Abstract
Cystathionine β-synthase (CBS) deficiency is a recessive inborn error of metabolism characterized by elevated serum total homocysteine (tHcy). Previously, our laboratory developed a mouse model of CBS deficiency, TgI278T Cbs−/− (abbreviated as Cbs−/−), characterized by low weight, low adiposity, decreased Scd-1 expression, facial alopecia, and osteoporosis. To determine the potential benefit of a methionine-restricted diet (MRD), we fed Cbs−/− and Cbs+/− control mice either an MRD or a regular diet (RD) from weaning till 240 d of age. Cbs−/− mice fed the MRD had a 77% decrease in tHcy, 28% increase in weight, 130% increase in fat mass, 82% increase in Scd-1 expression, and 10.6% increase in bone density and entirely lacked the alopecia phenotype observed in age-matched Cbs−/− mice fed the RD. At the end of the study, Cbs−/− mice fed the MRD were phenotypically indistinguishable from Cbs+/− mice fed the RD. Notably, whereas the MRD diet was highly beneficial to Cbs−/− mice, it had nearly opposite effect on Cbs+/− mice. These studies show that a low-methionine diet can correct the phenotypic consequences of loss of CBS and provide a striking example of how genotype and diet can interact to influence phenotype in mammals.—Gupta, S., Melnyk, S.B., Kruger, W.D. Cystathionine β-synthase-deficient mice thrive on a low-methionine diet.
Keywords: osteoporosis, alopecia, homocysteine, metabolism, inborn errors
Cystathionine β-synthase (CBS) deficiency is a monogenic inborn error of metabolism caused by mutation in the CBS gene, which encodes an enzyme that catalyzes the conversion of homocysteine to cystathionine. Affected patients accumulate homocysteine and methionine in plasma and have numerous complications, including thrombosis, ectopia lentis, osteoporosis, brittle and thin skin, fine fair hair, fatty liver, mental retardation, and psychiatric disturbances (1, 2). In addition, the appearance of patients with CBS deficiency often resembles that of patients with Marfan's syndrome (3).
The goal of treatment of patients with CBS deficiency is to decrease total homocysteine (tHcy) levels, because lower tHcy levels are associated with improved outcomes (4). For many patients, this can only be achieved by limiting the amount of methionine in the diet. Because homocysteine is derived from methionine, reduced dietary methionine intake (or reduced protein intake in general) will tend to keep tHcy levels low. However, patients with CBS deficiency also have low plasma total cysteine (tCys) levels because CBS catalyzes the first step in the cysteine biosynthetic pathway. Because early studies of children with CBS deficiency fed a cystine-supplemented, low-methionine diet demonstrated significant biochemical control and some degree of phenotypic improvement (5–7), it is now customary to supplement the low-methionine diet with cystine (cysteine disulfide). Thus, it is not entirely clear whether the patient phenotypes are being driven entirely by excess tHcy and methionine or by decreased cysteine.
To understand the pathogenic mechanism of CBS deficiency, Watanabe et al. (8) created a mouse that contained a CBS-knockout allele (Cbs−). It was found that Cbs−/− homozygotes had a neonatal lethal phenotype due to liver dysfunction. Our laboratory found that it was possible to circumvent this lethality problem by insertion of a transgene that expresses a cDNA encoding for a human CBS protein containing the I278T mutation under control of a zinc-inducible metallothionein promoter (9). Expression of this mutant CBS protein was able to rescue the neonatal lethal phenotype, but did not rescue the elevated tHcy levels. This mouse, like human patients, has extremely elevated plasma tHcy, elevated plasma methionine, and reduced plasma tCys. In addition, it has a variety of well-characterized phenotypes including osteoporosis, decreased mean survival, decreased weight gain, low percentage of body fat, and facial alopecia (10, 11). In a previous study, we showed that Cbs−/− mice fed a diet supplemented with the cysteine analog N-acetylcysteine (NAC) did not have any improvement in the phenotypes despite an increase in tCys levels (11). This finding led us to hypothesize that the main drivers of the Cbs−/− phenotypes are related to the elevated tHcy and methionine. Because homocysteine is derived solely from methionine, we decided to examine the effects of dietary methionine restriction in Cbs−/− mice. As a control, we also examined the effects of methionine restriction in sibling Cbs+/− mice. Our findings show that a low-methionine diet can reverse all of the phenotypes in Cbs−/− animals and illustrate the importance of gene-diet interactions in mammalian biology.
MATERIALS AND METHODS
Mouse model
The mouse model of CBS deficiency, Tg-I278T Cbs−/−, was described previously and is on the C57BL6 strain background (9). Transgene-positive Cbs−/− and Cbs+/− animals were created by mating either transgene-positive Cbs−/− or Cbs+/− males with transgene-positive Cbs+/− females on zinc sulfate water to induce the human transgene. Transgene induction during the neonatal period prevents lethality because of a lack of endogenous mouse CBS protein in pups. At 10 d of age, animals were genotyped as described previously (12). Weaning was done at d 30 of age, when mice were fed ad libitum with one of the 2 diets indicated below. Mice were weighed and photographed every 30 d. Mice were euthanized at 240 d of age, at which time tissue was extracted, and the corpses were then stored at −80°C for bioanalysis and body scans. Livers were fixed and stained with hematoxylin and eosin as described previously (9).
Dietary intervention
After weaning, control and Cbs−/− mice were fed a regular diet (RD) or a methionine-restricted diet (MRD) until 240 d of age. Both the regular (Teklad 2018SX) and the methionine-deficient diet (TD.07758) were obtained from Harlan Teklad (Madison, WI, USA). The MRD (0.5 g of Met/kg) contained 8.5% of the methionine contained in the RD (6 g of Met/kg). The cystine content of the RD and MRD was 3 and 3.5 g/kg, respectively. The energy content of the RD was 3.1 kcal/g with 24% of the energy coming from protein, 18% from fat, and 58% from carbohydrate. The energy content of the MRD was 3.9 kcal/g with 15.1% of the energy coming from protein, 18.3% from fat, and 66.6% from carbohydrate.
Dual-energy X-ray absorptiometry (DEXA) analysis
DEXA analysis was performed using a Lunar PIXimus II densitometer (GE Healthcare, Piscataway, NJ, USA) as described previously (11). DEXA data used for the RD mice were consolidated values obtained from the previous work (11) and additional values obtained along with the current study.
Measurement of bioanalytes in serum and liver tissues
Serum tHcy, tCys, and methionine levels were measured by using a 30-amino acid analyzer (Biochrom, Cambridge, UK) as described previously (10, 11). Serum was reduced in the presence of dithiothreitol, and the reaction was then stopped by sulfosalicylic acid. The supernatant obtained after centrifugation was used to analyze tHcy (a sum total of free and disulfide-bonded homocysteine), tCys (a sum total of free and disulfide-bonded cysteine), and methionine. Liver methionine, S-adenosyl methionine (AdoMet), S-adenosyl homocysteine (AdoHcy), and cysteine were measured using HPLC and electrochemical detection as described previously (13).
Western blotting
Liver homogenates (20% w/v) were made in radioimmunoprecipitation assay buffer (Thermo Scientific, Waltham, MA, USA) in the presence of a protease inhibitor cocktail tablet (Complete Mini; Roche, Mannheim, Germany) as described previously (11). The protein concentration was measured by the BCA protein assay kit (Thermo Scientific), and 30 μg of total protein extract was used to immunoblot stearoyl-coenzyme A desaturase (Scd-1) by using goat polyclonal antibody (1:200, sc-14719; Santa Cruz Biotechnology, Dallas, TX, USA) and secondary anti-goat antibody (1:5000, sc-2020; Santa Cruz Biotechnology). β-Actin was probed as a control by using a mouse monoclonal primary antibody (A-5441; Sigma-Aldrich, St. Louis, MO, USA). Western blotting was performed as described previously (11) using 10% bis-Tris SDS gel and N-morpholino propanesulfonic acid (MOPS) buffer (Invitrogen Life Technologies, Grand Island, NY, USA).
Statistical analysis
For growth curve analysis, we fitted the data from each group using a cubic polynomial model with relative weighting using the nonlinear curve-fitting functions in Prism 4.0 (GraphPad Software Inc., San Diego, CA). Because of the large difference between the genders, males and females were analyzed separately. The mean goodness of fit (R2) for all 8 curves was 0.82 (range, 0.74−0.94). Confidence intervals were determined from the se using Prism 4.0.
For all other analysis, significance was determined using analysis of variance (ANOVA) followed by Tukey's multiple comparison tests using Prism 4.0. To compare only 2 sets of samples, a 2-sided Student's t test was used. For all tests, a value of P < 0.05 was considered significant.
RESULTS
Methionine restriction has differential effects on growth of Cbs−/− and Cbs+/− mice
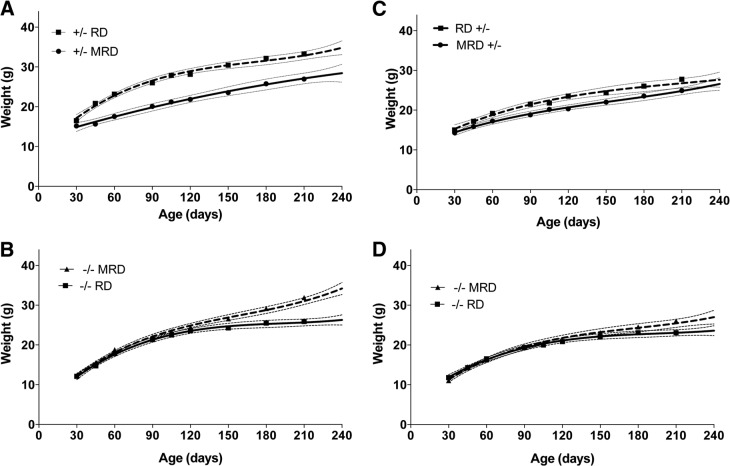
Previously, we observed that Cbs−/− mice fed the RD weighed significantly less than control mice at all ages from weaning (d 30) to 420 d of age (11). To examine the potentially beneficial effects of the MRD, we fed a cohort of newly weaned Cbs−/− and Cbs+/− mice the MRD and followed weight gain until 240 d of age. At the time of weaning, both male and female Cbs−/− mice weighed significantly less than age- and gender-matched control mice (Fig. 1A). However, by 45 d of age, the weight difference between the control and Cbs−/− mice was no longer significant, and by 90 d of age, Cbs−/− male mice weighed significantly more than the control mice. The female Cbs−/− mice caught up with the controls by d 60 but did not surpass the control animals by a statistically significant degree (Fig. 1B).
Figure 1.
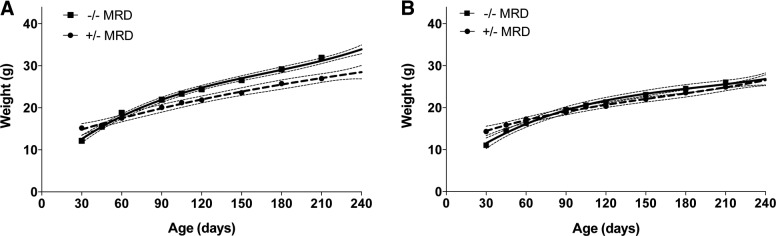
Comparison of the weights of Cbs−/− and Cbs+/− mice fed the MRD. A) Male mice were fed the MRD at 30 d of age and weighed 1×/mo until 240 d of age. Solid line, Cbs−/− mice (n=10); hatched line, Cbs+/− mice (n=7). Symbol at each time point shows the mean weight, with the error bar showing the 95% confidence interval of each curve. B) Identical to panel A, except that female mice were compared (n=9 for both).
We next compared the growth data from mice fed the MRD with our previously published growth data obtained from mice fed the RD, which were collected in an identical manner (11). We found that MRD feeding resulted in a significant decrease in weight gain in male Cbs+/− mice, with mice fed the MRD weighing 17% less at 240 d of age than mice fed the RD (Fig. 2A). However, in Cbs−/− animals, the opposite was observed, with mice fed the MRD weighing 28% more than mice fed the RD at 240 d of age (Fig. 2B). Cbs+/− female mice also tended to gain weight more slowly with MRD feeding, but the difference was not as dramatic as that for the males (Fig. 2C). Female Cbs−/− mice also showed increased weight gain with MRD feeding compared with that with RD feeding, but, again, the effect was not as robust as that in the males (Fig. 2D).
Figure 2.
Comparison of the effects of RD and MRD stratified by genotype. A) Comparison of growth curves of male Cbs+/− mice fed the RD (hatched line, n≥12 for each point) vs. MRD (solid line, n=7). The symbol at each time point shows the mean weight, with the error bars showing the 95% confidence interval of each curve. B) Comparison of growth curves of male Cbs−/− mice fed the RD (solid line, n≥8) and the MRD (hatched line, n=10). C) Comparison of growth curves of female Cbs+/− mice fed the RD (hatched line, n≥12) vs. the MRD (solid line, n=9). D) Comparison of growth curves of female Cbs−/− mice fed the RD (solid line, n≥13) and MRD (hatched line, n=9).
To assess the treatment efficacy of the MRD, we compared weight gain of Cbs−/− mice fed the MRD with that of Cbs+/− mice fed the RD (Fig. 3A, B). Comparisons for both males and females showed that Cbs−/− animals fed the MRD at weaning weighed the same as Cbs+/− animals fed the RD by the end of the study. Thus, the MRD treatment entirely corrected the weight loss phenotype of Cbs−/− mice.
Figure 3.
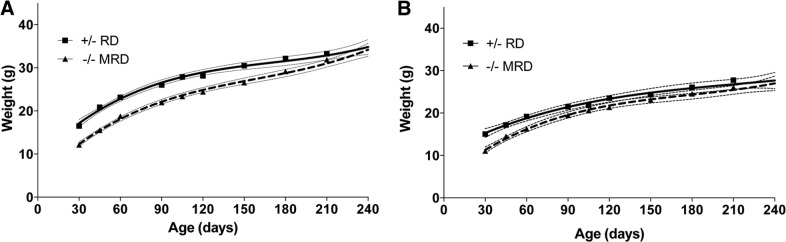
Comparison of the growth of Cbs+/− mice fed the RD vs. Cbs−/− mice fed the MRD. A) Male Cbs+/− mice fed the RD (solid line) and Cbs−/− mice fed the MRD (hatched line). B) Same as panel A, but female mice were compared.
Methionine restriction elevates fat mass in CBS-deficient mice
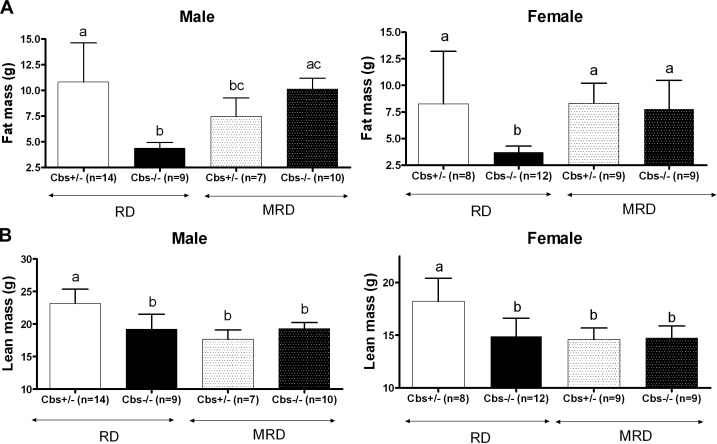
Previous studies showed that Cbs−/− mice fed the RD had a 45−60% reduction in fat mass compared with that of control Cbs+/− mice fed the RD (11). Lean mass, on the other hand, showed a much smaller decline. In the current study, we examined the effect of the MRD on body composition using DEXA analysis (Fig. 4A, B). In Cbs−/− animals, the MRD restored fat mass to levels nearly identical to those observed in Cbs+/− mice fed the RD. Notably, whereas male Cbs−/− mice showed a marked increase in fat mass when fed the MRD, male Cbs+/− mice fed the MRD showed a decrease in fat mass. This decrease in fat mass, however, was not observed in female Cbs+/− animals. Lean mass in Cbs−/− mice was unaffected by the MRD but was significantly lower (20 and 24% in females and males, respectively) in Cbs+/− mice fed the MRD. These studies indicate that the MRD restores weight to Cbs−/− mice primarily by increasing fat formation and that the MRD results in decreased weight in Cbs+/− mice by reductions in both fat and lean mass.
Figure 4.
DEXA body composition studies on 240-d-old Cbs−/− and Cbs+/− mice fed the RD and MRD. Effects on male and female mice are shown separately. A) Effect of genotype and diet on fat mass. B) Effect of genotype and diet on lean mass. Significance was determined by ANOVA followed by Tukey's multiple comparison tests. All graphs show means ± sd. Significance is shown by letters at the top of the error bars; a single common letter indicates nonsignificance.
Methionine restriction rescues loss of Scd-1 in CBS-deficient mice
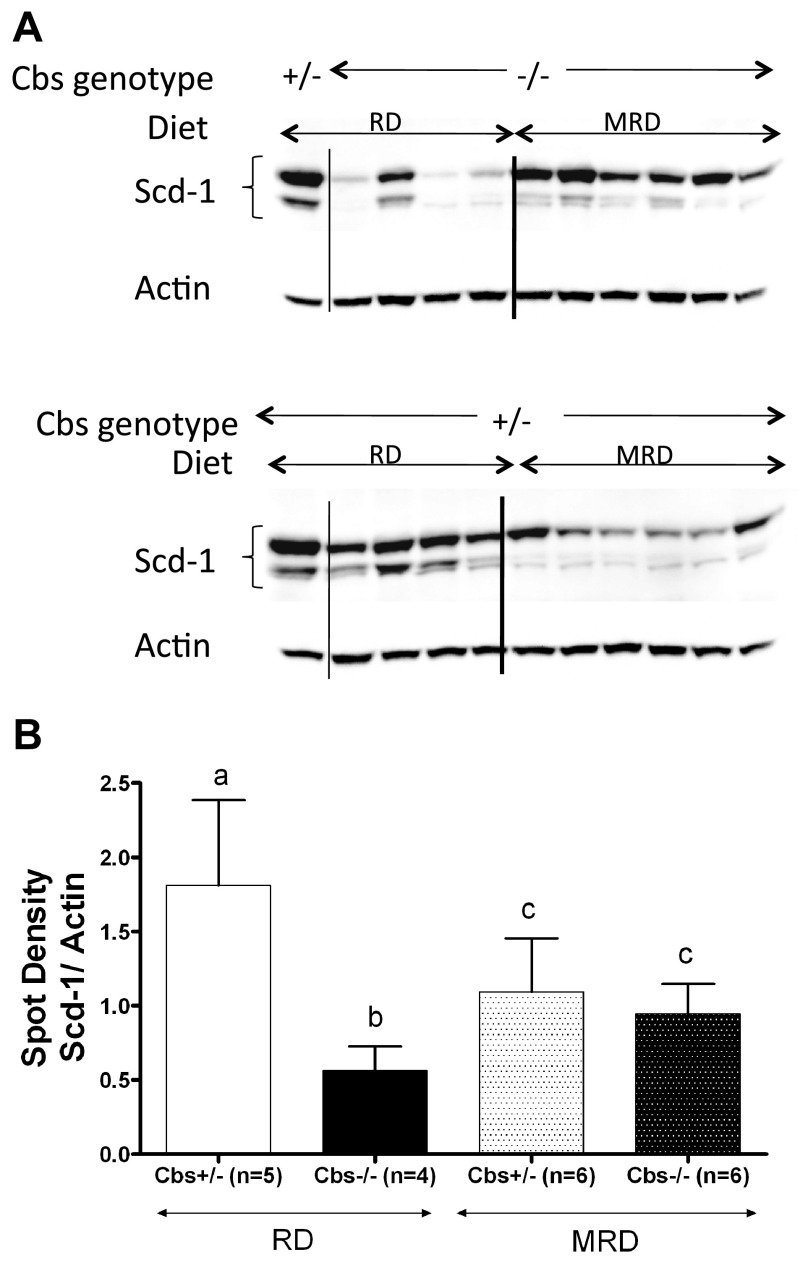
Previously, we showed that Scd-1, a key lipogenic enzyme involved in fat regulation (14), was down-regulated in Cbs−/− mice fed the RD and speculated that this might be related to the loss of fat mass in these animals (11). Therefore, we examined the effect of the MRD on Scd-1 protein levels. We found that the MRD in Cbs−/− mice caused a significant increase in Scd-1 protein levels (Fig. 5). However, in Cbs+/− mice, levels of Scd-1 were decreased by the MRD. These findings show that steady-state levels of Scd-1 are differentially affected by MRD, depending on the CBS genotype.
Figure 5.
Effect of MRD on Scd-1 levels in Cbs−/− and Cbs+/− male mice compared with that of RD. A) Liver Scd-1 runs as a doublet centered at 37 kDa as assessed by Western blotting. β-Actin was used as loading control. B) Densitometry graph of the Western blot. All graphs show means ± sd. Significant differences between columns, as determined by ANOVA, are indicated by different letters at the top of the error bar; a single common letter indicates nonsignificance.
Methionine restriction rescues hair loss and osteoporosis in CBS-deficient mice
Facial alopecia is the most striking phenotype of Cbs−/− mice fed the RD, occurring between 105 and 120 d of age in both males and females (11, 15). In addition to alopecia in older animals, it is also possible to distinguish younger Cbs−/− animals because of a slight difference in the sheen of the hair (unpublished results). Notably, it has been observed that some human patients with CBS deficiency have light skin and light hair, and this phenotype can occasionally be reversed with homocysteine-lowering treatment (2). Here, we found that MRD completely prevented facial alopecia in both male and female Cbs−/− mice (Fig. 6A). In addition, there was no difference in the sheen or hair color of younger Cbs−/− mice fed the MRD.
Figure 6.
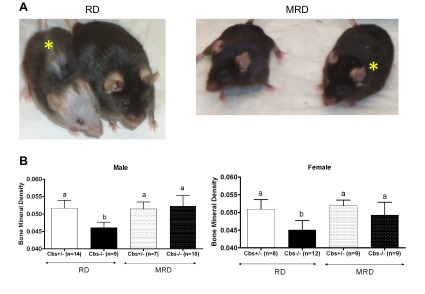
Correction of alopecia and osteoporosis phenotypes of Cbs−/− animals by MRD. A) Photo on the left shows 240-d-old mice fed the RD; photo on the right shows animals of the same age fed the MRD. Animal marked with the yellow asterisk is the Cbs−/− animal; the other animal in each photo is a sibling Cbs+/− animal. B) BMD of male and female Cbs−/− and Cbs+/− mice fed the RD and MRD as determined by DEXA. All graphs show means ± sd. Significant differences between columns are indicated by different letters at the top of the error bar; a single common letter indicates nonsignificance.
Another phenotype of Cbs−/− mice is osteoporosis (10, 11). To examine this phenotype, we used DEXA to measure bone mineral density (BMD) in Cbs−/− and Cbs+/− mice fed the MRD and RD (Fig. 6B). In Cbs−/− mice, the MRD completely reversed the BMD defect, raising BMD in both male and female mice to levels indistinguishable from those of Cbs+/− animals fed the RD. The MRD had no significant affect of BMD in Cbs+/− mice. These results show that MRD can reverse the osteoporosis defect in Cbs−/− mice.
Methionine restriction reverses metabolic defects in CBS-deficient mice
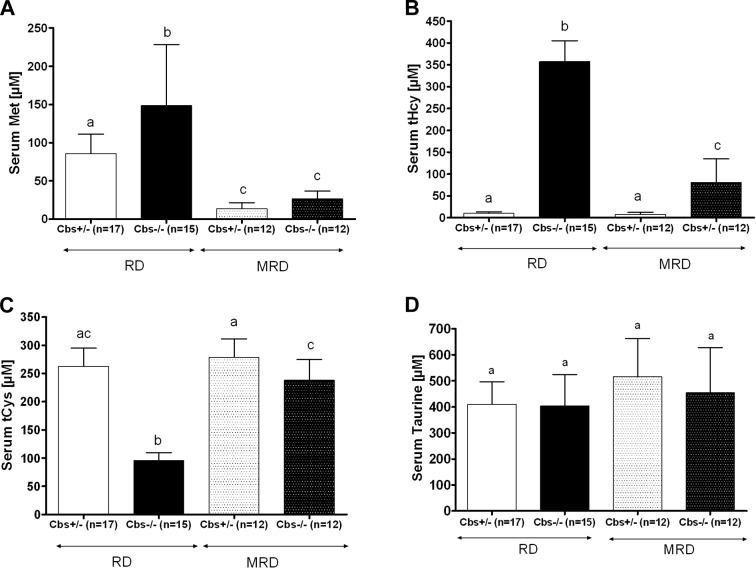
Given the effectiveness of the MRD in reversing the phenotypes of Cbs−/− mice, we were interested in how effective the diet was for lowering serum methionine and tHcy levels. After 210 d of the MRD, serum methionine levels decreased ∼80% in both Cbs+/− and Cbs−/− mice compared with levels in mice fed the RD (Fig. 7A). With regard to serum tHcy, we found that in Cbs−/− mice, the MRD lowered tHcy 77% [from 357±48 μM (RD) to 81±54 μM (MRD); Fig. 7B). However, it should also be noted that Cbs−/− mice fed the MRD still had a nearly 10-fold elevation in tHcy compared with that in Cbs+/− mice fed either the RD or MRD.
Figure 7.
Effect of Cbs genotype and diet on serum sulfur amino acids. A) Methionine. B) tHcy. C) tCys. D) Taurine. All measures were taken at 240 d of age. All graphs show means ± sd. Significant differences between columns, as determined by ANOVA, are indicated by different letters at the top of the error bar; a single common letter indicates nonsignificance.
Because methionine can be converted to cysteine and other thiol-containing molecules via the transsulfuration pathway, we also examined the serum levels of cysteine and taurine. Surprisingly, we discovered that the low levels of serum tCys in Cbs−/− mice were increased to near-normal levels when mice were fed the MRD (Fig. 7C). Control mice fed the MRD showed no increase in their serum tCys levels, indicating that the increase in Cbs−/− tCys was not simply due to the small increase in cysteine content in the MRD (see Materials and Methods).
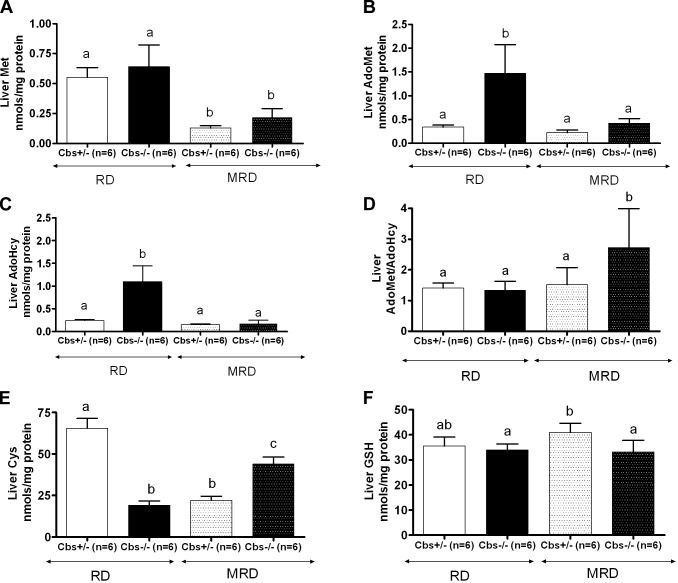
We also measured the liver levels of several methionine-related metabolites including methionine, AdoMet, AdoHcy, cysteine, and glutathione (GSH). Similar to levels in serum, liver methionine levels were decreased in both Cbs+/− and Cbs−/− mice fed the MRD (Fig. 8A). Cbs−/− mice fed the RD had significant elevations in both liver AdoMet and AdoHcy compared with those of Cbs+/− mice fed the RD, and MRD feeding normalized these levels (Fig. 8B, C). It should also be noted that the MRD had no effect on either AdoMet or AdoHcy in Cbs+/− animals. We also examined the cellular AdoMet/AdoHcy ratio, which is thought to be critical in controlling cellular transmethylation reactions (16). Notably, Cbs−/− mice fed the RD had no significant difference in the AdoMet/AdoHcy ratio (Fig. 8D), because both metabolites increased by similar levels. However, when Cbs−/− mice were fed the MRD, the AdoMet/AdoHcy ratio was somewhat elevated compared with that of the 3 other groups, mostly because the AdoMet levels in these mice are slightly elevated.
Figure 8.
Effect of Cbs genotype and diet on liver amino acid pools. The concentrations of free methionine, AdoMet, AdoHcy, cysteine, and GSH in the livers of 240-d-old animals were determined as described under Materials and Methods. A) Methionine. B) AdoMet. C) AdoHcy. D) AdoMet/AdoHcy ratio. E) Cysteine (Cys). F) GSH. All graphs show means ± sd. Significant differences between columns, as determined by ANOVA, are indicated by different letters at the top of the error bar; a single common letter indicates nonsignificance.
Similar to serum levels, liver cysteine levels were decreased in Cbs−/− mice fed the RD but showed a significant increase when mice were fed the MRD (Fig. 8E). Notably, in Cbs+/− mice, we observed the opposite effect, i.e., liver cysteine levels decreased in response to the MRD. We did not observe any significant change in liver GSH levels (Fig. 8F).
Liver pathology
We also assessed the effect of genotype and diet on the pathology of the livers in the animals used in this study. Hematoxylin and eosin-stained sections were evaluated for the level of steatosis blindly using a 4-point scale (Supplemental Fig. S1). We found that there was no significant difference in the mean levels of steatosis present in Cbs−/− and Cbs+/− animals fed the RD. When animals were fed the MRD, we observed a significant increase in the mean levels of steatosis in both groups, with the Cbs−/− animals fed the MRD having the highest level of steatosis. These results indicate that the levels of steatosis present in the liver do not correlate with any of the homocysteine-related phenotypes observed in this study. Notably, these results contrast with those reported by Maclean et al. (17) in their HO mouse model of homocystinuria in which HO mice appear to have reduced levels of steatosis when fed a low-methionine diet compared with those in C57BL/6J control animals.
DISCUSSION
The current study was primarily designed to determine whether dietary methionine restriction could reverse the phenotypes of CBS deficiency. To address this question, we have used the Tg-I278T Cbs−/− mouse model of CBS deficiency that exhibits several phenotypes, including decreased weight gain, decreased fat mass deposition, facial alopecia, and osteoporosis. Using this mouse model, we examined the effect of a methionine-restricted diet that contains only 8% of the methionine found in normal mouse chow. We found that both male and female Cbs−/− animals fed the MRD for 210 d no longer had significant differences in weight or fat mass compared with control Cbs+/− animals fed the RD. In addition, the MRD entirely corrected the osteoporosis and facial alopecia phenotypes. In fact, 240-d-old Cbs−/− mice fed the MRD were phenotypically indistinguishable from control 240-d-old Cbs+/− mice fed the RD. These findings clearly show that an MRD diet can essentially cure the phenotypes of Cbs−/− and reinforce the view that methionine restriction is beneficial in human patients with CBS deficiency.
Notably, despite the correction of the assayed physical phenotypes, Cbs−/− mice fed the MRD still had significantly elevated tHcy compared with that in Cbs+/− mice fed the RD (81 vs. 8 μM). This finding is consistent with our earlier work showing that Tg-I278T Cbs−/− mice (mean tHcy of ∼290 μM) had much more severe phenotypes than Tg-hCBS Cbs−/− mice (mean tHcy of 180 μM; ref. 10). Taken together, these findings support the view that there is a distinct threshold effect when it comes to homocysteine-induced phenotypes. The same phenomena may also be occurring in human patients with CBS deficiency. It has been well documented that treatment of patients with CBS deficiency with a combination of B vitamins, dietary methionine (protein) restriction, and betaine supplementation can significantly reduce the incidences of vascular events, the major cause of morbidity in patients with CBS deficiency, despite the fact that post-treatment homocysteine levels are still several times higher than the levels found in the normal population (18–20).
Because CBS deficiency also causes hypocysteinemia, it was assumed by early investigators that extra cysteine might also be beneficial (7). The findings reported here, along with our earlier NAC supplementation studies (11), question the need for extra cysteine supplementation currently integrated with methionine restriction therapy in patients with CBS deficiency. In our earlier work, we showed that treatment of Cbs−/− mice with NAC had no beneficial effects on weight gain, alopecia, or osteoporosis phenotypes, despite raising tCys levels (11). The data presented here show that we were able to restore serum tCys in Cbs−/− mice to near-normal levels by methionine restriction alone, suggesting that the low levels of serum cysteine are not related directly to the transsulfuration defect. A possible explanation for the increase in tCys in Cbs−/− mice fed the MRD is that methionine restriction is preventing the loss of cysteine by blocking the formation of homocysteine-cysteine disulfide. Because plasma homocystine and homocysteine-cysteine mixed disulfide are reabsorbed in the kidney via the luminal cystine/basic amino acid transporter, increased urinary homocystine, and mixed disulfide excretion occur when the capacity of the transporter is exceeded (21). We hypothesize that in CBS deficiency, a significant amount of cysteine is lost because of this mechanism. Consistent with this idea is the observation that patients with CBS deficiency have been shown to excrete large amounts of homocysteine-cysteine disulfide in the urine (22). Notably, elevated cysteine levels has been observed in HO mice in which tHcy has been lowered by treatment with betaine (23).
An interesting aspect of this study was the identification of a powerful interaction effect that occurred between diet and the Cbs genotype. With regard to weight gain, MRD resulted in a 17% decrease in weight in Cbs+/− mice, whereas in Cbs−/− mice, it caused a 28% increase. Similarly, DEXA analysis revealed that MRD caused Cbs−/− animals to gain fat mass, whereas Cbs+/− mice lost fat mass (although only statistically significant in males). Finally, the effect of MRD on the steady-state levels of the lipid biosynthetic enzyme Scd-1 also vary by genotype, increasing in Cbs−/− mice and decreasing in Cbs+/− mice. These data clearly demonstrate that a diet that can be beneficial on one genetic background (Cbs−/−) can actually be harmful on another background (Cbs+/−) and illustrates the importance of gene-environment interactions in understanding the causation of phenotypes.
A possible explanation for why Cbs−/− mice grow better than Cbs+/− mice fed the MRD involves less “methionine wastage” because of an inactive transsulfuration pathway. Dietary methionine that is used for methyl group donation ends up as homocysteine, which can have 2 metabolic fates: remethylation to methionine or CBS-catalyzed condensation with serine to form cystathionine. Thus, homocysteine represents a key branch point in methionine metabolism. In mammals, it is estimated that as much as half of all homocysteine is converted to cysteine via CBS and the transsulfuration pathway (24, 25). Notably, this conversion of homocysteine to cysteine occurs even when subjects consume a methionine-free diet (26). We suspect that in Cbs+/− mice, in which the transsulfuration pathway is intact, a significant percentage of homocysteine is converted to cysteine and, thus, can no longer be converted to methionine. In contrast, in Cbs−/− mice, the lack of transsulfuration prevents the loss of methionine, and, thus, they are able to better use the growth-limiting quantities in the MRD. Given our results, one might speculate that if a mammal was faced with a dietary environment in which methionine was specifically rate-limiting for growth (i.e., cysteine was available from other sources), mutations in the CBS allele might be commonplace. Notably, it has been recently reported that the Tao people, a group of aboriginal people in Taiwan, have an exceptionally high frequency of CBS mutations (27). Whether this may be related to dietary selection is not known.
Previously, we have shown that the reduced fat mass in Cbs−/− mice fed the RD is associated with reduced levels of Scd-1 (11). Because genetic deficiency of Scd-1 is linked to both reduced body adiposity and resistance to diet and genetically induced obesity (28), it has been suggested that low Scd-1 levels might be the cause of the low fat mass phenotype in Cbs−/− animals (11). Here, we observed that Cbs−/− mice fed the MRD had elevated Scd-1 levels compared with those of Cbs−/− fed the RD and that MRD caused down-regulation of Scd-1 in Cbs+/− mice. This latter observation is consistent with reports from other groups that methionine restriction in wild-type rodents results in loss of fat mass and down-regulation of Scd-1 (29–31). However, we did not observe a strong correlation between Scd-1 levels and either weight or fat mass in any of the mice used in these studies (Supplemental Fig. S2), suggesting that Scd-1 levels are not the major determinant of fat mass in these mice.
To summarize, there are 3 major conclusions that can be drawn from the studies described here. First, an MRD can entirely eliminate all of the deleterious phenotypes in Cbs−/− mice, even though it does not restore tHcy to normal levels. This finding reinforces the importance of methionine restriction in the treatment of CBS deficiency and suggests that it is not necessary to reduce tHcy levels to “normal” levels for treatment to be effective. Second, decreased plasma tCys levels in Cbs−/− mice are corrected by an MRD, suggesting that low tCys in patients with CBS deficiency is not due to lack of de novo cysteine production but rather is due to problems with loss of cysteine in the urine. Finally, this work demonstrates a very clear example of the gene-diet interaction in mice, in which the effect of a change in diet is entirely different, depending on the underlying genotype.
Supplementary Material
Acknowledgments
The authors thank the staff of the genomics, laboratory animal, and biostatistics facilities of Fox Chase Cancer Center for their assistance. The authors specifically acknowledge Fang Zhu for statistical support. The authors also thank Dr. Michael Tordoff and his laboratory for help in use of the PIXimus II DEXA. This equipment was provided by funds awarded to the Monell Chemical Senses Center under a grant from the Pennsylvania Department of Health. The department specifically disclaims responsibility for any analyses, interpretations or conclusions of the study.
This work was supported by grants from the Hempling Foundation for Homocystinuria Research, the U.S. National Institutes of Health (CA06927 and R01GM098772), and an appropriation from the Commonwealth of Pennsylvania.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- AdoHcy
- S-adenosyl homocysteine
- AdoMet
- S-adenosyl methionine
- ANOVA
- analysis of variance
- BMD
- bone mineral density
- CBS
- cystathionine β-synthase
- DEXA
- dual-energy X-ray absorptiometry
- GSH
- glutathione
- MRD
- methionine-restricted diet
- NAC
- N-acetylcysteine
- RD
- regular diet
- tCys
- total cysteine
- tHcy
- total homocysteine
- Scd-1
- stearoyl-coenzyme A desaturase
REFERENCES
- 1. Mudd S. H., Skovby F., Levy H. L., Pettigrew K. D., Wilcken B., Pyeritz R. E., Andria G., Boers G. H., Bromberg I. L., Cerone R., Fowler B., Gröbe H., Schmidt H., Schweitzer L. (1985) The natural history of homocystinuria due to cystathionine beta-synthase deficiency. Am. J. Hum. Genet. 37, 1–31 [PMC free article] [PubMed] [Google Scholar]
- 2. Reish O., Townsend D., Berry S. A., Tsai M. Y., King R. A. (1995) Tyrosinase inhibition due to interaction of homocyst(e)ine with copper: the mechanism for reversible hypopigmentation in homocystinuria due to cystathionine beta-synthase deficiency. Am. J. Hum. Genet. 57, 127–132 [PMC free article] [PubMed] [Google Scholar]
- 3. Brenton D. P., Dow C. J., James J. I., Hay R. L., Wynne-Davies R. (1972) Homocystinuria and Marfan's syndrome. A comparison. J. Bone Joint Surg. Br. 54, 277–298 [PubMed] [Google Scholar]
- 4. Yap S., Naughten E. (1998) Homocystinuria due to cystathionine β-synthase deficiency in Ireland: 25 years' experience of a newborn screened and treated population with reference to clinical outcome and biochemical control. J. Inherit. Metab. Dis. 21, 738–747 [DOI] [PubMed] [Google Scholar]
- 5. Sardharwalla I. B., Jackson S. H., Hawke H. D., Sass-Kortsak A. (1968) Homocystinuria: a study with low-methionine diet in three patients. Can. Med. Assoc. J. 99, 731–740 [PMC free article] [PubMed] [Google Scholar]
- 6. Perry T. L., Dunn H. G., Hansen S., MacDougall L., Warrington P. D. (1966) Early diagnosis and treatment of homocystinuria. Pediatrics 37, 502–505 [PubMed] [Google Scholar]
- 7. Komrower G. M., Lambert A. M., Cusworth D. C., Westall R. G. (1966) Dietary treatment of homocystinuria. Arch. Dis. Child. 41, 666–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Watanabe M., Osada J., Aratani Y., Kluckman K., Reddick R., Malinow M. R., Maeda N. (1995) Mice deficient in cystathionine β-synthase: animal models for mild and severe homocyst(e)inemia. Proc. Natl. Acad. Sci. U. S. A. 92, 1585–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang L., Chen X., Tang B., Hua X., Klein-Szanto A., Kruger W. D. (2005) Expression of mutant human cystathionine β-synthase rescues neonatal lethality but not homocystinuria in a mouse model. Hum. Mol. Genet. 14, 2201–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gupta S., Kühnisch J., Mustafa A., Lhotak S., Schlachterman A., Slifker M. J., Klein-Szanto A., High K. A., Austin R. C., Kruger W. D. (2009) Mouse models of cystathionine β-synthase deficiency reveal significant threshold effects of hyperhomocysteinemia. FASEB J. 23, 883–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gupta S., Kruger W. D. (2011) Cystathionine β-synthase deficiency causes fat loss in mice. PLoS One 6, e27598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang L., Jhee K. H., Hua X., DiBello P. M., Jacobsen D. W., Kruger W. D. (2004) Modulation of cystathionine β-synthase level regulates total serum homocysteine in mice. Circ. Res. 94, 1318–1324 [DOI] [PubMed] [Google Scholar]
- 13. Melnyk S., Pogribna M., Pogribny I. P., Yi P., James S. J. (2000) Measurement of plasma and intracellular S-adenosylmethionine and S-adenosylhomocysteine utilizing coulometric electrochemical detection: alterations with plasma homocysteine and pyridoxal 5′-phosphate concentrations. Clin. Chem. 46, 265–272 [PubMed] [Google Scholar]
- 14. Paton C. M., Ntambi J. M. (2009) Biochemical and physiological function of stearoyl-CoA desaturase. Am. J. Physiol. Endocrinol. Metab. 297, E28–E37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Robert K., Maurin N., Ledru A., Delabar J., Janel N. (2004) Hyperkeratosis in cystathionine β synthase-deficient mice: an animal model of hyperhomocysteinemia. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 280, 1072–1076 [DOI] [PubMed] [Google Scholar]
- 16. Yi P., Melnyk S., Pogribna M., Pogribny I. P., Hine R. J., James S. J. (2000) Increase in plasma homocysteine associated with parallel increases in plasma S-adenosylhomocysteine and lymphocyte DNA hypomethylation. J. Biol. Chem. 275, 29318–29323 [DOI] [PubMed] [Google Scholar]
- 17. Maclean K. N., Greiner L. S., Evans J. R., Sood S. K., Lhotak S., Markham N. E., Stabler S. P., Allen R. H., Austin R. C., Balasubramaniam V., Jiang H. (2012) Cystathionine protects against endoplasmic reticulum stress-induced lipid accumulation, tissue injury, and apoptotic cell death. J. Biol. Chem. 287, 31994–32005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wilcken D. E., Wilcken B. (1997) The natural history of vascular disease in homocystinuria and the effects of treatment. J. Inherit. Metab. Dis. 20, 295–300 [DOI] [PubMed] [Google Scholar]
- 19. Yap S., Naughten E. R., Wilcken B., Wilcken D. E., Boers G. H. (2000) Vascular complications of severe hyperhomocysteinemia in patients with homocystinuria due to cystathionine β-synthase deficiency: effects of homocysteine-lowering therapy. Semin. Thromb. Hemost. 26, 335–340 [DOI] [PubMed] [Google Scholar]
- 20. Yap S., Boers G. H., Wilcken B., Wilcken D. E., Brenton D. P., Lee P. J., Walter J. H., Howard P. M., Naughten E. R. (2001) Vascular outcome in patients with homocystinuria due to cystathionine β-synthase deficiency treated chronically: a multicenter observational study. Arterioscler. Thromb. Vasc. Biol. 21, 2080–2085 [DOI] [PubMed] [Google Scholar]
- 21. Brosnan J. T. (2001) Homocysteine and the kidney. In Homocysteine in Health and Disease (Carmel R., Jacobsen D.W., eds) pp 176–182, Cambridge University Press, Cambridge, UK [Google Scholar]
- 22. Carson N. A., Cusworth D. C., Dent C. E., Field C. M., Neill D. W., Westall R. G. (1963) Homocystinuria: a new inborn error of metabolism associated with mental deficiency. Arch. Dis. Child. 38, 425–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maclean K. N., Sikora J., Kozich V., Jiang H., Greiner L. S., Kraus E., Krijt J., Overdier K. H., Collard R., Brodsky G. L., Meltesen L., Crnic L. S., Allen R. H., Stabler S. P., Elleder M., Rozen R., Patterson D., Kraus J. P. (2010) A novel transgenic mouse model of CBS-deficient homocystinuria does not incur hepatic steatosis or fibrosis and exhibits a hypercoagulative phenotype that is ameliorated by betaine treatment. Mol. Genet. Metab. 101, 153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Storch K. J., Wagner D. A., Burke J. F., Young V. R. (1988) Quantitative study in vivo of methionine cycle in humans using [methyl-2H3]- and [1-13C]methionine. Am. J. Physiol. 255, E322–E331 [DOI] [PubMed] [Google Scholar]
- 25. Benight N. M., Stoll B., Chacko S., da Silva V. R., Marini J. C., Gregory J. F., 3rd, Stabler S. P., Burrin D. G. (2011) B-vitamin deficiency is protective against DSS-induced colitis in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 301, G249–G259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Storch K. J., Wagner D. A., Burke J. F., Young V. R. (1990) [1-13C; methyl-2H3]methionine kinetics in humans: methionine conservation and cystine sparing. Am. J. Physiol. 258, E790–E798 [DOI] [PubMed] [Google Scholar]
- 27. Lu Y. H., Huang Y. H., Cheng L. M., Yu H. C., Hsu J. H., Wu T. J. T., Lo M. Y., Lin A., Lin C. Y., Wu J. Y., Niu D. M. (2012) Homocystinuria in Taiwan: an inordinately high prevalence in an Austronesian aboriginal tribe, Tao. Mol. Genet. Metab. 105, 590–595 [DOI] [PubMed] [Google Scholar]
- 28. Cohen P., Miyazaki M., Socci N. D., Hagge-Greenberg A., Liedtke W., Soukas A. A., Sharma R., Hudgins L. C., Ntambi J. M., Friedman J. M. (2002) Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science 297, 240–243 [DOI] [PubMed] [Google Scholar]
- 29. Keating A. K., Freehauf C., Jiang H., Brodsky G. L., Stabler S. P., Allen R. H., Graham D. K., Thomas J. A., Van Hove J. L., Maclean K. N. (2011) Constitutive induction of pro-inflammatory and chemotactic cytokines in cystathionine β-synthase deficient homocystinuria. Mol. Genet. Metab. 103, 330–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Perrone C. E., Mattocks D. A., Jarvis-Morar M., Plummer J. D., Orentreich N. (2010) Methionine restriction effects on mitochondrial biogenesis and aerobic capacity in white adipose tissue, liver, and skeletal muscle of F344 rats. Metabolism 59, 1000–1011 [DOI] [PubMed] [Google Scholar]
- 31. Rizki G., Arnaboldi L., Gabrielli B., Yan J., Lee G. S., Ng R. K., Turner S. M., Badger T. M., Pitas R. E., Maher J. J. (2006) Mice fed a lipogenic methionine-choline-deficient diet develop hypermetabolism coincident with hepatic suppression of SCD-1. J. Lipid Res. 47, 2280–2290 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.