Abstract
Autotaxin (ATX) is a secreted lysophospholipase D (lysoPLD) that binds to integrin adhesion receptors. We dissected the roles of integrin binding and lysoPLD activity in stimulation of human breast cancer and mouse aortic vascular smooth muscle cell migration by ATX. We compared effects of wild-type human ATX, catalytically inactive ATX, an integrin binding-defective ATX variant with wild-type lysoPLD activity, the isolated ATX integrin binding N-terminal domain, and a potent ATX selective lysoPLD inhibitor on cell migration using transwell and single-cell tracking assays. Stimulation of transwell migration was reduced (18 or 27% of control, respectively) but not ablated by inactivation of integrin binding or inhibition of lysoPLD activity. The N-terminal domain increased transwell migration (30% of control). ATX lysoPLD activity and integrin binding were necessary for a 3.8-fold increase in the fraction of migrating breast cancer cell step velocities >0.7 μm/min. ATX increased the persistent directionality of single-cell migration 2-fold. This effect was lysoPLD activity independent and recapitulated by the integrin binding N-terminal domain. Integrin binding enables uptake and intracellular sequestration of ATX, which redistributes to the front of migrating cells. ATX binding to integrins and lysoPLD activity therefore cooperate to promote rapid persistent directional cell migration.—Wu, T., Kooi, C. V., Shah, P., Charnigo, R., Huang, C., Smyth, S. S., Morris, A. J. Integrin-mediated cell surface recruitment of autotaxin promotes persistent directional cell migration.
Keywords: lysophospholipase, lysophosphatidic acid, cell motility, cell adhesion
Autotaxin (ATX), or ectonucleotide phosphatase/pyrophosphatase 2 (ENPP2) is a secreted lysophospholipase D (lysoPLD) that was originally identified as an autocrine motility factor for melanoma cells (1). ATX catalyzes production of the bioactive lipid mediator lysophosphatidic acid (LPA; 1-acyl,2-hydroxy-sn-glycerol 3-phosphate) by hydrolysis of lysophospholipids (2). LPA acts on G-protein-coupled receptors to promote growth, migration, and survival of many cell types (3). Although soluble ATX can generate LPA in biological fluids, including blood plasma and malignant effusions associated with breast and ovarian cancers (4), recruitment of ATX to the cell surface is emerging as an important process that enables localized LPA production to mediate cell-specific signaling responses (5). Binding of ATX to integrin class adhesion receptors on the surface of lymphocytes and endothelial cells in high endothelial venules promotes LPA-dependent transendothelial lymphocyte migration and homing to peripheral lymph nodes (6, 7). Agonist-stimulated binding of ATX to platelet integrins increases LPA synthesis by enabling access of the enzyme to lipid substrates generated during platelet activation (8, 9). These effects of ATX and LPA on platelets and blood cells may contribute to vascular inflammation during the initiation and progression of cardiovascular disease (10).
ATX expression is elevated in several cancers, and ATX promotes LPA-mediated effects on cancer cell migration, growth, and survival in vitro (4). The role of ATX in breast cancer initiation and progression is of particular interest because transgenic overexpression of ATX and certain LPA receptors in mammary epithelium is sufficient to induce a high incidence of invasive breast tumors in mice (11), and LPA signaling promotes breast cancer cell metastasis to bone, also in mouse models (12). These observations focused efforts on the development of potent selective small molecule ATX inhibitors that may prove to be effective cancer therapies (13–15). Integrin cell adhesion receptors are also well established to play a critical role in cancer metastasis and tumor angiogenesis (16). Both of these processes require directional cell migration, which is critically dependent on spatially and temporally regulated trafficking of key regulatory molecules to the leading edge of the migrating cell (17). Intracellular integrin trafficking is essential for focal adhesion turnover that underlies polarized breast cancer cell migration, invasion, and metastasis (18, 19). However, the role of integrins in the widely documented effects of ATX on growth, migration, and survival of breast and other cancer cells is presently not known. Building on the recently reported structures of ATX (20, 21) and the related enzyme ENPP1 (22), we used rationally designed ATX variants, isolated ATX domains, and a highly potent pharmacological inhibitor of ATX lysoPLD activity (13) to dissect the role of integrin binding and LPA signaling in the mechanisms by which ATX promotes MDA-MB-231 breast cancer cell and mouse aortic vascular smooth muscle cell (mAVSMC) migration. Our results identify LPA-dependent and -independent effects of ATX on migration of these cells measured using transwell and single-cell tracking assays. We show that integrin-mediated cell surface binding resulting in ATX uptake and intracellular trafficking are critical for the ability of ATX to promote rapid directionally persistent MDA-MB-231 cell migration.
MATERIALS AND METHODS
Antibodies and reagents
Rat anti-ATX monoclonal IgG 4F1 was generously provided by Junken Aoki (Sendai University, Shibati, Japan). Other antibodies, reagents, and their sources are as follows: mouse anti-paxillin monoclonal IgG 5H11 (Millipore, Billerica, MA, USA), rhodamine red X570-conjugated goat anti-rat IgG (Invitrogen, Carlsbad, CA, USA), DyLight549-conjugated goat anti-mouse IgG (Thermo Scientific, Waltham, MA, USA), Alexa Fluor 555-conjugated goat anti-mouse IgG (Invitrogen), Alexa Fluor 680-conjugated goat anti-rabbit IgG (Li-COR, Lincoln, NE, USA, and Molecular Probes, Eugene, OR, USA), and Alexa Fluor 647-conjugated goat anti-rat (Abcam, Cambridge, MA, USA). The β3 mouse monoclonal IgG 7E3, fibronectin, echistatin, and all other general reagents were from previously described sources (8, 9, 23).
Cell lines and fluorescence microscopy
αIIbβ3-overexpressing CHO cells were a gift from Dr. Zhenyu Li (University of Kentucky) and were grown in α-MEM containing 5% FBS. MDA-MB-231 cells were grown in high-glucose DMEM containing 5% FBS. Primary mouse aorta vascular smooth muscle cells were isolated and cultured as described previously (24). For indirect immunofluorescence measurements, MDA-MB-231 cells (from American Type Culture Collection, Manassas, VA, USA) were plated on Nunc Lab-Tek 8-well chambered no. 1.5 borosilicate cover glasses (Nunc, Roskilde, Denmark). Cells were fixed with 3.7% PFA, permeabilized with 0.1% Triton X-100 and 2% BSA in PBS for 20 min, and then blocked with 2% BSA in PBS. Primary antibodies were used at 5–10 mg/ml and incubated overnight at 4°C. Specimens were incubated with Fluorophore-conjugated secondary antibodies at room temperature for 1 h. DAPI was used to counterstain nuclei. Specimens were analyzed with a Nikon inverted microscope configured for either laser scanning microscopy (Nikon A1R resonance scanning confocal microscope with spectral detector; Nikon, Tokyo, Japan), total internal reflection microscopy, or transmitted light live cell wide-field imaging.
Expression and purification of recombinant proteins
Wild-type and mutant ATX proteins and the ATX somatomedin B-like 1,2 (SMB1,2) domain were generated by transient transfection of suspension cultures of CHO-S cells with plasmid vectors, purified as described previously and exchanged into PBS by repeated centrifugal filtration (9, 20). Purified recombinant ATX was labeled using a microscale Alexa Fluor 488 protein-labeling kit (Invitrogen). ATX concentrations and labeling efficiency were monitored by absorbance and fluorescence measurements. Structural integrity and function of Alexa Fluor 488-labeled ATX and ATX variants were confirmed by SDS-PAGE and enzyme activity assays. In some cases, recombinant proteins were subjected to trypsin digestion, and peptides were analyzed by mass spectrometry-based proteomic methods, as described in detail in Supplemental Material.
Determination of ATX lysoPLD and nucleotide phosphodiesterase activities
ATX lyso-PLD activity was determined using detergent-solubilized substrates using either a spectrophotometric coupled enzymatic assay with minor adaptations for use in a microplate reader or by measuring C17 LPA production from C17 lysoPC using selected ion monitoring mode HPLC tandem mass spectrometry (9). ATX nucleotide phosphodiesterase activity was measured colorimetrically using the nucleotide derivative PNP-TMP as a substrate (20).
Static cell adhesion assay
Adhesion of calcein-AM-labeled CHO or MDA-MB-231 cells to ATX, ATX fragments, mutants, or variants and fibrinogen or BSA immobilized on microtiter plates was determined as described previously (9). The number of adherent cells was determined by measuring fluorescence (excitation/emission=494/517 nm) and reference to a standard curve generated by fluorescence measurements, using independently quantitated numbers of cells (8, 9).
Transwell migration assays
MDA-MB-231 cell migration was measured in a modified Boyden chamber, as described previously (24) with adaptations from other reports (25–27). Mouse aortic vascular smooth muscle cells were isolated, and their migration was measured exactly as described previously (24). Briefly, 8-μm-pore polycarbonate filters (Neuro Probe, Gaithersburg, MD, USA) were coated with 0.001% (w/v) fibronectin (Sigma-Aldrich, St. Louis, MO, USA). Cells (1–3×105 cells in 200 μl per well) were suspended in serum-free RPMI 1640 medium containing 0.1% (w/v) BSA and allowed to adhere to the membrane for 1 h. Migration-promoting stimuli or inhibitors were added to the lower chamber in the same culture medium. After an incubation at 37°C for times ranging from 3 to 36 h migrated cells were stained (Diff-Quick staining kit, International Reagents). The number of cells that migrated to the bottom side of the chamber was determined by either counting the number of cell nuclei or by measuring the optical density of the staining reagent at 590 nm, using a microplate reader (VersaMax; Molecular Devices, Sunnyvale, CA, USA).
Time-lapse single-cell motility assay and cell trajectory analysis
Cells were treated with trypsin and resuspended in DMEM medium containing 0.1% fatty acid-free BSA (Sigma) and plated low densities on glass-bottomed dishes (MatTek, Ashland, MA, USA) that had been coated with 5 μg/ml fibronectin and cultured for 3 h in a CO2 incubator. After addition of motility stimulators or inhibitors, cell motility was measured with a Nikon Biostation IMQ. Wide-field stitched images (6×6 mm) were acquired every 10 min for 6 h. The movement of individual cells was analyzed using NIS-Element AR Software (Nikon; refs. 28, 29). Images and movies of migrating cells were also acquired using a Nikon Eclipse Ti TIRF microscope and live cell incubation system as described previously (29, 30).
Statistical analysis of single-cell migration data
Data in Figs. 3E and 4B–F were analyzed by 1-way ANOVA after log transformation. Data in Fig. 3D were analyzed by generalized linear mixed modeling, which accounted for correlations among the individual step velocities for each cell. Results of pairwise statistical comparisons are indicated in the figures using italic capital letters; 2 groups not sharing a letter are significantly different. Box plots show rectangles with 25th, 50th, and 75th percentiles and whiskers extending to 10th and 90th percentiles. Statistical significance was defined by a value of P < 0.05. Analyses were conducted and box plots generated using SAS 9.3 (SAS Institute, Cary, NC, USA).
Figure 3.
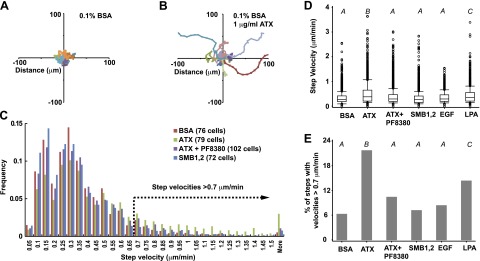
ATX stimulates migration of single MDA-MB-231 cells. A, B) Trajectories of 16 randomly selected MDA-MB-231 cells measured by monitoring the position of the cell nucleus every 10 min for 6 h in medium containing 0.1% fatty acid-free BSA with (B) and without (A) 1 μg/ml ATX. C) Individual MDA-MB-231 cells were tracked for 6 h by measuring the position of the cell nucleus every 10 min. Histogram shows the frequency distribution of step velocities measured for 37 tracked steps of the indicated number of MDA-MB-231 cells, determined in the presence of either BSA as control, 1 μg/ml ATX, 1 μg/ml ATX combined with 1 μM PF8380, or the ATX SMB1,2 domain. D) Effect of BSA, ATX, ATX in the presence of 1 μM PF8380, the ATX SMB1,2 domain (see C for number of cells analyzed), 100 ng/ml EGF (100 cells), or 1 μM 18:1 LPA (100 cells) on migration step velocities of individual MDA-MB-231 cells. E) Effect of BSA, ATX, ATX in the presence of 1 μM PF8380, the ATX SMB1,2 domain (see C for number of cells analyzed), 100 ng/ml EGF (100 cells), or 1 μM 18:1 LPA (100 cells) on the fraction of step velocities > 0.7 μm/min. D) Data were analyzed by generalized linear mixed modeling. E) Data were analyzed by 1-way ANOVA (see Materials and Methods for details). Results of pairwise comparisons are indicated using capital italic letters; 2 groups not sharing a letter are significantly different (P<0.05). Box plots show rectangles with 25th, 50th, and 75th percentiles and whiskers extending to the 10th and 90th percentiles.
Figure 4.
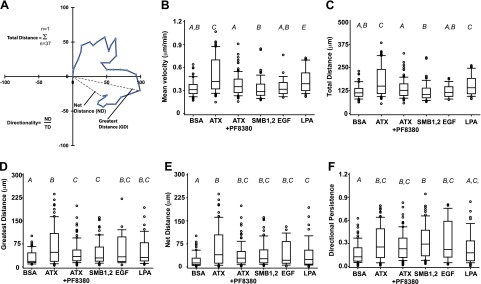
ATX increases the directional persistence of MDA-MD-231 cell migration. A) Illustration of data from a model single-cell tracking experiment showing the parameters measured to determine the total, net, and greatest distance moved and directionality. B–F) Effect of BSA, ATX, ATX in the presence of 1 μM PF8380, the ATX SMB1,2 domain (see Fig. 3C for number of cells analyzed), 100 ng/ml EGF (100 cells), or 1 μM 18:0 LPA (100 cells) on the mean migration velocity (B), total distance migrated (C), greatest distance migrated (D), net distance migrated (E), and directional persistence (F) of single MDA-MB-231 cell migration. Data were analyzed by 1-way ANOVA after log transformation. Results of pairwise comparisons are indicated using capital italic letters; 2 groups not sharing a letter are significantly different (P<0.05). Box plots show rectangles with 25th, 50th, and 75th percentiles and whiskers extending to 10th and 90th percentiles.
RESULTS
Identification of an integrin-binding-deficient ATX variant with near-wild-type PLD activity
ATX has a modular structure with tightly associated catalytic phosphodiesterase (PDE) and nuclease-like (NUC) domains and N-terminal tandem SMB1,2 domains (5). The isolated ATX SMB1,2 domain binds to β3 integrins on platelets and cultured cells through interactions that involve charged residues at the SMB2 domain surface (9, 20). The extensive interface between the SMB1 domain and the PDE domain forms a hydrophobic channel that has been proposed to play a critical role in substrate binding or product release by enabling ATX to access cell surface substrates or deliver LPA to cell surface receptors (21). In the course of studies to investigate the SMB1,2 PDE domain interaction we generated an ATX variant with an engineered disulfide bond designed to lock the SMB1 domain and PDE domains together by substituting the closely opposed SMB1 domain residues lysine 81 and PDE domain residue valine 279 with cysteine (Fig. 1A, B; note that valine 279 in human ATX corresponds to serine 279 in rat ATX, for which we have a structure). The variant protein ATXLys81/Cys;Val279/Cys (ATXLock) was efficiently expressed and secreted by CHO cells and behaved as a monomer during gel filtration chromatography (not shown). Proteomic analysis of tryptic peptides from reduced and nonreduced wild-type and ATXLock indicate that the engineered protein contains the predicted dilsulfide bond (Supplemental Fig. S1). Km values for hydrolysis of nucleotide and lysophospholipid substrates by ATXLock were comparable to those of the wild-type enzyme, although Vmax values against these substrates were modestly decreased (Fig. 1C, D). The spectrum of LPA species generated by ATXLock when incubated with human plasma as a source of lysoPC substrates was also not significantly different from that observed with wild-type ATX, indicating that selectivity of ATXLock for different lysoPC chain lengths was also unaltered (not shown). As reported previously (9), β3-integrin-expressing CHO (CHO-β3) cells bound to wild-type ATX immobilized on microtiter plates to a comparable extent to that observed with the positive control fibrinogen. These cells bound to a lesser, but still significant, extent to the isolated ATX SMB1,2 domain. In all cases, binding of CHO-β3 cells to these immobilized ligands was promoted by the integrin activator Mn2+ and blocked by the β3 integrin antagonist peptide echistatin. Surprisingly, CHO-β3 cells did not bind significantly to ATXLock (Fig. 1E). Fluorescently labeled MDA-MB-231 breast cancer cells also bound to wild-type ATX and ATX SMB1,2, in an Mn2+-promoted manner (although, in contrast to observations with CHO-β3 cells, binding of MDA-MB-231 cells was comparatively lower to ATX than to the fibrinogen control. As with CHO-β3 cells, binding of MDA-MB-231 cells to ATXLock was not significantly higher than to the BSA negative control (Fig. 1F). Nonconservative substitutions of residues in the SMB2 domain that attenuated binding of CHO-β3 cells and platelets to ATX (9, 20) also attenuated binding to MDA-MB-231 cells, providing further evidence binding of ATX to these cells involves comparable mechanisms (not shown). Taken together, these observations identify ATXLock as an integrin-binding-defective ATX variant with near-wild-type lipid and nucleotide phosphodiesterase activities. Because binding of MDA-MB-231 cells to wild-type ATX was promoted by Mn2+ and inhibited by echistatin, it is likely that this binding is mediated by integrin αVβ3, which is further supported by observations that blocking antibodies targeting this integrin also inhibit MDA-MB-231 interactions with ATX (see Fig. 5).
Figure 1.
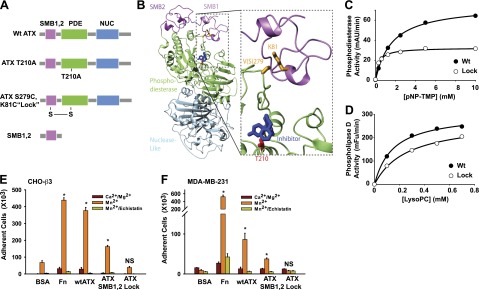
An ATX variant containing an engineered disulfide bond linking the SMB1 and PDE domains retains near-wild-type catalytic activity but does not bind to β3 integrins. A) Schematic diagram of wild-type ATX and the ATX variants and fragments used in these studies. B) Rat ATX structure identifying residues mutated to introduce the SMB1-PDE domain-linking disulfide residue. Note that V279 in the rat sequence corresponds to S279 in human ATX, which was used for our studies. C, D) Comparison of nucleotide phosphodiesterase (C) and lysoPLD (D) activities of wild-type (wt) ATX and ATXLock (means of duplicate determinations). E, F) Binding of β3-integrin-overexpressing CHO (CHO-β3; E) or MDA-MB-231 (F) cells to wild-type ATX, ATX SMB1,2, ATXLock, and fibrinogen (Fn) and BSA as positive and negative controls, respectively, determined in buffer containing either 10 μM Ca2+ and Mg2+, 500 μM Mn2+ or 500 μM Mn2+, and 20 μM echistatin, as described in Materials and Methods. Data are means ± sd of triplicate determinations from experiments repeated ≥3 times. Statistical significance evaluated by Student's t test. N.S. not significant (P>0.05). *P < 0.001.
Figure 5.
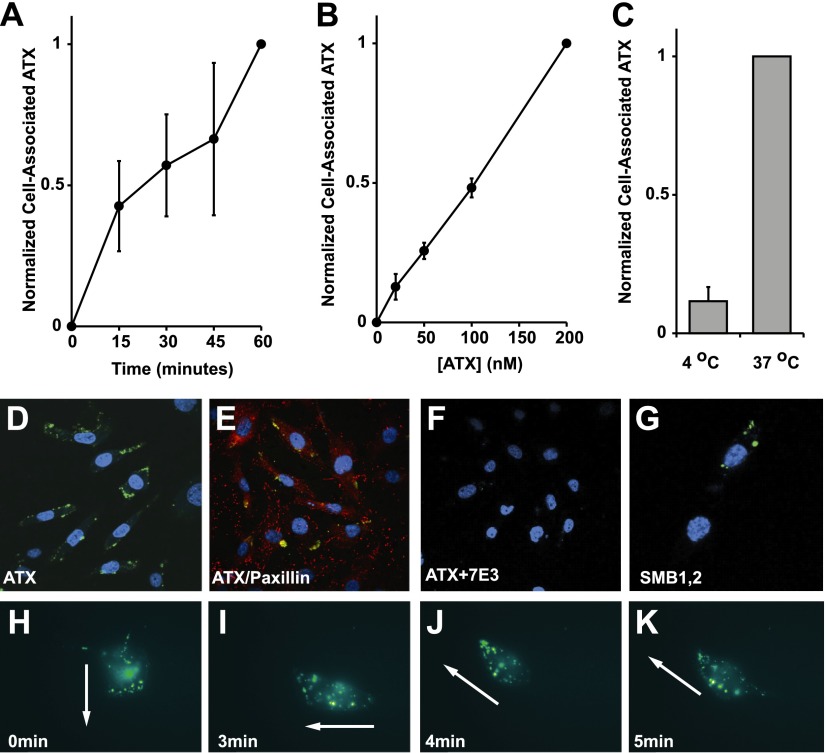
Uptake, intracellular accumulation, and redistribution of exogenous ATX by MDA-MB-231 cells. A–C) Time (A), ATX-concentration (B), and temperature dependence (C) of accumulation of Alexa Fluor 488-labeled ATX by MDA-MB-231 cells (means± sd of triplicate determinations from a representative experiment). D) Intracellular localization of Alexa Fluor 488-labeled ATX (green). E) Visualization of intracellular Alexa Fluor 488-labeled ATX (green) and the focal adhesion marker paxilin (red). F) Association of Alex Fluor 488-ATX with MDA-MB-231 cells is inhibited by preincubation with the β3-integrin-blocking antibody 7E3. G) Intracellular localization of Alexa Fluor 488-labeled ATX SMB1,2 domain (green). Cells are counterstained with DAPI (blue) to visualize the nucleus. H–K) Still images from Supplemental Movie S1 show Alexa Fluor 488 localization (green) in a single migrating MDA-MB-231 as it changes direction, at 0 min (H), 3 min (I), 4 min (J), and 5 min (K). Arrows indicate direction of cell migration at indicated times.
Stimulation of MDA-MB-231 cell migration by ATX in transwell assays is only partially dependent on lysoPLD activity
ATX stimulates migration of a wide variety of cell types through membrane filters in transwell assays, and these effects are well established to be largely dependent on lysoPLD activity and LPA receptor-mediated cellular responses (31). As previously reported (2), in incubations containing low levels of serum as a source of lysophospholipid substrates, ATX produced concentration-dependent increases in MDA-MB-231 cell transwell migration with maximal effects (∼9-fold increase over control) observed at 0.5-1 μg/ml (Fig. 2A). In comparison to vehicle control, increasing concentrations of the small-molecule ATX inhibitor PF8380 produced a 73% decrease in MDA-MB-231 cell migration in response to 1 μg/ml ATX that was maximal at 0.1 μM but did not decrease further at higher concentrations of the inhibitor (Fig. 2B). As reported previously (13) and confirmed by ourselves (not shown) the Ki for inhibition of ATX by PF8380 is ∼10 nM. Consistent with this high potency, ATX activity measured under the conditions used for the transwell migration assays was inhibited to undetectable levels by PF8380 concentrations in excess of 1 μM (Fig. 2B). This result suggests that promotion of MDA-MB-231 cell migration by ATX is partly independent of LPA generation.
Figure 2.
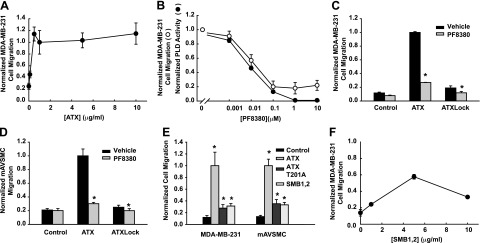
Integrin binding is necessary for lysoPLD-dependent and -independent effects of ATX on MDA-MB-231 cell migration in transwell assays. A) Effects of increasing concentrations of ATX on MDA-MB-231 cell migration. B) Effects of increasing concentrations of the ATX lysoPLD inhibitor PF8380 on MDA-MB-231 cell transwell migration (open circles) and ATX lysoPLD activity (solid circles). C) Comparison of effects of 1 μg/ml ATX and 1 μg/ml ATXLock on MDA-MB-231 cell migration in the presence or absence of vehicle or 1 μM PF8380. D) Comparison of effects of 1 μg/ml ATX and 1 μg/ml ATXLock on mAVSMC migration in the presence of vehicle or 1 μM PF8380. E) Comparison of effects of 1 μg/ml ATX, 1 μg/ml ATXT201A, and 1 μg/ml SMB1,2 on MDA-MB-231 cell and mAVSMC migration. F) Effects of increasing concentrations of ATX SMB1,2 on MDA-MB-231 cell migration. The number of cells migrating under the indicated experimental conditions was normalized to the number of cells migrating in response to 1 μg/ml ATX. Data are means ± sd of triplicate determinations from experiments repeated ≥3 times. Statistical significance evaluated by Student's t test. N.S., not significant (P>0.05). *P < 0.001.
Integrin binding is necessary for lysoPLD-dependent and -independent effects of ATX on cell migration
To investigate the role of integrin binding in stimulation of cell migration by ATX we compared effects of 1 μg/ml wild-type ATX and ATXLock on transwell migration of MDA-MB-231 cells. To extend these observations to another cell type we conducted parallel experiments using primary mAVSMCs, which we have previously reported exhibit robust transwell migration in response to LPA (24). Despite having near-wild-type lysoPLD activity the ATXLock variant stimulated MDA-MB-231 cell migration only 1.6-fold (Fig. 2C). Inhibition of lysoPLD activity with 1 μM PF8380 decreased migration in response to both wild-type ATX and ATX lock, suggesting that the modest effect of ATXLock on migration is primarily mediated by LPA. The pattern of migration responses of mAVSMCs to wild-type ATX, ATXLock, and PF8380 treatment was similar to that observed with MDA-MB-231 cells. These data indicate that stimulation of MDA-MB-231 and mAVSMC migration by ATX in transwell assays is largely dependent on LPA generation but provide new evidence that ATX binding to cell surface integrins is necessary for ATX ability to stimulate MDA-MB-231 cell and mAVSMC migration with full efficacy.
Role of integrin binding in lysoPLD-independent effects of ATX on MDA-MB-231 cell migration
As shown in Fig. 1E, F, CHO-β3 and MDA-MB-231 cells both bind to the isolated ATX SMB1,2 domain, so we compared effects of this ATX fragment to effects of wild-type ATX and catalytically inactive ATX T210A on MDA-MB-231 cell and mAVSMC migration in transwell assays. In comparison to 1 μg/ml wild-type ATX, 1 μg/ml of either ATXT210A or SMB1,2 promoted moderate but still significant increases in transwell migration of both cell types (Fig. 2E, F). The effect of SMB1,2 on MDA-MB-231 cell migration was concentration dependent, and a maximal effect (∼40% of that observed with ATX) was observed at a concentration of 5 μg/ml. Taken together, our observations using ATXT210A and the SMB1,2 domain substantiate our studies using PF8380 providing evidence for a component of the effect of ATX on MDA-MB-231 cell and mAVSMC migration that is independent of lysoPLD activity. The explanation for the reduced potency with which the SMB1,2 domain promotes transwell cell migration in comparison to wild-type ATX is presently not known.
ATX stimulates directional single-cell MDA-MB-231 cell migration through LPA-dependent and -independent mechanisms
The transwell migration assays described above cannot provide information about effects of ATX on the speed and directional persistence of MDA-MB-231 cell migration and are complicated by the direct effects of ATX as an adhesive ligand for these cells. We therefore used single-cell time-lapse live cell imaging video microscopy to characterize effects of ATX on MDA-MB231 cell migration. We compared effects of ATX (in the presence and absence of the ATX inhibitor PF8380) and the ATX SMB1,2 domains on MDA-MB-231 cell migration and used epidermal growth factor and LPA, which have been previously shown to promote migration of these cells in both transwell and single-cell tracking assays (27, 32) as controls. Wide field images of cells were acquired every 10 min for 6 h and processed to monitor the location of the nucleus of 70–100 randomly selected individual cells. Figure 3A, B shows trajectories for 16 randomly selected cells in medium containing either 0.1% fatty acid-free BSA as control or 1 μg/ml ATX. In comparison to control, cell motility was clearly increased in the presence of ATX. To enable robust statistical comparisons of the effects of experimental manipulations on single-cell MDA-MB-231 cell migration, we next examined the frequency distribution of step velocities (the speed of migration of an individual cell during each 10-min observation period) exhibited by ∼100 individual cells. Consistent with other reports using single-cell tracking to study migration (28), these data revealed considerable heterogeneity in the velocity of cell migration during these individual observed steps (Fig. 3C). A comparison between the individual step velocities exhibited by all cells within our sample revealed that ATX produced a modest but significant increase in the migration step velocity of individual MDA-MB-231cells. This effect was sensitive to inhibition with PF8380 and not observed with the ATX SMB1,2 domain (Fig. 3D). LPA, but not EGF, also increased MDA-MB-231 cell step velocities above control. By inspection of Fig. 3C, it is apparent that individual MDA-MB-231 cell migration is stochastic during the 6-h experiment such that the majority of step velocities are low and consequently difficult to measure accurately because they result in small migration distances during the 10-min observation period. By setting an arbitrary threshold of >0.7 μm/min for analysis of these data to define “fast-moving” steps that can be more precisely measured, ATX produced a 3.8-fold increase in the percentage of cells exhibiting step velocities > 0.7 μm/min, and this increase was abolished in the presence of 1 μM PF8380. Again, only LPA but not EGF increased fraction of step velocities >0.7 μm/min but to a lesser extent (1.2-fold) than was observed with ATX. The estimated correlation between successive step velocities for all cells within our observation group was 0.30 with P > 0.0001, indicating that a fast-moving step is more often preceded by another fast-moving step than by a slow step. Migrating MDA-MB-231 cells therefore appear to exhibit episodes of fast migration, although further studies will be required to investigate the effect of migration promoting stimuli on the duration of these. As shown in Fig. 4A, these single-cell tracking data can also be analyzed to calculate the mean step velocity as well as the total, net, and greatest distance moved by the cells and the persistence of directionality of migration (the net distance moved divided by the total distance moved), which is a way to quantitate the tendency of an individual cell to maintain its direction of migration between observed steps. Again, ATX and to a lesser extent LPA increased the mean migration velocity and total distance migrated (Fig. 4C), while all of the stimuli examined (including ATX in the presence of PF8380 and the ATX SMB1,2 domain) increased the greatest and net distance migrated by single MDA-MB-231 cells. Of particular interest, ATX either alone or in the presence of PF8380 and the ATX SMB1,2 domain but not the ATX product LPA produced significant increases in the directional persistence of single MDA-MB-231 cell migration. Taken together, these observations provide evidence for distinct mechanisms by which ATX can promote single MDA-MB-231 cell migration. Production of LPA, which is inhibited by PF8380, increases the migration velocity of the cells (observed most clearly as an increase in migration steps with velocities>0.7 μm/min), while a lysoPLD-independent mechanism that persists in the presence of PF8380 and can be recapitulated by incubation of cells with the isolated ATX SMB1,2 domains increases the persistent directionality of migration.
Uptake and polarized redistribution of ATX by migrating MDA-MB-231 cells
To augment our observations of MDA-MB-231 cell adhesion to ATX we examined the association of soluble Alexa Fluor 488-labeled ATX with MDA-MB-231 cells by flow cytometry. Association of ATX with these cells was time and concentration dependent but surprisingly was not saturable at ATX concentrations up to 200 nM (22 μg/ml). Association of ATX with MDA-MB-231 cells was reduced by ∼80% when the cells were incubated at 4°C, suggesting the possibility that ATX was being internalized (Fig. 5A–C). When MDA-MB-231 cell-associated ATX was visualized by fluorescence microscopy we observed a bright discontinuous punctate localization close to the cell periphery. A similar localization pattern was observed when cells were incubated with the Alexa Fluor 488-labeled ATX SMB1,2 domain, and incorporation of fluorescently labeled ATX into these structures was inhibited in the presence of the β3-integrin-blocking antibody 7E3 (Fig. 5D, F, G). These puncta did not colocalize significantly with the focal adhesion marker paxillin (Fig. 5E) or with markers for early (EEA-1) or late (Rab 7) endosomes (not shown). Because this uptake and intracellular accumulation of ATX would be occurring during our studies of the effects of ATX on MDA-MB-231 cell migration we used TIRF microscopy to image cell-associated Alexa Fluor 488- labeled ATX during migration of single MDA-MB-231 cells. We observed significant location of ATX at the leading edge of migrating cells, and this localization pattern was preserved in cells during relatively rare instances where we observed the cell changing the direction of migration, as shown in Fig. 5 and Supplemental Movie S1. Taken together, these findings indicate that integrin binding enables uptake and intracellular accumulation of ATX in a juxta membrane compartment that is oriented toward the leading edge of migrating cells under conditions where ATX promotes rapid directionally persistent cell migration.
DISCUSSION
Our results indicate that localized production of LPA at the surface of MDA-MB-231 cells is necessary for potent stimulation of migration. Support for this conclusion comes from studies using the ATXLock mutant, which retains near-wild-type lysoPLD activity but does not bind to cell surface β3 integrins. The rationale for making this ATX variant came from efforts to understand the role of the hydrophobic channel formed by the PDE SMB1,2 domain interface in substrate binding and product release. While these domains are closely opposed and form multiple contacts in the crystal structure of ATX (20), the SMB1,2 domains do not contact the PDE domain in the crystal structure of the closely related ENPP1 enzyme (22). Instead, they are present but not visible, suggesting flexibility that allows multiple packing orientations in the crystals (20). The difference in orientation of the SMB1,2 domains in ATX and ENPP1 has been attributed to steric effects of an “insertion loop” that is present in ENPP1 but deleted in ATX to form a pocket that determines selectivity for lipid substrates (22). This adaptation discriminates ATX, which is a secreted soluble protein, from ENPP1 and other ENPP family members that are anchored at the cell surface by their N-terminal transmembrane sequences. Our observations raise the possibility that the interaction between the SMB and PDE domains in ATX could be regulated to control integrin binding. Further structural and biophysical studies will be needed to address this possibility.
Widely reported effects of ATX on cell migration are established to be substantially dependent on availability of lysophospholipid substrates for the lysoPLD activity of ATX, which generates LPA to act on LPA receptors expressed by the migrating cells (2). Our observations from transwell migration assays and single-cell tracking studies are also consistent with an important role for ATX lysoPLD activity in increasing the velocity and distance migrated by two distinct cell types, MDA-MB-231 breast cancer and mAVSMCs, but provide new evidence that a component of the mechanism by which ATX promotes migration of these cells is independent of catalysis. This conclusion is based on our finding that ∼20% of the effect of ATX on transwell migration of both cell types we studied is insensitive to a potent inhibitor of ATX lysoPLD activity and by observations that both a catalytically inactive ATX mutant and the isolated ATX SMB1,2 domains can stimulate migration of MDA-MB-231 cells and mAVSMCs in transwell assays. To provide more detailed information about how ATX increases cell migration than can be obtained from transwell assays, we undertook the first reported study of the effects of ATX on single-cell migration. The data obtained revealed mechanistically separate effects of ATX on the velocity and directional persistence of MDA-MB-231 cell migration. Our results indicate that the lysoPLD activity of ATX acting on cell-derived substrates results in increased episodes of rapid migration by single MDA-MB-231 cells. Since the effects of ATX on rapid migration steps of these cells are also dependent on integrin binding and are observed under conditions where ATX is actively accumulated by migrating cells and redistributed to the leading edge we propose that they involve localized LPA production and signaling actions on LPA receptors at the front of migrating cells. Interestingly, ATX administered in the presence of an ATX inhibitor or the isolated ATX SMB1,2 domains did not promote rapid single MDA-MB-231 cell migration but instead increased the persistence of constitutive directional migration of these cells to an extent that was comparable to that observed in cells treated with wild-type ATX. Although the mechanism remains to be established, these observations suggest that the interaction of ATX with MDA-MB-231 cell integrins mediated by the SMB1,2 domains promotes an LPA-independent response that maintains the directionality of migrating cells. These effects might explain the ability of the isolated ATX SMB1,2 domain to promote migration of MDA-MB-231 cells in transwell assays and could be related to catalytic activity-independent effects of ATX on neuronal cell morphology and focal adhesion organization that have been reported by others (33). Taken together, our results support a model in which these LPA-dependent and -independent mechanisms cooperate to drive rapid directionally persistent MDA-MB-231 cell migration. While trafficking of β3 integrins is clearly important for establishing polarity and direction in migrating cells (18), and our studies indicate that ATX interactions with these cells that are important for stimulation of directional cell migration also involve β3 integrins, more work will be needed to ascertain the relationship between these two processes. In particular, the identity of the intracellular compartment that accumulates extracellularly administered ATX and its relationship to endocytic compartments known to be important in integrin recycling needs to be better defined.
Our finding that ATX promotes directionally persistent cell migration could explain the observation that ATX is essential for normal blood vessel formation in mice and zebrafish. In this latter system, ATX deficiency results in aberrant vascular connections between segmental arteries that sprout from the dorsal aorta during development because polarized vascular endothelial cell migration becomes disorganized (34, 35). The ATX/LPA axis plays important roles in cancer, cardiovascular disease and vascular injury responses and lung fibrosis. In each case, an important component of the underlying mechanism involves effects of ATX on cell migration (14). The development of small-molecule ATX inhibitors typified by the PF8380 compound used in this study has been driven by efforts to target alterations in LPA metabolism and signaling that underlie these diseases. In addition to directly inhibiting ATX activity, our findings suggest that pharmacological inhibition of ATX interactions with integrins might also be an effective strategy to ablate localized LPA signaling. For example, small molecule integrin antagonists might act synergistically with inhibitors of ATX lysoPLD activity, and it is also possible that some of the clinical effects of integrin antagonists, particularly those used in cancer therapy (36), might involve effects on ATX and LPA signaling.
Supplementary Material
Acknowledgments
This work was supported by grants from the U.S. National Institutes of Health and the U.S. Department of Veterans Affairs to A.J.M. and S.S.S. T.W. was the recipient of an American Heart Association postdoctoral fellowship. This material is also based on work supported in part by resources at the Lexington Veterans Affairs Medical Center.
The authors are grateful to Manjula Sunkara, Fanmuyi Yang, and Frederick Onono for their contributions to the project.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- ATX
- autotaxin
- ATXLock
- ATXLys81/Cys;Val279/Cys
- CHO-β3
- β3-integrin-expressing CHO
- ENPP
- ectonucleotide phosphatase/pyrophosphatase
- LPA
- lysophosphatidic acid (1-acyl,2-hydroxy-sn-glycerol 3-phosphate)
- lysoPLD
- lysophospholipase D
- mAVSMC
- mouse aortic vascular smooth muscle cell
- PDE
- phosphodiesterase
- SMB1,2
- somatomedin B-like 1,2
REFERENCES
- 1. Stracke M. L., Krutzsch H. C., Unsworth E. J., Arestad A., Cioce V., Schiffmann E., Liotta L. A. (1992) Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J. Biol. Chem. 267, 2524–2529 [PubMed] [Google Scholar]
- 2. Umezu-Goto M., Kishi Y., Taira A., Hama K., Dohmae N., Takio K., Yamori T., Mills G. B., Inoue K., Aoki J., Arai H. (2002) Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J. Cell Biol. 158, 227–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lin M. E., Herr D. R., Chun J. (2010) Lysophosphatidic acid (LPA) receptors: signaling properties and disease relevance. Prostaglandins Other Lipid Mediat. 91, 130–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Houben A. J., Moolenaar W. H. (2011) Autotaxin and LPA receptor signaling in cancer. Cancer Metastasis Rev. 30, 557–565 [DOI] [PubMed] [Google Scholar]
- 5. Moolenaar W. H., Perrakis A. (2011) Insights into autotaxin: how to produce and present a lipid mediator. Nat. Rev. Mol. Cell Biol. 12, 674–679 [DOI] [PubMed] [Google Scholar]
- 6. Bai Z., Cai L., Umemoto E., Takeda A., Tohya K., Komai Y., Veeraveedu P. T., Hata E., Sugiura Y., Kubo A., Suematsu M., Hayasaka H., Okudaira S., Aoki J., Tanaka T., Albers H. M., Ovaa H., Miyasaka M. (2013) Constitutive lymphocyte transmigration across the basal lamina of high endothelial venules is regulated by the autotaxin/lysophosphatidic acid axis. J. Immunol. 190, 2036–2048 [DOI] [PubMed] [Google Scholar]
- 7. Kanda H., Newton R., Klein R., Morita Y., Gunn M. D., Rosen S. D. (2008) Autotaxin, an ectoenzyme that produces lysophosphatidic acid, promotes the entry of lymphocytes into secondary lymphoid organs. Nat. Immunol. 9, 415–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pamuklar Z., Federico L., Liu S., Umezu-Goto M., Dong A., Panchatcharam M., Fulkerson Z., Berdyshev E., Natarajan V., Fang X., van Meeteren L. A., Moolenaar W. H., Mills G. B., Morris A. J., Smyth S. S. (2009) Autotaxin/lysopholipase D and lysophosphatidic acid regulate murine hemostasis and thrombosis. J. Biol. Chem. 284, 7385–7394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fulkerson Z., Wu T., Sunkara M., Kooi C. V., Morris A. J., Smyth S. S. (2011) Binding of autotaxin to integrins localizes lysophosphatidic acid production to platelets and mammalian cells. J. Biol. Chem. 286, 34654–34663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morris A. J., Smyth S. S. (2013) Lysophosphatidic acid and cardiovascular disease: seeing is believing. J. Lipid Res. 54, 1153–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu S., Umezu-Goto M., Murph M., Lu Y., Liu W., Zhang F., Yu S., Stephens L. C., Cui X., Murrow G., Coombes K., Muller W., Hung M. C., Perou C. M., Lee A. V., Fang X., Mills G. B. (2009) Expression of autotaxin and lysophosphatidic acid receptors increases mammary tumorigenesis, invasion, and metastases. Cancer Cell 15, 539–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. David M., Wannecq E., Descotes F., Jansen S., Deux B., Ribeiro J., Serre C. M., Gres S., Bendriss-Vermare N., Bollen M., Saez S., Aoki J., Saulnier-Blache J. S., Clezardin P., Peyruchaud O. (2010) Cancer cell expression of autotaxin controls bone metastasis formation in mouse through lysophosphatidic acid-dependent activation of osteoclasts. PloS ONE 5, e9741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gierse J., Thorarensen A., Beltey K., Bradshaw-Pierce E., Cortes-Burgos L., Hall T., Johnston A., Murphy M., Nemirovskiy O., Ogawa S., Pegg L., Pelc M., Prinsen M., Schnute M., Wendling J., Wene S., Weinberg R., Wittwer A., Zweifel B., Masferrer J. (2010) A novel autotaxin inhibitor reduces lysophosphatidic acid levels in plasma and the site of inflammation. J. Pharmacol. Exp. Ther. 334, 310–317 [DOI] [PubMed] [Google Scholar]
- 14. Albers H. M., Ovaa H. (2012) Chemical evolution of autotaxin inhibitors. Chem. Rev. 112, 2593–2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Albers H. M., Dong A., van Meeteren L. A., Egan D. A., Sunkara M., van Tilburg E. W., Schuurman K., van Tellingen O., Morris A. J., Smyth S. S., Moolenaar W. H., Ovaa H. (2010) Boronic acid-based inhibitor of autotaxin reveals rapid turnover of LPA in the circulation. Proc. Natl. Acad. Sci. U. S. A. 107, 7257–7262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Desgrosellier J. S., Cheresh D. A. (2010) Integrins in cancer: biological implications and therapeutic opportunities. Nat. Rev. Cancer 10, 9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huttenlocher A. (2005) Cell polarization mechanisms during directed cell migration. Nat. Cell Biol. 7, 336–337 [DOI] [PubMed] [Google Scholar]
- 18. Bridgewater R. E., Norman J. C., Caswell P. T. (2012) Integrin trafficking at a glance. J. Cell Sci. 125, 3695–3701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Felding-Habermann B., O'Toole T. E., Smith J. W., Fransvea E., Ruggeri Z. M., Ginsberg M. H., Hughes P. E., Pampori N., Shattil S. J., Saven A., Mueller B. M. (2001) Integrin activation controls metastasis in human breast cancer. Proc. Natl. Acad. Sci. U. S. A. 98, 1853–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hausmann J., Kamtekar S., Christodoulou E., Day J. E., Wu T., Fulkerson Z., Albers H. M., van Meeteren L. A., Houben A. J., van Zeijl L., Jansen S., Andries M., Hall T., Pegg L. E., Benson T. E., Kasiem M., Harlos K., Kooi C. W., Smyth S. S., Ovaa H., Bollen M., Morris A. J., Moolenaar W. H., Perrakis A. (2011) Structural basis of substrate discrimination and integrin binding by autotaxin. Nat. Struct. Mol. Biol. 18, 198–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nishimasu H., Okudaira S., Hama K., Mihara E., Dohmae N., Inoue A., Ishitani R., Takagi J., Aoki J., Nureki O. (2011) Crystal structure of autotaxin and insight into GPCR activation by lipid mediators. Nat. Struct. Mol. Biol. 18, 205–212 [DOI] [PubMed] [Google Scholar]
- 22. Kato K., Nishimasu H., Okudaira S., Mihara E., Ishitani R., Takagi J., Aoki J., Nureki O. (2012) Crystal structure of Enpp1, an extracellular glycoprotein involved in bone mineralization and insulin signaling. Proc. Natl. Acad. Sci. U. S. A. 109, 16876–16881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Salous A. K., Panchatcharam M., Sunkara M., Mueller P., Dong A., Wang Y., Graf G. A., Smyth S. S., Morris A. J. (2013) Mechanism of rapid elimination of lysophosphatidic acid and related lipids from the circulation of mice. J. Lipid Res. 54, 2775–2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Panchatcharam M., Miriyala S., Salous A., Wheeler J., Dong A., Mueller P., Sunkara M., Escalante-Alcalde D., Morris A. J., Smyth S. S. (2013) Lipid phosphate phosphatase 3 negatively regulates smooth muscle cell phenotypic modulation to limit intimal hyperplasia. Arterioscler. Thromb. Vasc. Biol. 33, 52–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Helbig G., Christopherson K. W., 2nd, Bhat-Nakshatri P., Kumar S., Kishimoto H., Miller K. D., Broxmeyer H. E., Nakshatri H. (2003) NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J. Biol. Chem. 278, 21631–21638 [DOI] [PubMed] [Google Scholar]
- 26. Muller A., Homey B., Soto H., Ge N., Catron D., Buchanan M. E., McClanahan T., Murphy E., Yuan W., Wagner S. N., Barrera J. L., Mohar A., Verastegui E., Zlotnik A. (2001) Involvement of chemokine receptors in breast cancer metastasis. Nature 410, 50–56 [DOI] [PubMed] [Google Scholar]
- 27. Price J. T., Tiganis T., Agarwal A., Djakiew D., Thompson E. W. (1999) Epidermal growth factor promotes MDA-MB-231 breast cancer cell migration through a phosphatidylinositol 3′-kinase and phospholipase C-dependent mechanism. Cancer Res. 59, 5475–5478 [PubMed] [Google Scholar]
- 28. Delorme-Walker V. D., Peterson J. R., Chernoff J., Waterman C. M., Danuser G., DerMardirossian C., Bokoch G. M. (2011) Pak1 regulates focal adhesion strength, myosin IIA distribution, and actin dynamics to optimize cell migration. J. Cell Biol. 193, 1289–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li X., Zhou Q., Sunkara M., Kutys M. L., Wu Z., Rychahou P., Morris A. J., Zhu H., Evers B. M., Huang C. (2013) Ubiquitylation of phosphatidylinositol 4-phosphate 5-kinase type I gamma by HECTD1 regulates focal adhesion dynamics and cell migration. J. Cell Sci. 126, 2617–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu Z., Li X., Sunkara M., Spearman H., Morris A. J., Huang C. (2011) PIPKIγ regulates focal adhesion dynamics and colon cancer cell invasion. PLoS ONE 6, e24775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Van Meeteren L. A., Moolenaar W. H. (2007) Regulation and biological activities of the autotaxin-LPA axis. Prog. Lipid Res. 46, 145–160 [DOI] [PubMed] [Google Scholar]
- 32. Alemayehu M., Dragan M., Pape C., Siddiqui I., Sacks D. B., Di Guglielmo G. M., Babwah A. V., Bhattacharya M. (2013) beta-Arrestin2 regulates lysophosphatidic acid-induced human breast tumor cell migration and invasion via Rap1 and IQGAP1. PloS ONE 8, e56174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yuelling L. M., Fuss B. (2008) Autotaxin (ATX): a multi-functional and multi-modular protein possessing enzymatic lysoPLD activity and matricellular properties. Biochim. Biophys. Acta 1781, 525–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yukiura H., Hama K., Nakanaga K., Tanaka M., Asaoka Y., Okudaira S., Arima N., Inoue A., Hashimoto T., Arai H., Kawahara A., Nishina H., Aoki J. (2011) Autotaxin regulates vascular development via multiple lysophosphatidic acid (LPA) receptors in zebrafish. J. Biol. Chem. 286, 43972–43983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Van Meeteren L. A., Ruurs P., Stortelers C., Bouwman P., van Rooijen M. A., Pradere J. P., Pettit T. R., Wakelam M. J., Saulnier-Blache J. S., Mummery C. L., Moolenaar W. H., Jonkers J. (2006) Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol. Cell. Biol. 26, 5015–5022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goodman S. L., Picard M. (2012) Integrins as therapeutic targets. Trends Pharmacol. Sci. 33, 405–412 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.