Abstract
The purpose of this study was to investigate the effect of bone marrow stromal cells (BMSCs) on tendon healing in a canine ex vivo model. Bone marrow was harvested and BMSCs were isolated and cultured according to established protocols. Cells were seeded into 0.5 mg/ml collagen gels and cultured for 24 h to allow gel contraction, and then implanted between the lacerated ends of repaired flexor digitorum profundus tendons. Tendons repaired with a gel patch alone and without a gel patch served as control groups. After 2 and 4 weeks in culture, the repaired tendons were evaluated for breaking strength and stiffness. Cell viability was assessed by labeling the cells with PKH26 red fluorescent cell linker. The maximal strength and stiffness of repaired tendons with the BMSC-seeded patch were significantly higher than the repaired tendons without a patch or with a patch without cells, at both 2 and 4 weeks (p < 0.05). Viable BMSC were present between the cut tendon ends at both 2 and 4 weeks. We conclude that BMSC-seeded gel patch transplantation has the potential to enhance flexor tendon healing, and we plan to investigate this effect in vivo.
Keywords: Bone marrow stromal cell, Tendon, Tendon healing, Canine, In vitro
1. Introduction
Injuries to the finger flexor tendons are common, especially in the young and working-age population and result in considerable disability, healthcare cost, and loss of working capability [1]. Primary repair following tendon injury has been commonly accepted as the gold standard to restore hand function [2,3]. However, complications such as adhesions, gap formation, and rupture following tendon repair are still common [4,5], and adhesion-free tendon healing is still a challenge [6].
Although both intrinsic [7,8] and extrinsic [9] processes are involved in flexor tendon healing, the extrinsic healing process is usually predominant because of the relatively poor vascularization of the tendon. However, extrinsic healing comes at the risk of adhesion formation between tendon and surrounding tissues. Intrinsic healing mainly relies upon the ability of the injured tendon to recruit tenocytes from the tendon surface, which migrate to the site of injury [10]. Yet, in the normal tendon, the cellular component comprises only 5% of tissue volume [11] and cellularity is further decreased at the cut ends of injured tendons. Furthermore even a single interrupted suture placed in a normal tendon produces a markedly acellular zone in the surrounding tendon [11], hence conventional tendon repair techniques often render injured tendons even more hypocellular.
Bone marrow stromal cells (BMSCs) are multipotential cells which can differentiate into a spectrum of cell types including fibroblasts and tenocytes [12,13], which have been widely used in tissue engineering to generate the tendon tissue [14,15]. To overcome flexor tendon hypocellualarity and potentially increase the intrinsic healing capabilities of tendon repairs, we proposed to investigate if BMSCs would enhance the flexor tendon intrinsic healing. We hypothesized that interposition of BMSC-seeded collagen gel constructs between the cut tendon ends would increase tendon healing strength in a canine ex vivo model.
2. Methods
2.1. Bone marrow stromal cell harvest
Bone marrow was harvested from mixed-breed dogs weighing between 25 and 30 kg. The dogs were euthanized for other, IACUC approved, studies. Immediately after euthanasia, 4.0 ml of bone marrow was aspirated from each tibia using a 20 ml syringe containing 1.0 ml heparin solution. Bone marrow containing heparin solution was added to 5.0 ml PBS and centrifuged at 1500 rpm for 5 min at room temperature. The supernatant PBS with heparin was removed and the bone marrow cells were divided into four 100 mm dishes in 8 ml of stromal cell growth medium. The medium consisted of minimal essential medium (MEM) with Earle’s salts (GIBCO, Grand Island, NY), 10% fetal calf serum and 5% antibiotics (Antibiotic-Antimycotic, GIBCO, Grand Island, NY). The bone marrow cells were incubated in a 37 °C, 5% CO2 humidified atmosphere. On day 5 after primary culture, the medium containing floating cells was removed and new medium was added to the remaining adherent cells. These adherent cells were defined as bone marrow stromal cells (BMSCs), based on previous reports [12]. The medium was then changed on alternate days. After reaching confluence on days 14–21, the cells were suspended by washing twice with sterile phosphate-buffered saline (PBS), harvested with 0.25% trypsin, counted and subcultured in Petri dishes. Cells between passages 2 and 4 were used for the experiments.
2.2. Preparation of cell-seeded collagen gel
Vitrogen bovine dermal collagen (Cohesion Technologies, Palo Alto, CA, U.S.A.)wasprepared following the company’s instructions. Briefly, 10 ml of sterile, chilled Vitrogen collagen were mixed with 3 ml of sterile MEM, 1.05 ml of sterile 0.167 M NaOH and 0.95 ml distilled H2O to adjust the pH to 7.4 ± 0.2, making 15 ml temporary collagen/MEM solution on ice. The solution was then stored at 4–6 °C for no longer than 1 h prior to use.
Confluent plates of BMSCs were washed twice with sterile PBS and then trypsinized. The cells were counted with a hemocytometer and centrifuged to remove the media and leave behind a cell pellet with a known number of cells. The amount of collagen and cell density was then adjusted to a final collagen concentration of 0.5 mg/ml and initial cell density 1.0 × 106 cells/ml. A 1 ml aliquot of the cell-seeded collagen solution was evenly distributed over the surface of a sterile 35 mm Petri dish with approximately 1 mm thick layer of solution.
After incubating at 37 °C in a 5% CO2 humidified incubator for 24 h for gel contraction, the BMSC-seeded collagen was cut to a similar cross-sectional shape as the tendon ends and used immediately. As a control, collagen gel was prepared similarly, without the addition of BMSCs in the final stages.
2.3. Tendon repair with implant
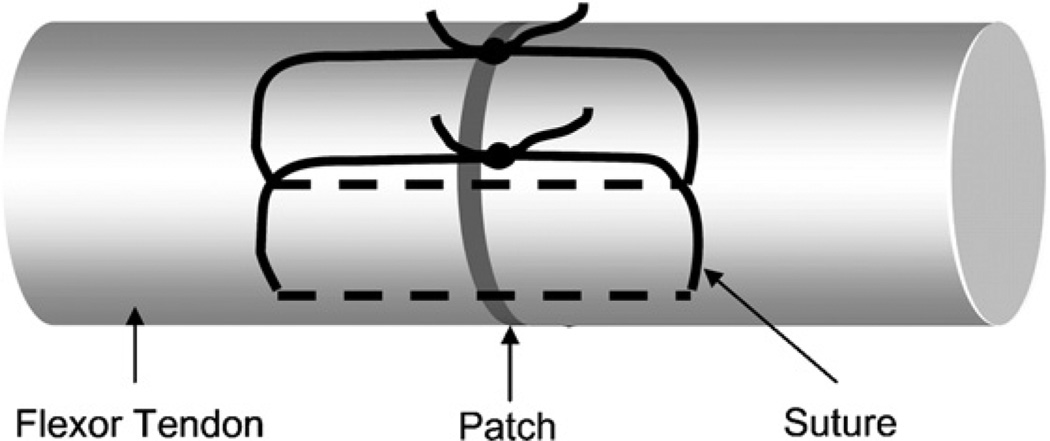
Forty eight FDP tendons from the 2nd, 3rd, 4th and 5th digits of 6 dogs were used in this study. After harvesting, tendons were immediately placed in tissue culture medium as detailed below. The tendons were randomly assigned to three groups: (1) repaired tendon with cell-seeded gel patch; (2) repaired tendon with gel patch without cells; and (3) repaired tendon without gel patch. Each tendon was transected in the middle of a 30 mm length centered at the proximal interphalangeal joint level. This section of the FDP tendon consists of two collagen bundles. A simple suture of 6/0 Ethilon (Ethicon, Somerville, NJ) was placed in each bundle. Before tightening the suture loop, the gel patch with or without BMSCs was implanted between the tendon end and then the sutures was knotted to close the repaired tendon (Fig. 1).
Fig. 1.
Flexor tendon repair using two single looped sutures with patch sandwiched in between.
2.4. Repaired tendon culture
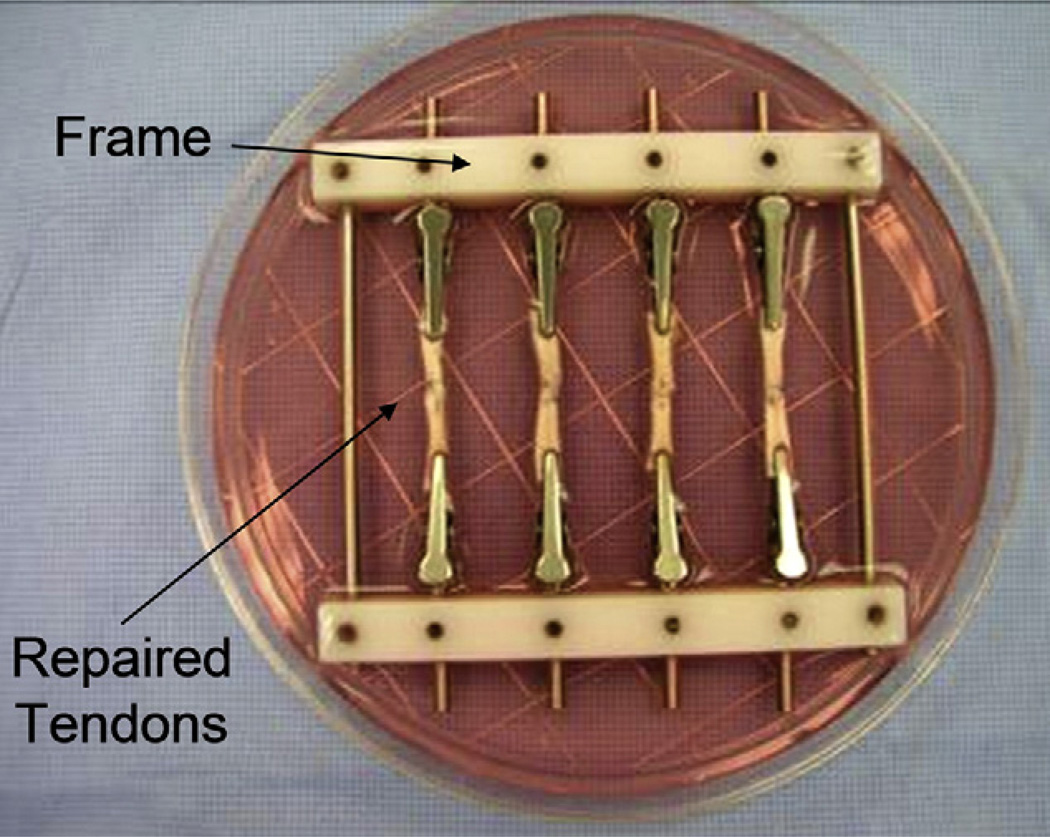
A custom-made frame with four pairs of clamps was designed to fit in a 100 mm Petri dish. The repaired tendons were held in the frame by directly clamping the tendon ends and the frame was placed in the Petri dish containing tissue culture medium (Fig. 2). The tissue culture medium consisted of minimal essential medium (MEM) with Earle’s salts (GIBCO, Grand Island, NY), 10% fetal calf serum and 5% antibiotics (Antibiotic-Antimycotic, GIBCO, Grand Island, NY). The tendons were incubated at 37 °C in a 5% CO2 humidified atmosphere. Culture medium was changed every 72 h.
Fig. 2.
Repaired tendons were clamped with custom-made frame and cultured in the medium.
2.5. Biomechanical testing
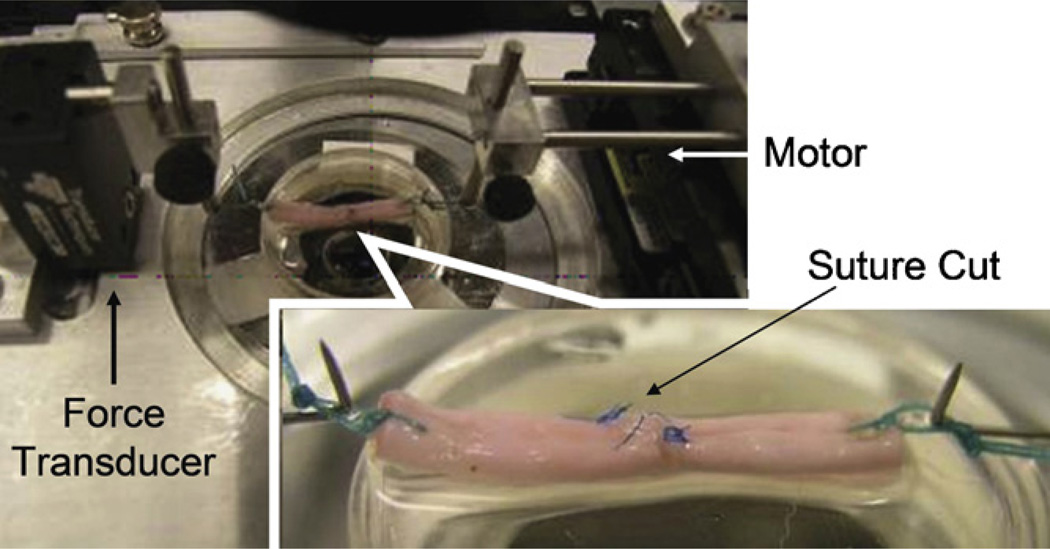
Specimens were removed from tissue culture at 14 or 28 days post-repair. A single suture loop was placed at each end of the repaired tendon for the purpose of mounting the tendon in a custom-designed mechanical micro-tester. The specimens were mounted in the testing apparatus with one suture loop connecting to a load transducer (Techniques Inc., Temecula, CA) and the other loop connecting to a motor and potentiometer (Parker Hannifin Corp., Rohnert Park, CA). The loop at each tendon end was 5 mm long, so that the whole testing specimen including the repaired tendon and suture loops was 40 mm long. Before testing, the two sutures at the repair site were cut carefully, without disrupting the repair site, in order to assess the patch healing strength rather than the suture holding strength (Fig. 3). For mechanical testing, the tendon was placed on a flat glass platform moistened with saline. The specimen was then distracted at a rate of 0.1 mm/s until the repair site was totally separated. The displacement and maximum strength measured by the transducer were recorded for data analysis.
Fig. 3.
Repaired tendons were mounted on the micro-tester for mechanical testing. Before distraction, the sutures were cut at both side of tendon to eliminate the suture holding strength.
2.6. Cell viability assessment
In order to assess BMSC cell viability and distinguish BMSCs from the tenocytes existing in the native tendon, some BMSCs were labeled with PKH26 red fluorescent cell linker (Sigma, St. Louis,MO) before seeding in the gel patch. Two FDP tendons in each group were seeded with labeled BMSCs. Following tissue culture for 2 or 4 weeks, the tendon samples were observed with a confocal microscope (LSM310 Zeiss, Germany).
2.7. Statistical analysis
Analysis of variance (ANOVA) was used to analyze the three groups at each of the two time points, followed by a Tukey–Kramer post hoc test for individual comparisons. Only p values of less than 0.05 were considered significant.
3. Results
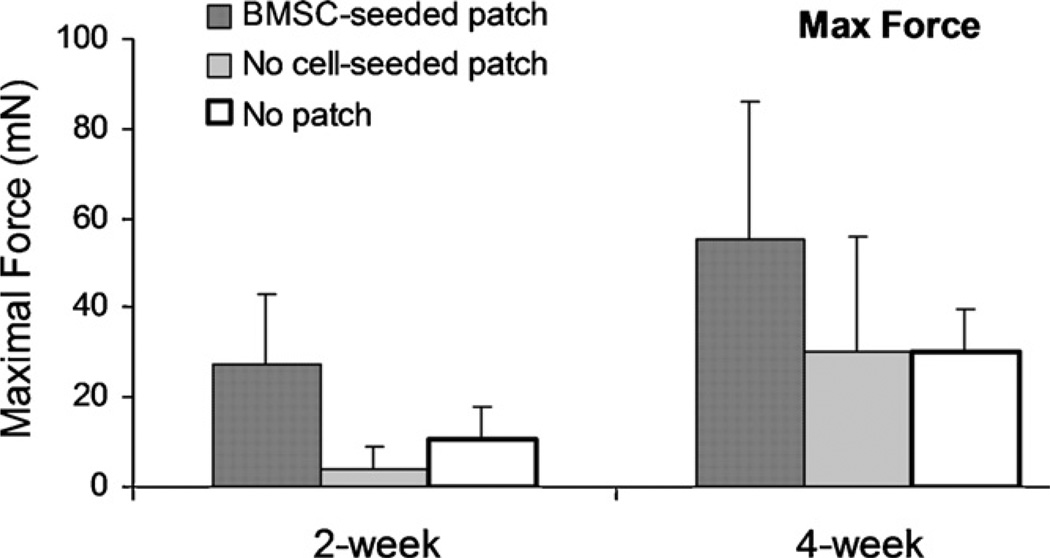
The maximal strength of the repaired tendons at 2 weeks was 10.5 mN (±7.1), 4.1 mN (±4.9), and 27.4 mN (±15.6) for repaired tendon without patch, with patch without cells and with cell-seeded patch, respectively. The maximal strength of repaired tendons at 4 weeks was 30.1 mN (±25.9), 30.2 mN (±25.9), and 55.4 mN (±30.5) for repaired tendon without patch, with patch without cell and with cell-seeded patch, respectively. The maximal strength of repaired tendons with the BMSC-seeded patch was significantly higher than the repaired tendons without patch or with patch without cells, at both 2 and 4 weeks (p < 0.05). There was no significant difference between the repaired tendons without patch and with patch without cells. The maximal strength of the repaired tendons at 4 weeks was significantly higher than the tendons at 2 weeks in all three groups (p < 0.05) (Fig. 4).
Fig. 4.
The maximal failure strength of the repaired tendon without suture holding (a < b < c, p < 0.05).
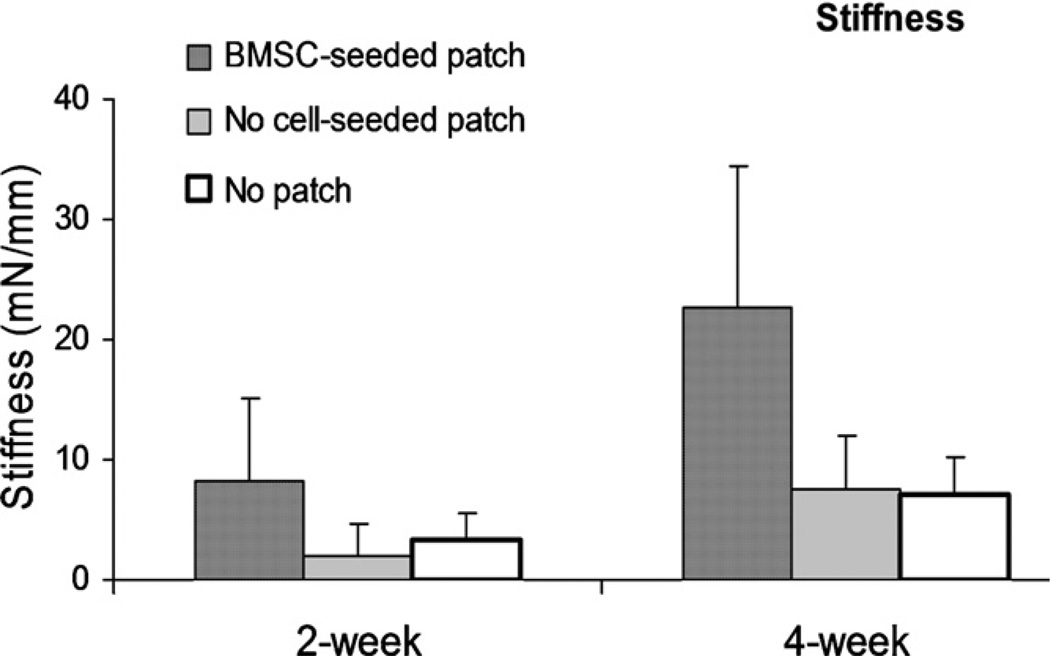
The stiffness of the repaired tendons with BMSC-seeded patch was significantly higher than the repaired tendons without patch and with patch without cells at both 2 and 4 weeks (p < 0.05). The stiffness was also significantly increased with time in all groups (Fig. 5).
Fig. 5.
The stiffness of the repaired tendon without suture holding (a < b < c, p < 0.05).
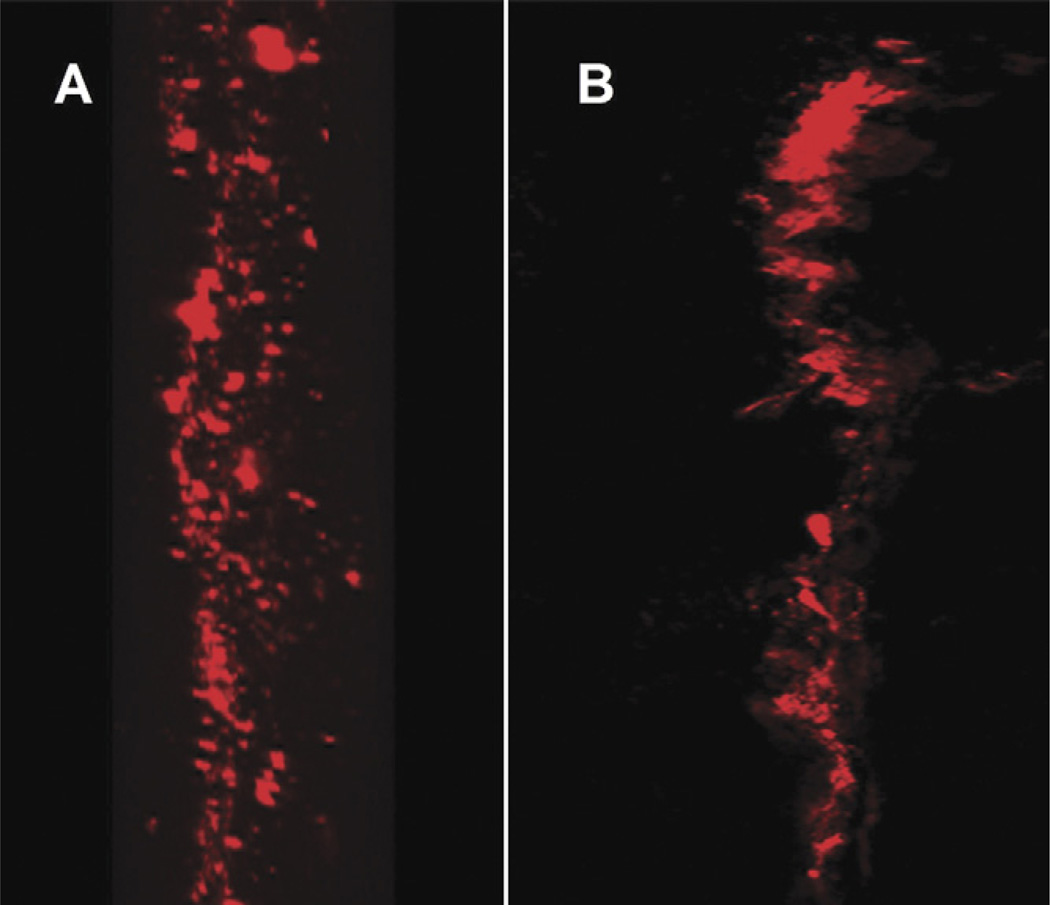
Qualitative observation by confocal microscopy revealed that labeled viable BMSC were present between the cut tendon ends after both 2 and 4 weeks of tissue culture (Fig. 6).
Fig. 6.
The labeled BMSCs with PKH26 cell linker were observed under confocal microscopy with red fluorescent at 2 weeks (A) and 4 weeks (B).
4. Discussion
Due to the hypocellular and hypovascular nature of the flexor tendon, intrinsic healing requires amuch longer time than in other connective tissues [16–18]. This hypocellularity is worsened after injury and surgical repair [19,20]. Furthermore, in tendon healing there is a risk of gap healing rather than a contact healing, due to the effect of muscle tension on the repaired tendon, especially with postoperative rehabilitation [20–23]. This effect may be potentiated by the inherent delay in healing associated with tendon hypovascularity. Silfverskiold et al. used X-rays to measure the distance between two metallic markers which had been inserted into the tendon during the tendon repair surgery. They found that a gap with a mean value of 3.2 mm was present at the final follow-up in 24 of 34 repaired FDP tendons in zone II [24]. Gap healing following flexor tendon injury and repair has also been observed in animal models [20,22,25]. Delivery of new cells directly to the injury site could therefore be a useful therapeutic strategy both to accelerate healing and reduce the risk of late gapping.
Bone marrow stromal cells (BMSCs) are multipotential cells which are able to differentiate into a spectrum of cell types, including fibroblasts and tenocytes [12,13,26]. Recently, Juncosa-Melvin et al. reported that BMSCs improved the biomechanical properties of collagen constructs designed to replace tendon grafts [27]. Recent studies have demonstrated that BMSCs can increase tendon regeneration in animal models of partial tendon injury in vivo [28–30]. Crovace et al. used an Achilles tendinitis model created by collagenase injection to study the effect of BMSCs. They found that BMSCs restored the fiber architecture, increased expression of type I collagen and cartilage oligomeric matrix protein (COMP) compared to the control group [28]. Smith and Smith treated 168 racehorses suffering from superficial digital flexor tendon injure with BMSC implantation. They demonstrated that BMSC treatment improved the outcomes and decreased the re-injury rate [29]. The current study using an ex vivo model of complete tendon injury confirmed that BMSC-seeded patch increased flexor tendon healing in this model as well. This ex vivo model may be useful to optimize the engineered patch with an appropriate stem cell type, density, and adjunctive growth factors prior to studying the results using living animals.
In the current study, we used a collagen gel as the vehicle to transplant the BMSCs directly into the lacerated flexor tendon repair site in a canine ex vivo model. The selections of the gel concentration and cell density were based on previous studies [31]. Chen et al. used a collagen gel lattice contraction model to study tenocyte behavior. They demonstrated that gel contraction and mechanical properties of the contracted collagen lattice were maximal with a 0.5 mg/ml initial gel concentration and 1.0 × 106 cells/ml [31]. Using this formulation, we have demonstrated that the healing strength and stiffness both increased in the BMSC groups compared with the two control groups.
The two control groups (with gel patch, but no cells and without gel patch or cells) were selected for the purpose of eliminating the possibilities of stimulation or isolation effects of the collagen gel on tendon healing. We did not find any significant difference between the two control groups in our ex in vivo model, although some reports have demonstrated that collagen gel alone could stimulate tendon healing [32,33].
As the sutures were cut before the tendon tensile testing, the suture holding strength was eliminated as a factor and we were able to measure the strength of the tendon–tendon healing directly. This is a novel method to mechanically evaluate tendon healing strength.
One of the limitations of the current study was that specific markers of tenocyte differentiation, such as tenomodulin and types I and III collagen expression were not examined, as we focused on the mechanical properties of healing. Future studies should assess these factors as well. Second, the tendon healing was studied using an ex vivo model, which would be different from an in vivo condition in biological response, nutrition and mechanical stimulation. Third, we did not use other cell sources, such as dermal fibroblasts, as another control group, to investigate the effect of different cell types on enhancement of tendon healing. Finally, the behavior of the BMSCs may differ from that of the tenocytes which were used to determine the gel and cell concentration. Therefore, the optimization of the gel and cell concentration of the BMSC needs to be further studied. In future studies, we will use the ex vivo model to investigate BMSC differentiation potential and functional behavior after implantation to repaired tendon ends using immunohistochemistry approaches. The effects of growth factors on the BMSC-seeded patch will be also considered. Once the ex vivo model is optimized, we plan to investigate the effect of cell-seeded patches in animal model in vivo.
5. Conclusion
In this study we directly implanted BMSCs between repaired flexor tendon ends using a collagen patch as the cell delivery vehicle. We also developed a method to directly assess the mechanical properties of the healing tissues. The repaired tendons with BMSCs had improved failure strength and stiffness. We believe that BMSC-seeded gel patch transplantation has the potential to enhance flexor tendon healing and plan to investigate its effect in vivo.
Acknowledgment
This study was funded by a grant from Mayo Foundation.
Footnotes
Conflict of interest
The authors confirm that there is no potential conflict of interest including employment, consultancies, stock ownership, honoraria, and paid expert testimony, and patent applications influencing this work.
References
- 1.Kelsey JL. Upper extremity disorders: frequency, impact, and cost. New York: Churchill Livingstone; 1997. [Google Scholar]
- 2.Kleinert HE, Schepel S, Gill T. Flexor tendon injuries. Surg Clin North Am. 1981;61(2):267–286. doi: 10.1016/s0039-6109(16)42381-9. [DOI] [PubMed] [Google Scholar]
- 3.Strickland JW. Development of flexor tendon surgery: twenty-five years of progress. J Hand Surgery-Am Vol. 2000;25(2):214–235. doi: 10.1053/jhsu.2000.jhsu25a0214. [DOI] [PubMed] [Google Scholar]
- 4.Small JO, Brennen MD, Colville J. Early active mobilisation following flexor tendon repair in zone 2 (see comments) J Hand Surg (Br) 1989;14(4):383–391. doi: 10.1016/0266-7681_89_90152-6. [DOI] [PubMed] [Google Scholar]
- 5.Harris SB, Harris D, Foster AJ, Elliot D. The aetiology of acute rupture of flexor tendon repairs in zones 1 and 2 of the fingers during early mobilization. J Hand Surg (Br) 1999;24(3):275–280. doi: 10.1054/jhsb.1998.0212. [DOI] [PubMed] [Google Scholar]
- 6.Manske PR. Flexor tendon healing. J Hand Surg. 1988;13B(3):237–245. doi: 10.1016/0266-7681_88_90077-0. [DOI] [PubMed] [Google Scholar]
- 7.Mass DP, Tuel RJ. Intrinsic healing of the laceration site in human superficialis flexor tendons in vitro. J Hand Surg (Am) 1991;16(1):24–30. doi: 10.1016/s0363-5023(10)80006-1. [DOI] [PubMed] [Google Scholar]
- 8.Boyer MI, Goldfarb CA, Gelberman RH. Recent progress in flexor tendon healing. The modulation of tendon healing with rehabilitation variables. J Hand Ther. 2005;18(2):80–85. doi: 10.1197/j.jht.2005.02.009. quiz 6. [DOI] [PubMed] [Google Scholar]
- 9.Jones ME, Mudera V, Brown RA, Cambrey AD, Grobbelaar AO, McGrouther DA. The early surface cell response to flexor tendon injury. J Hand Surg (Am) 2003;28(2):221–230. doi: 10.1053/jhsu.2003.50044. [DOI] [PubMed] [Google Scholar]
- 10.Gelberman RH, Vande Berg JS, Lundborg GN, Akeson WH. Flexor tendon healing and restoration of the gliding surface. An ultrastructural study in dogs. J Bone Joint Surg (Am) 1983;65(1):70–80. [PubMed] [Google Scholar]
- 11.Tozer S, Duprez D. Tendon and ligament: development, repair and disease. Birth Defects Res C Embryo Today. 2005;75(3):226–236. doi: 10.1002/bdrc.20049. [DOI] [PubMed] [Google Scholar]
- 12.Liechty KW, MacKenzie TC, Shaaban AF, Radu A, Moseley AM, Deans R, et al. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6(11):1282–1286. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- 13.Prockop DJ, Sekiya I, Colter DC. Isolation and characterization of rapidly self-renewing stem cells from cultures of human marrow stromal cells. Cytotherapy. 2001;3(5):393–396. doi: 10.1080/146532401753277229. [DOI] [PubMed] [Google Scholar]
- 14.Awad HA, Boivin GP, Dressler MR, Smith FNL, Young RG, Butler DL. Repair of patellar tendon injuries using a cell-collagen composite. J Orthop Res. 2003;21(3):420–431. doi: 10.1016/S0736-0266(02)00163-8. [DOI] [PubMed] [Google Scholar]
- 15.Kryger GS, Chong AK, Costa M, Pham H, Bates SJ, Chang J. A comparison of tenocytes and mesenchymal stem cells for use in flexor tendon tissue engineering. J Hand Surg (Am) 2007;32(5):597–605. doi: 10.1016/j.jhsa.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Harrison RK, Jones ME, Clayton E, Grobbelaar AO, Sanders R. Mapping of vascular endothelium in the human flexor digitorum profundus tendon. J Hand Surg (Am) 2003;28(5):806–813. doi: 10.1016/s0363-5023(03)00301-0. [DOI] [PubMed] [Google Scholar]
- 17.Manske PR, Gelberman RH, Vande Berg JS, Lesker PA. Intrinsic flexor-tendon repair. A morphological study in vitro. J Bone Joint Surg (Am) 1984;66(3):385–396. [PubMed] [Google Scholar]
- 18.Banes AJ, Donlon K, Link GW, Gillespie Y, Bevin AG, Peterson HD, et al. Cell populations of tendon: a simplified method for isolation of synovial cells and internal fibroblasts: confirmation of origin and biologic properties. J Orthop Res. 1988;6(1):83–94. doi: 10.1002/jor.1100060111. [DOI] [PubMed] [Google Scholar]
- 19.Buckman RF, Jr, Hufnagel HV, Olivier G, Buckman PD, Zuidema GD. Some effects of Bunnell suture on otherwise uninjured tendons in subhuman primates. Surgery. 1977;82(5):660–666. [PubMed] [Google Scholar]
- 20.Bertone AL, Stashak TS, Smith FW, Norrdin RW. A comparison of repair methods for gap healing in equine flexor tendon. Vet Surg. 1990;19(4):254–265. doi: 10.1111/j.1532-950x.1990.tb01181.x. [DOI] [PubMed] [Google Scholar]
- 21.Silfverskiold KL, May EJ. Gap formation after flexor tendon repair in zone. II. Results with a new controlled motion programme. Scand J Plast Reconstr Surg Hand Surg. 1993;27(4):263–268. [PubMed] [Google Scholar]
- 22.Jann H, Blaik M, Emerson R, Tomioka M, Stein L, Moll D. Healing characteristics of deep digital flexor tenorrhaphy within the digital sheath of horses. Vet Surg. 2003;32(5):421–430. doi: 10.1053/jvet.2003.50059. [DOI] [PubMed] [Google Scholar]
- 23.Silva MJ, Boyer MI, Gelberman RH. Recent progress in flexor tendon healing. J Orthop Sci. 2002;7(4):508–514. doi: 10.1007/s007760200090. [DOI] [PubMed] [Google Scholar]
- 24.Silfverskiold KL, May EJ, Tornvall AH. Gap formation during controlled motion after flexor tendon repair in zone. II. A prospective clinical study. J Hand Surg (Am) 1992;17(3):539–546. doi: 10.1016/0363-5023(92)90368-y. [DOI] [PubMed] [Google Scholar]
- 25.Valdes-Vazquez MA, McClure JR, Oliver JL, 3rd, Ramirez S, Seahorn TL, Haynes PF. Evaluation of an autologous tendon graft repair method for gap healing of the deep digital flexor tendon in horses. Vet Surg. 1996;25(4):342–350. doi: 10.1111/j.1532-950x.1996.tb01423.x. [DOI] [PubMed] [Google Scholar]
- 26.Fu W, Lu Y, Piao Y. Culture and pluripotentiality of human marrow mesenchymal stem cells. Chung Hua Hsueh Yeh Hsueh Tsa Chi. 2002;23(4):202–204. [PubMed] [Google Scholar]
- 27.Juncosa-Melvin N, Shearn JT, Boivin GP, Gooch C, Galloway MT, West JR, et al. Effects of mechanical stimulation on the biomechanics and histology of stem cell-collagen sponge constructs for rabbit patellar tendon repair. Tissue Eng. 2006;12(8):2291–2300. doi: 10.1089/ten.2006.12.2291. [DOI] [PubMed] [Google Scholar]
- 28.Crovace A, Lacitignola L, Francioso E, Rossi G. Histology and immunohistochemistry study of ovine tendon grafted with cBMSCs and BMM-NCs after collagenase-induced tendinitis. Vet Comp Orthop Traumatol. 2008;21(4):329–336. doi: 10.3415/vcot-07-05-0050. [DOI] [PubMed] [Google Scholar]
- 29.Smith RK, Smith RKW. Mesenchymal stem cell therapy for equine tendinopathy. Disabil Rehabil. 2008;30(20–22):1752–1758. doi: 10.1080/09638280701788241. [DOI] [PubMed] [Google Scholar]
- 30.Fortier LA, Smith RK, Fortier LA, Smith RKW. Regenerative medicine for tendinous and ligamentous injuries of sport horses. Vet Clin North Am-Equine Pract. 2008;24(1):191–201. doi: 10.1016/j.cveq.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Chen MY, Sun Y, Zhao C, Zobitz ME, An KN, Moran SL, et al. Factors related to contraction and mechanical strength of collagen gels seeded with canine endotenon cells. J Biomed Mater Res B Appl Biomater. 2007;82(2):519–525. doi: 10.1002/jbm.b.30757. [DOI] [PubMed] [Google Scholar]
- 32.Mian M, Aloisi R, Benetti D, Rosini S, Fantozzi R. Potential role of heterologous collagen in promoting cutaneous wound repair in rats. Int J Tissue React. 1992;14(Suppl.):43–52. [PubMed] [Google Scholar]
- 33.Ishikawa H, Koshino T, Takeuchi R, Saito T. Effects of collagen gel mixed with hydroxyapatite powder on interface between newly formed bone and grafted achilles tendon in rabbit femoral bone tunnel. Biomaterials. 2001;22(12):1689–1694. doi: 10.1016/s0142-9612(00)00331-8. [DOI] [PubMed] [Google Scholar]