristalsis is lost when HCN-dependent pacemaker depolarizations are abolished. Specifically, in the absence of HCN-dependent pacemaker activity, the ureteral smooth muscle twitches, undergoing near simultaneous activation of localized muscle segments distal to the PKJ. These data demonstrating desynchronized muscular activity of the UUT in the absence of HCN-dependent pacemaker depolarizations are consistent with the desynchronized muscular activity observed in other autonomic muscles with pacemaker dysfunction, such as the heart and gut. In the heart, loss of coordinated contractions resultant from pacing defects, including arrhythmias, have been shown to lead to incomplete contractions characterized as quivering and flutter (52, 53). In the gut, loss of peristaltic waves associated with pacemaker defects has been shown to lead to spontaneously generated contractions of localized gut segments (54, 55). Thus, the desynchronized muscle activity observed in the absence of HCN-dependent UUT pacemaker depolarizations is consistent with loss of pacemaker function, as observed in other musculature. In further support of these findings, abnormal ureter peristalsis has been documented in gli-null mice (40), which develop a contraction-competent UUT smooth muscle coat but lack HCN3+ cells.
Our finding that HCN channels underlie UUT pacemaker activity is consistent with the role of HCN channels in pacemakers of the brain and heart, as well as recent studies demonstrating de novo pacemaker activity in bioengineered pacemaker cells. HCN1-4 (56, 57) and HCN2 and HCN4 (7) have been identified in the brain and heart, respectively, and our studies and others (40) demonstrate that HCN3 is abundantly expressed at the PKJ, and do not rule out the possibility that other HCN isoforms are expressed at this site at lower levels (26). In the brain, studies have demonstrated that HCN channels drive the spontaneous depolarization of neuronal pacemakers that regulate sensory input in the thalamus (58–60). In the heart, HCN channel block via selective antagonist and genetic ablation studies demonstrate that spontaneous cardiac pacemaker depolarizations are driven by HCN channel conductance (9, 37, 38, 60–63). Moreover, HCN4 channel mutations in humans impair cardiac pacemaker excitability, resulting in bradycardia where a significantly decreased heart rate can result in lethal cardiac arrhythmias (64–66).
That HCN channels underlie pacemaker activity, as demonstrated in the brain, heart, and our studies of the UUT, has been further confirmed by bioengineering studies showing that HCN channels are sufficient to transform quiescent cell types into pacemakers (67). Transfection of HCN channels induces spontaneous, rhythmic pacemaker depolarization in cell types that normally maintain a constant resting membrane potential including atrial and ventricular myocytes (68–70), as well as human mesenchymal stem cells (71). Strikingly, human mesenchymal stem cells transfected with HCN channels can integrate into donor canine hearts and pace the heartbeat (71).
In addition to the HCN channels that drive their pacemaker activity, cardiac pacemakers also selectively coexpress TTC channels (43, 44) that are activated by small HCN-dependent depolarizations. Studies using organic TTC channel inhibitors show that the depolarizing conductance of TTC channels modulates the frequency of heart contractions, thus regulating the heart rate (45, 46). In this study we show an analogous ion channel profile and function in UUT pacemakers. Our protein expression studies show that TTC channels are restricted to the PKJ and are coexpressed by HCN+ cells at this site. Moreover, our functional data demonstrate that inhibition of TTC channel activity decreases the frequency of contraction in the UUT, as it does in the heart. Collectively, these data show for the first time that at least 2 ion channels, HCN and TTC channels, play similar functional roles in UUT and cardiac pacemakers.
These findings provide insight into physiological similarities between pacemaker activity recorded in the heart and UUT. Studies by Orbelli and Von Brucke (15) dating back to 1910 first noted similarities in electromyograms of the UUT and heart. Subsequent studies using surface electrodes in isolated ureters showed that spontaneous electrical activity at the PKJ occurs via gradual depolarization after hyperpolarization (16, 72, 73), which was confirmed by direct voltage recordings in autorhythmic cells of the PKJ (20, 21). Similarly, the spontaneous electrical activity of cardiac pacemakers occurs via gradual depolarization after hyperpolarization (7, 8, 10). Our studies show for the first time that the electrophysiological similarities between UUT and heart pacemaker activity can be attributed to analogous ion channel expression and function and reveal a conserved mechanism controlling autorhythmicity in these two distinct muscle types. Moreover, our findings have translational applications that include novel diagnostics to detect fetuses susceptible to urinary tract motility defects prior to permanent renal damage.
Supplementary Material
Acknowledgments
This work was supported by U.S. National Institutes of Health grant RO1 DK45218, awarded to D.H., and British Heart Foundation Centre of Research Excellence, University of Oxford, grant RE/08/004, awarded to G.B.
The authors thank Dr. Norio Hashimoto (Nissan Chemical Industries, Minamisaitama, Japan) for the generous gift of R(−)efondipine. The authors thank Dr. Jeremy Graham and Mr. Mark Henson for providing expertise in construction of ratiometric optical mapping apparatuses; Dr. David Christini, Dr. Emre Aksay, and Dr. Willemijn Groenendaal for helpful suggestions on experimental techniques; and Dr. Harel Weinstein, Dr. Jeremy Dittman, Dr. Tom Schultheiss, Dr. Lauren Acinapura, Mr. Chad Kurylo, Ms. Lauretta Lacko, and Mr. James Mtui for helpful manuscript discussions.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- HCN
- hyperpolarization-activated cation
- HCN3
- hyperpolarization-activated cation isoform 3
- PKJ
- pelvis-kidney junction
- SMA
- smooth muscle actin
- TTC
- T-type calcium
- UUT
- upper urinary tract
REFERENCES
- 1. Monfredi O., Dobrzynski H., Mondal T., Boyett M. R., Morris G. M. (2010) The anatomy and physiology of the sinoatrial node–a contemporary review. Pacing Clin. Electrophysiol. 33, 1392–1406 [DOI] [PubMed] [Google Scholar]
- 2. Huizinga J. D., Lammers W. J. (2009) Gut peristalsis is governed by a multitude of cooperating mechanisms. Am. J. Physiol. Gastroint. Liver Physiol. 296, G1–G8 [DOI] [PubMed] [Google Scholar]
- 3. Weiss R. M., Tamarkin F. J., Wheeler M. A. (2006) Pacemaker activity in the upper urinary tract. J. Smooth Musc. Res. 42, 103–115 [DOI] [PubMed] [Google Scholar]
- 4. Barbuti A., DiFrancesco D. (2008) Control of cardiac rate by “funny” channels in health and disease. Ann. N. Y. Acad. Sci. 1123, 213–223 [DOI] [PubMed] [Google Scholar]
- 5. Mostafa R. M., Moustafa Y. M., Hamdy H. (2010) Interstitial cells of Cajal, the maestro in health and disease. World J. Gastroenterol. 16, 3239–3248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alberti C. (2012) Congenital ureteropelvic junction obstruction: physiopathology, decoupling of tout court pelvic dilatation-obstruction semantic connection, biomarkers to predict renal damage evolution. Eur. Rev. Med. Pharmacol. Sci. 16, 213–219 [PubMed] [Google Scholar]
- 7. Ludwig A., Zong X., Stieber J., Hullin R., Hofmann F., Biel M. (1999) Two pacemaker channels from human heart with profoundly different activation kinetics. EMBO J. 18, 2323–2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ishii T. M., Takano M., Xie L. H., Noma A., Ohmori H. (1999) Molecular characterization of the hyperpolarization-activated cation channel in rabbit heart sinoatrial node. J. Biol. Chem. 274, 12835–12839 [DOI] [PubMed] [Google Scholar]
- 9. BoSmith R. E., Briggs I., Sturgess N. C. (1993) Inhibitory actions of ZENECA ZD7288 on whole-cell hyperpolarization activated inward current (If) in guinea-pig dissociated sinoatrial node cells. Br. J. Pharmacol. 110, 343–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Biel M., Wahl-Schott C., Michalakis S., Zong X. (2009) Hyperpolarization-activated cation channels: from genes to function. Physiol. Rev. 89, 847–885 [DOI] [PubMed] [Google Scholar]
- 11. Huizinga J. D., Thuneberg L., Kluppel M., Malysz J., Mikkelsen H. B., Bernstein A. (1995) W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature 373, 347–349 [DOI] [PubMed] [Google Scholar]
- 12. Hirota S., Isozaki K., Nishida T., Kitamura Y. (2000) Effects of loss-of-function and gain-of-function mutations of c-kit on the gastrointestinal tract. J. Gastroenterol. 35(Supp. 12), 75–79 [PubMed] [Google Scholar]
- 13. Zhu M. H., Kim T. W., Ro S., Yan W., Ward S. M., Koh S. D., Sanders K. M. (2009) A Ca(2+)-activated Cl(-) conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J. Physiol. 587, 4905–4918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Engelmann T. W. (1869) Zur Physiologie des Ureters. Pflügers Arch. Gesamte Physiol. Menschen Tiere 2, 243–293 [Google Scholar]
- 15. Orbelli L., Von Brucke E. (1910) Beitrage zur Physiologie der autonom innervierten Muskulatur. Pflügers Arch. Gesamte Physiol. Menschen Tiere 133, 23 [Google Scholar]
- 16. Bozler E. (1942) The activity of the pacemaker previous to the discharge of a muscular impulse. Am. J. Physiol. 136, 9 [Google Scholar]
- 17. Gosling J. A. (1970) Atypical muscle cells in the wall of the renal calix and pelvis with a note on their possible significance. Experientia 26, 769–770 [DOI] [PubMed] [Google Scholar]
- 18. Dixon J. S., Gosling J. A. (1982) The musculature of the human renal calices, pelvis and upper ureter. J. Anat. 135, 129–137 [PMC free article] [PubMed] [Google Scholar]
- 19. Klemm M. F., Exintaris B., Lang R. J. (1999) Identification of the cells underlying pacemaker activity in the guinea-pig upper urinary tract. J. Physiol. 519(Pt. 3), 867–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lang R. J., Hashitani H., Tonta M. A., Parkington H. C., Suzuki H. (2007) Spontaneous electrical and Ca2+ signals in typical and atypical smooth muscle cells and interstitial cell of Cajal-like cells of mouse renal pelvis. J. Physiol. 583, 1049–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lang R. J., Hashitani H., Tonta M. A., Bourke J. L., Parkington H. C., Suzuki H. (2010) Spontaneous electrical and Ca2+ signals in the mouse renal pelvis that drive pyeloureteric peristalsis. Clin. Exp. Pharmacol. Physiol. 37, 509–515 [DOI] [PubMed] [Google Scholar]
- 22. Dickens E. J., Hirst G. D., Tomita T. (1999) Identification of rhythmically active cells in guinea-pig stomach. J. Physiol. 514(Pt. 2), 515–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hirst G. D., Ward S. M. (2003) Interstitial cells: involvement in rhythmicity and neural control of gut smooth muscle. J. Physiol. 550, 337–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bramich N. J., Brading A. F. (1996) Electrical properties of smooth muscle in the guinea-pig urinary bladder. J. Physiol. 492(Pt. 1), 185–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hashitani H., Edwards F. R. (1999) Spontaneous and neurally activated depolarizations in smooth muscle cells of the guinea-pig urethra. J. Physiol. 514(Pt. 2), 459–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hurtado R., Bub G., Herzlinger D. (2010) The pelvis-kidney junction contains HCN3, a hyperpolarization-activated cation channel that triggers ureter peristalsis. Kidney Int. 77, 500–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hagiwara N., Irisawa H., Kameyama M. (1988) Contribution of two types of calcium currents to the pacemaker potentials of rabbit sino-atrial node cells. J. Physiol. 395, 233–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Doerr T., Denger R., Trautwein W. (1989) Calcium currents in single SA nodal cells of the rabbit heart studied with action potential clamp. Pflügers Arch. 413, 599–603 [DOI] [PubMed] [Google Scholar]
- 29. Ono K., Iijima T. (2010) Cardiac T-type Ca(2+) channels in the heart. J. Mol. Cell. Cardiol. 48, 65–70 [DOI] [PubMed] [Google Scholar]
- 30. Bub G., Tateno K., Shrier A., Glass L. (2003) Spontaneous initiation and termination of complex rhythms in cardiac cell culture. J. Cardiovasc. Electrophysiol. 14, S229–236 [DOI] [PubMed] [Google Scholar]
- 31. Hall C. E., Hurtado R., Hewett K. W., Shulimovich M., Poma C. P., Reckova M., Justus C., Pennisi D. J., Tobita K., Sedmera D., Gourdie R. G., Mikawa T. (2004) Hemodynamic-dependent patterning of endothelin converting enzyme 1 expression and differentiation of impulse-conducting Purkinje fibers in the embryonic heart. Development 131, 581–592 [DOI] [PubMed] [Google Scholar]
- 32. Knisley S. B., Justice R. K., Kong W., Johnson P. L. (2000) Ratiometry of transmembrane voltage-sensitive fluorescent dye emission in hearts. Am. J. Physiol. 279, H1421–1433 [DOI] [PubMed] [Google Scholar]
- 33. Xu C., Loew L. M. (2003) The effect of asymmetric surface potentials on the intramembrane electric field measured with voltage-sensitive dyes. Biophys. J. 84, 2768–2780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liau J., Dumas J., Janks D., Roth B. J., Knisley S. B. (2004) Cardiac optical mapping under a translucent stimulation electrode. Ann. Biomed. Eng. 32, 1202–1210 [DOI] [PubMed] [Google Scholar]
- 35. Nishizawa H., Suzuki T., Shioya T., Nakazato Y., Daida H., Kurebayashi N. (2009) Causes of abnormal Ca2+ transients in guinea pig pathophysiological ventricular muscle revealed by Ca2+ and action potential imaging at cellular level. PloS ONE 4, e7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hurtado R., Mikawa T. (2006) Enhanced sensitivity and stability in two-color in situ hybridization by means of a novel chromagenic substrate combination. Dev. Dyn. 235, 2811–2816 [DOI] [PubMed] [Google Scholar]
- 37. Marshall P. W., Rouse W., Briggs I., Hargreaves R. B., Mills S. D., McLoughlin B. J. (1993) ICI D7288, a novel sinoatrial node modulator. J. Cardiovasc. Pharmacol. 21, 902–906 [DOI] [PubMed] [Google Scholar]
- 38. Shin K. S., Rothberg B. S., Yellen G. (2001) Blocker state dependence and trapping in hyperpolarization-activated cation channels: evidence for an intracellular activation gate. J. Gen. Physiol. 117, 91–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gross D., Loew L. M., Webb W. W. (1986) Optical imaging of cell membrane potential changes induced by applied electric fields. Biophys. J. 50, 339–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cain J. E., Islam E., Haxho F., Blake J., Rosenblum N. D. (2011) GLI3 repressor controls functional development of the mouse ureter. J. Clin. Invest. 121, 1199–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schmidt-Nielsen B. (1987) The renal pelvis. Kidney Int. 31, 621–628 [DOI] [PubMed] [Google Scholar]
- 42. Silverstein D. M., Thornhill B. A., Leung J. C., Vehaskari V. M., Craver R. D., Trachtman H. A., Chevalier R. L. (2003) Expression of connexins in the normal and obstructed developing kidney. Pediatr. Nephrol. 18, 216–224 [DOI] [PubMed] [Google Scholar]
- 43. Bohn G., Moosmang S., Conrad H., Ludwig A., Hofmann F., Klugbauer N. (2000) Expression of T- and L-type calcium channel mRNA in murine sinoatrial node. FEBS Lett. 481, 73–76 [DOI] [PubMed] [Google Scholar]
- 44. Niwa N., Yasui K., Opthof T., Takemura H., Shimizu A., Horiba M., Lee J. K., Honjo H., Kamiya K., Kodama I. (2004) Cav3.2 subunit underlies the functional T-type Ca2+ channel in murine hearts during the embryonic period. Am. J. Physiol. 286, H2257–H2263 [DOI] [PubMed] [Google Scholar]
- 45. Masumiya H., Shijuku T., Tanaka H., Shigenobu K. (1998) Inhibition of myocardial L- and T-type Ca2+ currents by efonidipine: possible mechanism for its chronotropic effect. Eur. J. Pharmacol. 349, 351–357 [DOI] [PubMed] [Google Scholar]
- 46. Madle A., Linhartova K., Koza J. (2001) Effects of the T-type calcium channel blockade with oral mibefradil on the electrophysiologic properties of the human heart. Med. Sci. Monitor 7, 74–77 [PubMed] [Google Scholar]
- 47. Furukawa T., Miura R., Honda M., Kamiya N., Mori Y., Takeshita S., Isshiki T., Nukada T. (2004) Identification of R(-)-isomer of efonidipine as a selective blocker of T-type Ca2+ channels. Br. J. Pharmacol. 143, 1050–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tanaka H., Komikado C., Shimada H., Takeda K., Namekata I., Kawanishi T., Shigenobu K. (2004) The R(-)-enantiomer of efonidipine blocks T-type but not L-type calcium current in guinea pig ventricular myocardium. J. Pharmacol. Sci. 96, 499–501 [DOI] [PubMed] [Google Scholar]
- 49. Huang L., Keyser B. M., Tagmose T. M., Hansen J. B., Taylor J. T., Zhuang H., Zhang M., Ragsdale D. S., Li M. (2004) NNC 55–0396 [(1S,2S)-2-(2-(N-[(3-benzimidazol-2-yl)propyl]-N-methylamino)ethyl)-6-fluoro-1,2,3,4-tetrahydro-1-isopropyl-2-naphtyl cyclopropanecarboxylate dihydrochloride]: a new selective inhibitor of T-type calcium channels. J. Pharmacol. Exp. Ther. 309, 193–199 [DOI] [PubMed] [Google Scholar]
- 50. Lee S. E., Ahn D. S., Lee Y. H. (2009) Role of T-type Ca channels in the spontaneous phasic contraction of pregnant rat uterine smooth muscle. Korean J. Physiol. Pharmacol. 13, 241–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dwyer T. M., Schmidt-Nielsen B. (2003) The renal pelvis: machinery that concentrates urine in the papilla. News Physiol. Sci. 18, 1–6 [DOI] [PubMed] [Google Scholar]
- 52. Goel R., Srivathsan K., Mookadam M. (2013) Supraventricular and ventricular arrhythmias. Primary Care 40, 43–71 [DOI] [PubMed] [Google Scholar]
- 53. Nattel S. (2002) New ideas about atrial fibrillation 50 years on. Nature 415, 219–226 [DOI] [PubMed] [Google Scholar]
- 54. Nakagawa T., Misawa H., Nakajima Y., Takaki M. (2005) Absence of peristalsis in the ileum of W/W(V) mutant mice that are selectively deficient in myenteric interstitial cells of Cajal. J. Smooth Musc. Res. 41, 141–151 [DOI] [PubMed] [Google Scholar]
- 55. Lennartsson J., Ronnstrand L. (2012) Stem cell factor receptor/c-Kit: from basic science to clinical implications. Physiol. Rev. 92, 1619–1649 [DOI] [PubMed] [Google Scholar]
- 56. Seifert R., Scholten A., Gauss R., Mincheva A., Lichter P., Kaupp U. B. (1999) Molecular characterization of a slowly gating human hyperpolarization-activated channel predominantly expressed in thalamus, heart, and testis. Proc. Natl. Acad. Sci. U. S. A. 96, 9391–9396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Notomi T., Shigemoto R. (2004) Immunohistochemical localization of Ih channel subunits, HCN1-4, in the rat brain. J. Comp. Neurol. 471, 241–276 [DOI] [PubMed] [Google Scholar]
- 58. Harris N. C., Constanti A. (1995) Mechanism of block by ZD 7288 of the hyperpolarization-activated inward rectifying current in guinea pig substantia nigra neurons in vitro. J. Neurophysiol. 74, 2366–2378 [DOI] [PubMed] [Google Scholar]
- 59. Steriade M., Timofeev I. (2003) Neuronal plasticity in thalamocortical networks during sleep and waking oscillations. Neuron 37, 563–576 [DOI] [PubMed] [Google Scholar]
- 60. Ludwig A., Budde T., Stieber J., Moosmang S., Wahl C., Holthoff K., Langebartels A., Wotjak C., Munsch T., Zong X., Feil S., Feil R., Lancel M., Chien K. R., Konnerth A., Pape H. C., Biel M., Hofmann F. (2003) Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. EMBO J. 22, 216–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rouse W., Johnson I. R. (1994) Haemodynamic actions of a novel sino-atrial node function modulator, ZENECA ZD7288, in the anaesthetized dog: a comparison with zatebradine, atenolol and nitrendipine. Br. J. Pharmacol. 113, 1064–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Stieber J., Herrmann S., Feil S., Loster J., Feil R., Biel M., Hofmann F., Ludwig A. (2003) The hyperpolarization-activated channel HCN4 is required for the generation of pacemaker action potentials in the embryonic heart. Proc. Natl. Acad. Sci. U. S. A. 100, 15235–15240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Herrmann S., Stieber J., Stockl G., Hofmann F., Ludwig A. (2007) HCN4 provides a ‘depolarization reserve’ and is not required for heart rate acceleration in mice. EMBO J. 26, 4423–4432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Laish-Farkash A., Glikson M., Brass D., Marek-Yagel D., Pras E., Dascal N., Antzelevitch C., Nof E., Reznik H., Eldar M., Luria D. (2010) A novel mutation in the HCN4 gene causes symptomatic sinus bradycardia in Moroccan Jews. J. Cardiovasc. Electrophysiol. 21, 1365–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Roubille F., Tardif J. C. (2013) New therapeutic targets in cardiology: heart failure and arrhythmia: HCN channels. Circulation 127, 1986–1996 [DOI] [PubMed] [Google Scholar]
- 66. Baruscotti M., Bottelli G., Milanesi R., DiFrancesco J. C., DiFrancesco D. (2010) HCN-related channelopathies. Pflügers Arch. 460, 405–415 [DOI] [PubMed] [Google Scholar]
- 67. Xiao Y. F., Sigg D. C. (2007) Biological approaches to generating cardiac biopacemaker for bradycardia. Acta Physiol. Sin. 59, 562–570 [PubMed] [Google Scholar]
- 68. Cho H. C., Kashiwakura Y., Marban E. (2007) Creation of a biological pacemaker by cell fusion. Circ. Res. 100, 1112–1115 [DOI] [PubMed] [Google Scholar]
- 69. Plotnikov A. N., Sosunov E. A., Qu J., Shlapakova I. N., Anyukhovsky E. P., Liu L., Janse M. J., Brink P. R., Cohen I. S., Robinson R. B., Danilo P., Jr., Rosen M. R. (2004) Biological pacemaker implanted in canine left bundle branch provides ventricular escape rhythms that have physiologically acceptable rates. Circulation 109, 506–512 [DOI] [PubMed] [Google Scholar]
- 70. Qu J., Plotnikov A. N., Danilo P., Jr., Shlapakova I., Cohen I. S., Robinson R. B., Rosen M. R. (2003) Expression and function of a biological pacemaker in canine heart. Circulation 107, 1106–1109 [DOI] [PubMed] [Google Scholar]
- 71. Potapova I., Plotnikov A., Lu Z., Danilo P., Jr., Valiunas V., Qu J., Doronin S., Zuckerman J., Shlapakova I. N., Gao J., Pan Z., Herron A. J., Robinson R. B., Brink P. R., Rosen M. R., Cohen I. S. (2004) Human mesenchymal stem cells as a gene delivery system to create cardiac pacemakers. Circ. Res. 94, 952–959 [DOI] [PubMed] [Google Scholar]
- 72. Irisawa H., Kobayashi M. (1962) Intracellular action potentials of the Guinea pig ureter. Proc. Japan. Acad. 38, 171–175 [Google Scholar]
- 73. Weiss R. M., Wagner M. L., Hoffman B. F. (1967) Localization of pacemaker for peristalsis in the intact canine ureter. Invest. Urol. 5, 42–48 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.