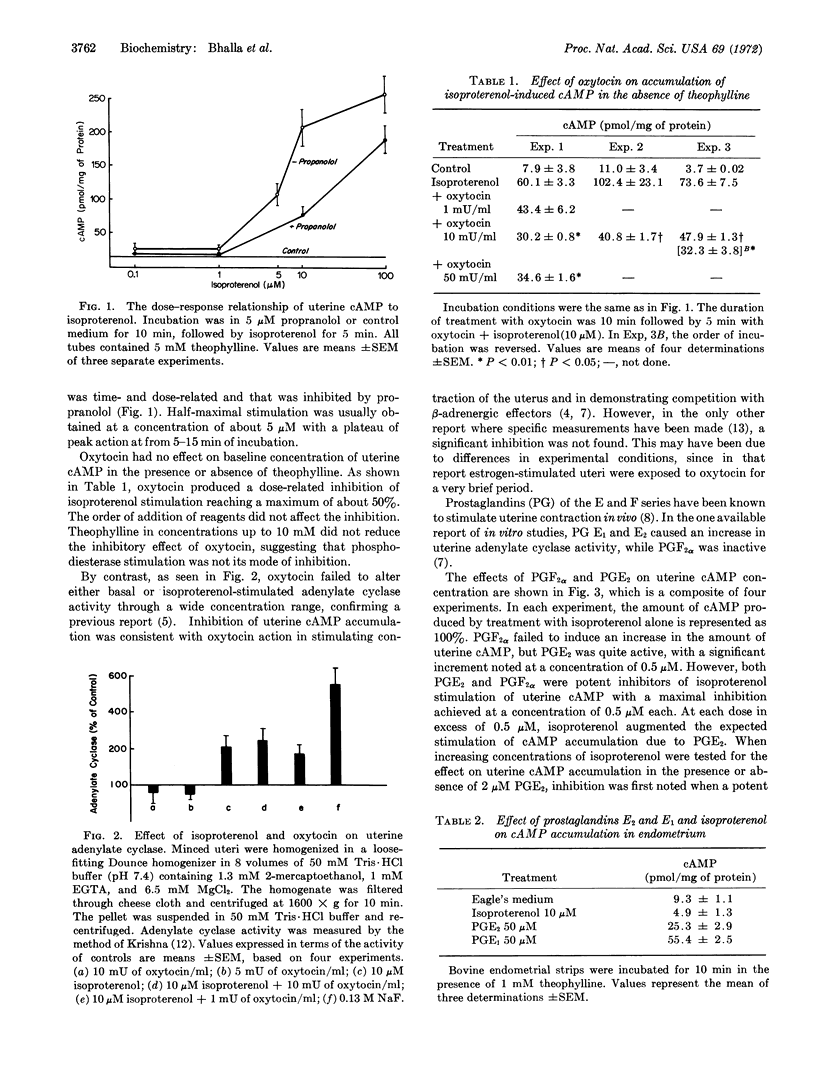
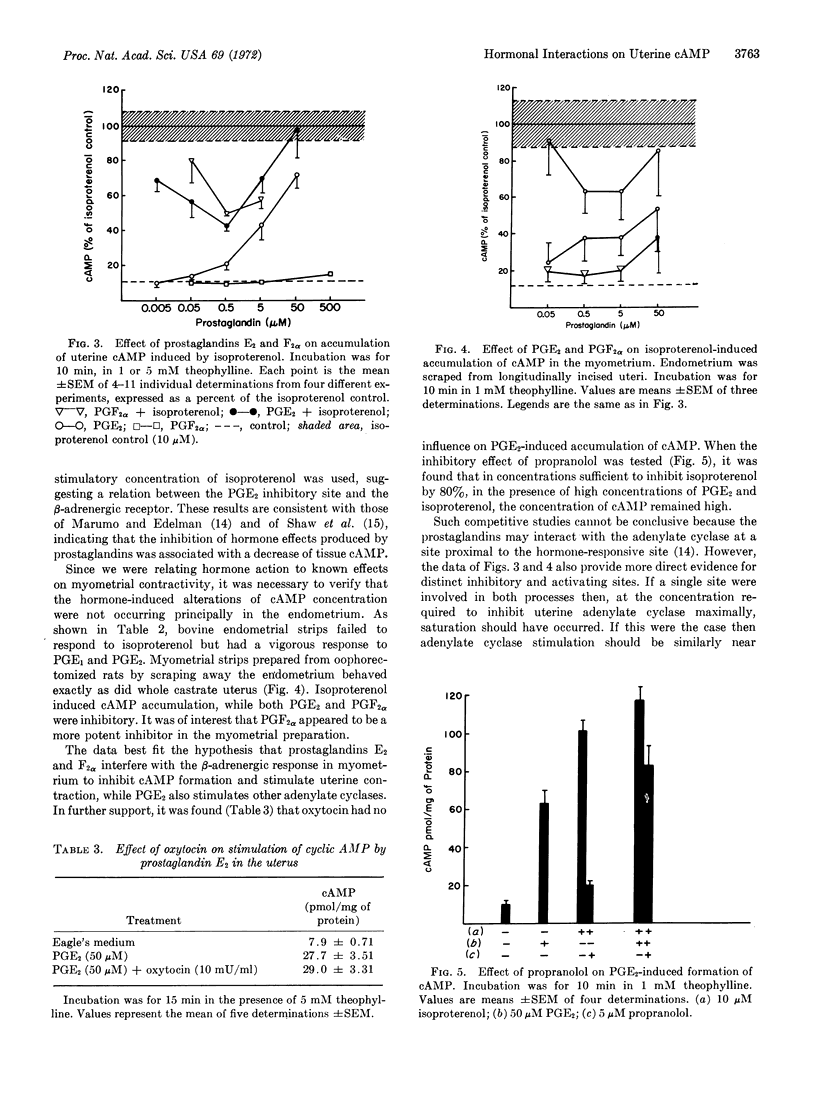
Abstract
Interactions of hormones stimulating and inhibiting uterine contraction were studied in vitro in uteri from oophorectomized rats. The β-adrenergic effector, isoproterenol, a potent inhibitor of contraction, produced a dose-related increase of adenylate cyclase and accumulation of adenosine 3′:5′-cyclic monophosphate (cAMP) that was inhibitable by propranolol. Oxytocin, which stimulates contraction, effectively inhibited accumulation of uterine cAMP induced by isoproterenol in the presence or absence of theophylline. Prostaglandins E2 and F2α, each at a maximum effective concentration of 0.5 μM, also inhibited accumulation of cAMP induced by isoproterenol, consistent with their effect in stimulation of uterine contraction. Prostaglandin E2, but not prostaglandin F2α, stimulated cAMP accumulation in a dose-related manner at concentrations in excess of 0.5 μM. Neither propranolol nor oxytocin inhibited that response. Bovine endometrial adenylate cyclase failed to respond to isoproterenol but was stimulated by prostaglandins E1 and E2. When myometrial preparations were studied, isoproterenol stimulation and prostaglandin effects were observed as for whole castrate uterus. The competitive physiological actions of β-adrenergic effectors on the one hand, and oxytocin and prostaglandins on the other hand, are based on their influences on a myometrial adenylate cyclase. Stimulation of uterine cAMP accumulation by prostaglandin E2 is due to action at a different and unrelated site.
Keywords: β-adrenergic effector, rats, adenylate cyclase, uterine contraction
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butcher R. W., Baird C. E. Effects of prostaglandins on adenosine 3',5'-monophosphate levels in fat and other tissues. J Biol Chem. 1968 Apr 25;243(8):1713–1717. [PubMed] [Google Scholar]
- Butcher R. W., Ho R. J., Meng H. C., Sutherland E. W. Adenosine 3',5'-monophosphate in biological materials. II. The measurement of adenosine 3',5'-monophosphate in tissues and the role of the cyclic nucleotide in the lipolytic response of fat to epinephrine. J Biol Chem. 1965 Nov;240(11):4515–4523. [PubMed] [Google Scholar]
- Harbon S., Clauser H. Cyclic adenosine 3',5' monophosphate levels in rat myometrium under the influence of epinephrine, prostaglandins and oxytocin, correlations with uterus motility. Biochem Biophys Res Commun. 1971 Sep 17;44(6):1496–1503. doi: 10.1016/s0006-291x(71)80255-3. [DOI] [PubMed] [Google Scholar]
- Karim S. M., Hillier K., Trussell R. R., Patel R. C. Induction of labour with prostaglandin E2. J Obstet Gynaecol Br Commonw. 1970 Mar;77(3):200–210. doi: 10.1111/j.1471-0528.1970.tb03507.x. [DOI] [PubMed] [Google Scholar]
- Krishna G., Weiss B., Brodie B. B. A simple, sensitive method for the assay of adenyl cyclase. J Pharmacol Exp Ther. 1968 Oct;163(2):379–385. [PubMed] [Google Scholar]
- Marumo F., Edelman I. S. Effects of Ca++ and prostaglandin E1 on vasopressin activation of renal adenyl cyclase. J Clin Invest. 1971 Aug;50(8):1613–1620. doi: 10.1172/JCI106649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitznegg P., Heim F., Meythaler B. Influence of endogenous and exogenous cyclic 3',5'-AMP on contractile responses induced by oxytocin and calcium in isolated rat uterus. Life Sci. 1970 Feb 1;9(3):121–128. doi: 10.1016/0024-3205(70)90356-5. [DOI] [PubMed] [Google Scholar]
- Polacek I., Daniel E. E. Effect of - and -adrenergic stimulation on the uterine motility and adenosine 3'5'-monophosphate level. Can J Physiol Pharmacol. 1971 Nov;49(11):988–998. doi: 10.1139/y71-137. [DOI] [PubMed] [Google Scholar]
- Sharma S. K., Talwar G. P. Action of cyclic adenosine 3',5'-monophosphate in vitro on the uptake and incorporation of uridine into ribonucleic acid in ovariectomized rat uterus. J Biol Chem. 1970 Apr 10;245(7):1513–1519. [PubMed] [Google Scholar]
- Shaw J., Gibson W., Jessup S., Ramwell P. The effect of PGE-1 on cyclic AMP and ion movements in turkey erythrocytes. Ann N Y Acad Sci. 1971 Apr 30;180:241–260. doi: 10.1111/j.1749-6632.1971.tb53195.x. [DOI] [PubMed] [Google Scholar]
- Triner L., Vulliemoz Y., Verosky M., Nahas G. G. Acetylcholine and the cyclic AMP system in smooth muscle. Biochem Biophys Res Commun. 1972 Mar 10;46(5):1866–1873. doi: 10.1016/0006-291x(72)90063-0. [DOI] [PubMed] [Google Scholar]
- Triner L., Vulliemoz Y., Verosky M., Nahas G. G. The effect of catecholamines on adenyl cyclase activity in rat uterus. Life Sci I. 1970 Jun 15;9(12):707–712. doi: 10.1016/0024-3205(70)90201-8. [DOI] [PubMed] [Google Scholar]
- Walton G. M., Garren L. D. An assay for adenosine 3',5'-cyclic monophosphate based on the association of the nucleotide with a partially purified binding protein. Biochemistry. 1970 Oct 13;9(21):4223–4229. doi: 10.1021/bi00823a026. [DOI] [PubMed] [Google Scholar]


