Abstract
Radiation exposure leads to diverse outcomes in vivo across different tissues and even within the same cell lineage. The diversity of radiation response in vivo is at least partially attributable to the status of the tumor suppressor p53, a master regulator of cellular response to stress, and activation of its transcriptional targets. In certain cells, such as hematopoietic progenitors and transit amplifying cells in the gastrointestinal epithelium, activation of p53 by radiation triggers the intrinsic pathway of apoptosis. However, in many other cells, activation of p53 by radiation does not result in apoptosis, which underscores the importance of understanding the role of p53 in regulating radiation response through alternative mechanisms. In this review, we summarize recent studies using genetically engineered mice to dissect the role of p53 in 1) cells where its activation is dissociated from the intrinsic pathway of apoptosis, such as hematopoietic stem cells and vascular endothelial cells and 2) tissues where activation of the intrinsic pathway of apoptosis does not promote the acute radiation syndrome, such as the gastrointestinal epithelium. We highlight findings showing that the apoptosis-independent response of p53 to radiation in vivo can contribute to death or survival in a cell-type dependent manner, which underscores the complexity by which p53 regulates the cellular and tissue response to radiation.
Keywords: Apoptosis, Cell cycle arrest, Normal tissue injury, p53, Radiation
Introduction
The tumor suppressor p53 is a master regulator of cellular response to radiation (1–3). p53 is a multifunctional transcription factor containing two transcriptional activation domains that can independently enhance transcription of downstream target genes, and a DNA binding domain responsible for sequence-specific binding of p53 to its response elements (4). Upon radiation exposure, activation of the DNA damage response increases the level of p53 protein in cells primarily by promoting protein translation (5) and inhibiting protein degradation (6). Accumulation of p53 protein in the nucleus induces a variety of downstream signaling pathways that mediate cellular response to stress (7, 8). Activation of p53-mediated signaling can cause cell cycle arrest and facilitate DNA repair, which promote cell survival, or induce the intrinsic pathway of apoptosis and cell senescence, which augment cell death. Therefore, p53 plays a crucial role in controlling cellular fate after irradiation.
Several factors influence the p53 response following irradiation, including the intensity of stress, the presence of co-factors that interact with p53, and DNA binding cooperativity of p53 (7, 9). Additionally, it has been shown in mice that total-body irradiation induces p53 and its downstream signaling in vivo in a tissue-dependent manner (8, 10, 11). For example, activation of p53 results in dramatically increased pre-mitotic apoptosis in tissues that have a rapid turnover rate such as the hematopoietic system and the gastrointestinal epithelium. To the contrary, in tissues with a slower turnover rate, such as the myocardium, accumulation of p53 following radiation does not cause a significant increase in pre-mitotic apoptosis, but instead induces genes that control cell-cycle checkpoints such as the cyclin-dependent kinase inhibitor p21. Moreover, recent studies in the hematopoietic system suggest that p53 activation results in a distinct response in stem cells versus progenitors (12, 13). Thus, these findings reveal the diversity of p53 response following radiation in vivo and underscore the significance of dissecting the mechanisms by which p53 controls radiation response in a cell-type specific manner.
To dissect the role of p53 in response to radiation in vivo, several groups have performed mechanistic studies using mice that either lack p53 or its transcriptional targets, or using the Cre-loxP system (14) to spatially and/or temporally disrupt p53 in mice. In this review, we summarize recent advances in understanding the role of p53-mediated signaling in regulating radiation response through mechanisms that are independent of apoptosis in the hematopoietic system, the gastrointestinal epithelium and vascular endothelial cells.
Role of p53 in controlling radiation response in hematopoietic stem and progenitor cells
Radiation causes acute and long-term toxicity in the hematopoietic compartment
The hematopoietic system is very sensitive to radiation. After irradiation, a rapid increase in pre-mitotic apoptosis ablates hematopoietic progenitor cells and more differentiated hematopoietic cells (15), leading to acute hematological radiation toxicity due to the short-term loss of functioning blood cells (16, 17). The overall response of the hematopoietic compartment is mediated by apoptosis in the acute phase following radiation exposure, coupled with long-term defects in hematopoietic stem cells (HSCs) after the recovery phase (18). The reduction in fitness of irradiated HSCs, which is associated with cell senescence (18–20), has been demonstrated using competitive repopulation assays. HSCs from mice that are exposed to lethal or sub-lethal doses of total-body irradiation have a dramatic decrease in long-term engraftment in the bone marrow compared to unirradiated HSCs (19, 21, 22). Collectively, these results demonstrate that radiation exposure causes short-term and long-term damage to the hematopoietic compartment. While acute hematological radiation toxicity is primarily attributed to apoptosis, chronic hematological toxicity is at least partially caused by apoptosis-independent mechanisms.
Loss of p53 ameliorates acute hematological radiation toxicity by blocking apoptosis
Radiation induces the DNA damage response to active p53 in hematopoietic stem cells and progenitors (HSPCs) (12, 13, 23). However, the response of p53 to radiation varies between stem and progenitor cells. While p53 activation engages radiation-induced apoptosis in hematopoietic progenitors, activation of p53 in short-term HSCs does not induce the intrinsic pathway of apoptosis. Moreover, radiation does not induce detectable levels of phosphorylated p53 in long-term HSCs (13). It has been shown that p53 is necessary to promote radiation-induced apoptosis in hematopoietic cells because deletion of p53 in mice (p53−/− mice) dramatically abrogates radiation-induced apoptosis and ameliorates acute hematological radiation toxicity (24–26). The essential role of p53-mediated apoptosis in acute hematopoietic toxicity is further demonstrated using mice that lack PUMA (p53 upregulated modulator of apoptosis), a transcriptional target of p53 that activates the intrinsic pathway of apoptosis (27, 28). Compared to PUMA+/+ mice, both PUMA+/− and PUMA−/− mice are resistant to acute hematological radiation toxicity due to a dramatic decrease in apoptosis in hematopoietic progenitor cells (29, 30).
Loss of p53 improves long-term engraftment potential of irradiated hematopoietic stem cells
It has been challenging to study long-term effects of radiation on the hematopoietic system in p53−/− mice due to the extremely high penetrance of spontaneous lymphomas (31, 32). Recent studies investigated how p53 controls long-term fitness of HSPCs after total-body irradiation using bone marrow chimeric mice that harbor only a small portion of p53-deficient cells. Marusyk et al. generated bone marrow chimera containing approximately 15% GFP-tagged p53−/− cells. After 2.5 Gy total-body irradiation, the percentage of p53−/− peripheral blood cells in chimeric mice increased significantly compared to unirradiated controls, indicating that p53 disruption confers radioresistance and facilitates clonal expansion of HSPCs (33). These results indicate that deletion of p53 in HSPCs improves their clonogenic capacity after irradiation over HSPCs with wild-type p53 because radiation-induced apoptosis is blocked in progenitors and because stem/progenitor cells are protected from radiation-induced loss of fitness.
To address a similar question, Bondar et al generated a novel conditional allele in which a GFP-tagged oncogenic p53 mutant R172H (mp53) can be temporally induced in the whole animal by tamoxifen. Injection with a single dose of tamoxifen created bone marrow chimeric mice that contain a small portion (<5%) of mp53 cells in peripheral blood. After exposure to 2.5 Gy total-body irradiation, the percentage of mp53 blood cells increased dramatically and even persisted 200 days after irradiation, demonstrating the expansion of mp53 cells in the long-term HSC pool (34). Interestingly, induction of mp53 either two days before or 7 days after irradiation, when the DNA damage response is diminished, significantly increased the percentage of mp53 cells in hematopoietic cells. These results indicate that in addition to the DNA damage response, stress stimuli that are secondary to radiation, such as increased reactive oxygen species (22, 35, 36), impair the fitness of HSPCs that have functional p53. In addition, deletion of cyclin-dependent kinase inhibitor 2A gene, which transcribes both p16INK4a and p19ARF in mice (37), partially improves the clonogenic capacity of p53-wild type HSPCs after irradiation, suggesting that senescence contributes to radiation-induced defects in HSPCs. Together, these findings indicate that a permanent change in p53 activity improves the fitness of HSPCs by blocking acute apoptosis, which is induced by the DNA damage response and by suppressing delayed senescence, which may be induced by an altered microenvironment after irradiation.
Deletion of p21 allows human fibroblasts to bypass senescence in response to DNA damage (38), suggesting that p21 may also play a role in regulating radiation-induced senescence in vivo. However, different groups have shown that loss of p21 exacerbates defects in long-term engraftment potential of irradiated HSCs (13, 39). These data indicate that p21 is necessary to protect HSCs against radiation. Interestingly, Insinga and colleagues found that radiation upregulated p21 in short-term HSCs and long-term HSCs via p53-dependent and p53-independent mechanisms, respectively (13). In addition, p21 protein actually suppressed radiation-induced p53 activation in long-term HSCs, because deletion of p21 in long-term HSCs increased phosphorylated of p53 protein and apoptosis after irradiation. These results indicate that p21 regulates the response to radiation in HSCs through mechanisms that either dependent or independent of p53. Further studies are warranted to understand the mechanisms by which radiation induces p21 in a p53-independent manner and how p21 suppresses p53 activation in long-term HSCs after irradiation.
Summary
A reduced level of p53 in the hematopoietic compartment promotes radiation resistance, making p53 a promising target for preventing the acute hematopoietic syndrome and/or residual bone marrow toxicity. However, the manner in which p21 cooperates with p53 to regulate radiation response in short-term and long-term HSCs remains to be better understood (Figure 1A). In addition, there is a concern about the long-term consequences of reducing p53 (such as thymic lymphoma) during radiation because of its function as a tumor suppressor (40). Therefore, further studies are warranted to evaluate the effect of temporarily blocking p53 during irradiation on radiation toxicity of the hematopoietic system and radiation-induced cancer.
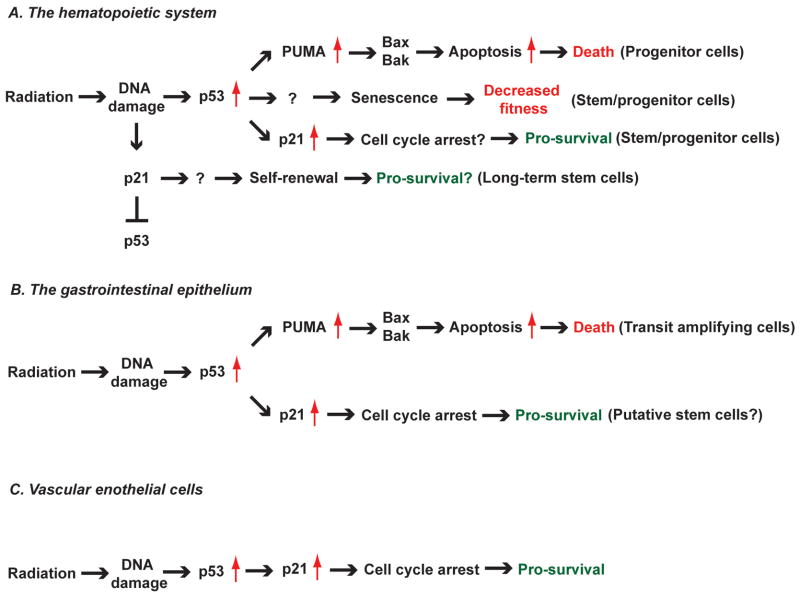
Figure 1. The diverse role of p53 in regulating cellular response to radiation in vivo.
Schematic diagram summarizing the results of studies investigating the role of genes in the p53 pathway in the cellular response to radiation. (A) Mice that lack p53 (26) or PUMA (29, 30) are resistant to hematopoietic radiation toxicity because of decreased radiation-induced apoptosis in hematopoietic progenitor cells. In addition, mice with hematopoietic cell-specific deletion of Bax and Bak are also resistant to radiation-induced acute hematopoietic toxicity (51), which recapitulates the phenotype observed in p53−/− and PUMA−/− mice. In addition to suppressing apoptosis, deletion of p53 increases the fitness of hematopoietic stem/progenitor cells after irradiation (33, 34) through mechanisms that are partially dependent on blocking radiation-induced senescence. Conversely, deletion of the cyclin-dependent kinase inhibitor p21 decreases the fitness of hematopoietic stem/progenitor cells after irradiation (13, 39), which is likely due to defects in cell cycle arrest. In long-term hematopoietic stem cells (LT-HSCs), radiation induces p21 in a p53-independent manner, which improves self-renewal of stem cells. Moreover, deletion of p21 increases p53 levels in LT-HSCs after irradiation, suggesting that p21 negatively regulates p53 in LT-HSCs (13). (B) Deletion of p53 in the whole animal (46, 47) or in the gastrointestinal (GI) epithelium (51) suppresses radiation-induced apoptosis, which is mediated by PUMA (54) and Bax/Bak (51), in transit amplifying cells in the GI epithelium. However, loss of p53 in the GI epithelium exacerbates the radiation-induced GI syndrome (26, 51) because of defects in p21-mediated cell cycle arrest, which leads to mitotic catastrophe in putative intestinal stem cells that are responsible for tissue regeneration after radiation. (C) Deletion of p53 sensitizes endothelial cells to radiation (77, 78) due to defects in p21-mediated cell cycle arrest. Damage of cardiac endothelial cells by radiation leads to myocardial hypoxia and cardiac ischemia, which cause radiation-induced cardiac injury (78).
Role of p53-mediated signaling in the radiation-induced gastrointestinal (GI) syndrome
Loss of p53 sensitizes mice to the radiation-induced GI syndrome
Exposure of the gastrointestinal (GI) tract to radiation causes acute gastrointestinal toxicity or the GI syndrome (41). The GI syndrome is caused by destruction of the GI epithelium, which leads to infection and loss of fluid and electrolytes (42, 43). The integrity of the small intestine is dependent on a constant state of renewal driven by the stem cells residing in the crypts. Radiation impairs the regeneration of intestinal epithelium predominantly by inducing cell death in crypt epithelial cells (44, 45). Crypt epithelial cells are highly sensitive to radiation-induced pre-mitotic apoptosis, which occurs within a few hours after irradiation (44). It has been shown that p53-mediated signaling plays a pivotal role in promoting pre-mitotic apoptosis of crypt epithelial cells because crypt epithelial cells in p53−/− mice are dramatically resistant to radiation-induced apoptosis that occurs 4 to 6 hours after irradiation (46, 47).
While crypt epithelial cells in p53−/− mice are resistant to radiation-induced apoptosis, p53−/− mice are surprisingly more sensitive to the radiation-induced GI syndrome (26). Detailed time course studies after radiation exposure show that p53−/− mice have a delayed onset of cell death in crypt epithelial cells that occurs approximately 24 hours after irradiation (48). Thus, it is possible that loss of p53 sensitizes crypt epithelial cells to mitotic death, which results from aberrant segregation of the genomic DNA during mitosis (49, 50). Mitotic catastrophe is frequently observed in cells that have a defect in cell cycle checkpoints (50). Indeed, in the first 24 hours after irradiation, crypt epithelial cells of p53+/+ mice show a decrease in cell proliferation; however, crypt epithelial cells of p53−/− mice have a defect in cell cycle arrest and continue to proliferate (26, 48). Collectively, these results indicate a diverse role of p53 in regulating the survival of crypt epithelial cells.
The intrinsic pathway of apoptosis in GI epithelial cells does not contribute to the radiation-induced GI syndrome
To specifically investigate the role of the intrinsic pathway of apoptosis in the radiation-induced GI syndrome, we utilized the Cre-loxP system to generate mice with GI epithelium-specific deletion of Bak (Bcl-2 homologous antagonist killer) and Bax (Bcl-2 associated X protein) (VillinCre; BaxFL/−; Bak−/−) (51). Bak and Bax are key pro-apoptotic proteins that govern mitochondrial outer membrane permeabilization to irreversibly initiate the intrinsic pathway of apoptosis (52, 53). Remarkably, deletion of both Bak and Bax in the GI epithelium decreased radiation-induced apoptosis in crypt epithelial cells, but it did not protect mice from the radiation-induced GI syndrome (51). In contrast, specific deletion of p53 in the GI epithelium significantly exacerbated the GI syndrome, which recapitulates the phenotype that was observed in p53−/− mice (51). Moreover, deletion of Bak and Bax did not rescue the radiation sensitivity of the GI tract resulting from loss of p53. Together, these results demonstrate that 1) survival from the GI syndrome is not increased by blocking the intrinsic pathway of apoptosis in GI epithelial cells and 2) loss of p53 sensitizes GI epithelial cells to radiation through mechanisms that are independent of pre-mitotic apoptosis.
Loss of PUMA protects mice from the GI syndrome via the cyclin-dependent kinase inhibitor p21
Other groups also investigated the role of p53-mediated apoptosis in controlling the radiation-induced GI syndrome using mice with whole animal knockout of PUMA (54). Remarkably, PUMA−/− mice not only showed a defect in radiation-induced apoptosis in the crypts, but also had improved survival from the GI syndrome (54). These results suggest that blocking PUMA-mediated apoptosis may protect mice from the GI syndrome, which appears to contradict the results using mice with GI epithelium-specific deletion of Bak and Bax. However, because PUMA functions upstream of Bak and Bax to initiate pre-mitotic apoptosis (10), it is possible that deletion of PUMA protects mice from the GI syndrome through mechanisms that are independent of its role in regulating apoptosis (55). Indeed, through mechanisms that are not well understood, the GI epithelium of PUMA−/− mice has elevated levels of the cyclin-dependent kinase inhibitor p21 (54, 56). Thus, up-regulation of p21 may function in the resistance to the radiation-induced GI syndrome resulting from deletion of PUMA.
The role of p21 in the radiation-induced GI syndrome has been examined in several studies using p21−/− mice. The results from these studies demonstrate that p21−/− mice are more sensitive to the radiation-induced GI syndrome than mice retaining functional p21 (26, 51, 56), indicating that p21-mediates signaling is necessary to prevent mice from developing the GI syndrome. To elucidate whether p21 is necessary for the resistance of PUMA−/− mice to the GI syndrome, Leibowitz et al. investigated the radiation-induced GI syndrome in p53−/−, PUMA−/−, p21−/− and PUMA−/−; p21−/− (double knockout) mice (56). Their results showed that PUMA−/−; p21−/− mice developed the radiation-induced GI syndrome significantly faster than PUMA−/− mice, indicating that p21 is also necessary to confer resistance to the GI syndrome in PUMA−/− mice. Remarkably, although p53−/−, p21−/− and PUMA−/−; p21−/− mice were more sensitive to the GI syndrome compared to wild-type mice; these mice all had a significantly higher number of regenerated crypts in the small intestine 72 hours after irradiation, which is likely due to compromised cell cycle arrest (57, 58). Defects in cell cycle arrest in these mice elicit a higher percentage of crypt cells that undergo aberrant mitosis or mitotic catastrophe, which results in delayed cell death after irradiation (56). Consistent with this model, we found that “super p53 mice”, which harbor an extra copy of p53 (59), are more resistant to the radiation-induced GI syndrome via a mechanism that is also dependent on p21 (14, 60). Taken together, these results demonstrate a pivotal role of the p53/p21 axis in protecting mice against the radiation-induced GI radiation syndrome by preventing crypt cells from premature mitotic entry after irradiation.
Mitotic catastrophe contributes to cell death in intestinal stem cells after irradiation
The increased sensitivity of p53−/− and p21−/− mice to GI syndrome reveals that certain types of intestinal stem cells (ISCs) (61) essential to regenerate the GI epithelium after radiation injury may be killed through mitotic catastrophe. Indeed, in the small intestine of wild-type mice, radiation not only induces pre-mitotic apoptosis, but also causes aberrant mitosis and mitotic death in crypt epithelial cells (51, 62). To elucidate the mechanisms by which ISCs die from radiation, a recent study(63) investigated the radiosensitivity of Lgr5+ crypt base columnar cells (CBCs), a group of ISCs that can reconstitute at least part of the GI tract (64). Hua and colleagues found that radiation exposure caused a dose-dependent decrease in CBCs in the small intestine. In addition, an irreversible loss of CBCs in the small intestine was observed at a radiation dose that caused the GI syndrome (15 Gy). Remarkably, the majority of CBCs were depleted around 1 to 3.5 days, rather than a few hours, after 15 Gy, suggesting that the majority of CBCs died from mitotic death after irradiation. Together, these results reveal a strong association between mitotic catastrophe of CBCs and the onset of the radiation-induced GI syndrome.
Summary
The diverse effect of p53-mediated signaling on radiosensitivity of the GI epithelium reveals the complex biology of the radiation-induced GI syndrome (Table 1). While some crypt epithelial cells are highly sensitive to radiation-induced apoptosis, which is largely dependent on p53 activation, blocking the intrinsic pathway of apoptosis in the GI epithelium does not significantly influence the GI syndrome. In contrast, studies with p53−/− and p21−/− mice demonstrate the significance of the p53/p21-mediated cell cycle arrest pathway in preventing mitotic catastrophe in crypt epithelial cells after irradiation (Figure 1B). Given that multiple types of ISCs may contribute to regeneration of the small intestine after radiation injury (65), future studies using mouse genetics to manipulate p53 expression in specific types of ISCs would provide insight into how p53-mediated apoptosis and cell cycle arrest cooperate to regulate the radiation-induced GI syndrome.
Table 1.
Summary of studies that use knockout mice to study the role of p53-mediated signaling in regulating the radiation-induced GI syndrome
| Deletion of genes | Radiation-induced pre-mitotic apoptosis | Radiation-induced GI syndrome | References |
|---|---|---|---|
| p53 (whole animal) | Decreased | Sensitive | 26, 46, 47, 56 |
| p53 (GI epithelium) | Decreased | Sensitive | 51 |
| Bax and Bak (GI epithelium) | Decreased | No change | 51 |
| PUMA (whole animal) | Decreased | Resistant | 54, 56 |
| p21 (whole animal) | No change | Sensitive | 26, 51, 56 |
| PUMA and p21 (whole animal) | Decreased | Sensitive | 56 |
Role of p53-mediated signaling in response of endothelial cells to radiation
The vascular endothelium is a critical to maintain the architecture and function of blood vessels. Damage to endothelial cells significantly contributes to the pathogenesis of acute and late effects of radiation (66, 67). For example, animal models show that radiation causes ultrastructural endothelial degeneration and a substantial decrease in microvessel density in the myocardium, which occurs prior to the onset of radiation-induced myocardial injury (68–72). Radiation causes endothelial cell death or dysfunction through a variety of mechanisms including apoptosis (73), senescence (74, 75) and mitotic death (75). In vitro studies using endothelial cells from different sources indicate that radiation induces expression of p53 protein and its transcriptional targets, such as the cyclin-dependent kinase inhibitor p21. However, the mechanism through which p53 influences radiation response in endothelial cells is controversial. Some studies indicate that blocking p53-mediated signaling improves survival of endothelial cells in vitro by suppressing apoptosis or senescence (74, 76), while others using endothelial cells isolated from p53−/− mice to show that deletion of p53 sensitizes endothelial cells to radiation in vitro (77).
Burdelya et al. evaluated the effect of blocking p53 in tumor stroma, which contains endothelial cells, on tumor response to radiation in vivo. They used mouse tumorigenic packaging cells that produce a retrovirus encoding a dominant-negative mutant p53 to generate xenograft tumors with p53-deficient stroma (77). Tumors with p53-deficient stroma showed markedly prolonged growth delay compared to tumors with p53-wild type stroma. A similar level of growth delay was also observed in tumors that were treated with a p53 inhibitor, PFTα, in combination with radiation. In addition, blocking p53 in tumor stroma resulted in a significant decrease in vessel density in tumors, suggesting that inhibition of p53 sensitizes tumor-associated endothelial cells to radiation in vivo.
To specifically investigate the effect of blocking p53 in endothelial cells on radiation-induced heart disease, we used the Cre-loxP system to delete p53 in endothelial cells using Tie2Cre and VE-Cadherin-Cre mice (78). We observed that after whole-heart irradiation, mice in which both alleles of p53 are deleted in endothelial cells (i.e. Tie2Cre; p53FL/− or VECre; p53FL/− mice) were sensitized to radiation-induced myocardial injury compared to mice that retained one allele of p53 in endothelial cells (i.e. Tie2Cre; p53FL/+ or VECre; p53FL/+ mice). After whole-heart irradiation, both Tie2Cre; p53FL/− and VECre; p53FL/− mice showed a focal decrease in microvessel density in the myocardium, which leads to cardiac ischemia and myocardial necrosis. The progression of myocardial necrosis resulted in systolic dysfunction and heart failure. In addition, in vitro studies using primary endothelial cells showed that after irradiation a higher percentage of p53-deficient endothelial cells displayed early entry into mitosis and contained micronuclei with positive γ-H2AX foci, which result from improper segregation of genomic DNA after radiation. Together, these results demonstrate that p53 protects cardiac endothelial cells from radiation in vivo by preventing the formation of aberrant mitosis or mitotic catastrophe.
Because radiation induces p21 expression in cardiac endothelial cells in a p53-depedent manner, we also studied radiation-induced heart disease in p21−/− mice. Remarkably, after whole heart irradiation p21−/− mice phenocopy the sensitivity of Tie2Cre; p53FL/− and VECre; p53FL/− mice to radiation-induced myocardial injury. Similar to Tie2Cre; p53FL/− and VECre; p53FL/− mice, p21−/− mice developed a reduction in microvessel density, increased vascular permeability and myocardial hypoxia prior to the onset of cardiac dysfunction. These data demonstrate a crucial role of the p53/p21 axis in protecting cardiac endothelial cells from radiation (Figure 1C).
Summary
Results from studies in mice indicate that blocking p53 in vivo through either pharmacological inhibition or genetic deletion dramatically increases radiosensitivity of endothelial cells in tumors and in the heart. These findings suggest that p53 may generally play a pro-survival role in endothelial cell in vivo. Thus, genetically engineered mice with endothelial cell-specific deletion of p53 may be useful tools to mechanistically study the impact of vascular injury on acute and late effects of radiation. Given the diversity of gene expression profiles in human endothelial cells isolated from different tissues (79), further studies are warranted to dissect how p53 functions in endothelial cells to regulate the radiation response of different organs.
Conclusion and Perspectives
Radiation activates p53-mediated signaling in a variety of cells; however, the consequence of p53 activation is cell-type dependent. Using genetically engineered mouse models to manipulate the expression of p53 in specific cell types in vivo, several groups have begun to mechanistically dissect the role of p53 in regulating radiation response of different organs in a cell-type specific manner. The findings summarized in this review demonstrate how the response of p53 to radiation can vary across different organs or even within the same cell lineage (Figure 1). The complexity by which p53 regulates cellular and tissue response to radiation underscores the importance of understanding the mechanisms through which individual cell types respond to radiation. These findings may be critical for developing better strategies to ameliorate normal tissue injury from radiation therapy or radiation disasters.
Acknowledgments
We thank David Van Mater and Katherine Castle for critically reviewing the manuscript. This work was supported by NIAID grant R01 AI080488 (D.G.K).
Footnotes
CONFLICT OF INTEREST
None
DISCLOSURE
The authors do not have any conflict of interest to disclose.
References
- 1.Gudkov AV, Komarova EA. The role of p53 in determining sensitivity to radiotherapy. Nature reviews Cancer. 2003;3:117–129. doi: 10.1038/nrc992. [DOI] [PubMed] [Google Scholar]
- 2.Gudkov AV, Komarova EA. Pathologies associated with the p53 response. Cold Spring Harbor perspectives in biology. 2010;2:a001180. doi: 10.1101/cshperspect.a001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindsay KJ, Coates PJ, Lorimore SA, et al. The genetic basis of tissue responses to ionizing radiation. Br J Radiol. 2007;80(Spec No 1):S2–6. doi: 10.1259/bjr/60507340. [DOI] [PubMed] [Google Scholar]
- 4.Brady CA, Attardi LD. p53 at a glance. Journal of cell science. 2010;123:2527–2532. doi: 10.1242/jcs.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takagi M, Absalon MJ, McLure KG, et al. Regulation of p53 translation and induction after DNA damage by ribosomal protein L26 and nucleolin. Cell. 2005;123:49–63. doi: 10.1016/j.cell.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 6.Brooks CL, Gu W. p53 ubiquitination: Mdm2 and beyond. Mol Cell. 2006;21:307–315. doi: 10.1016/j.molcel.2006.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlereth K, Charles JP, Bretz AC, et al. Life or death: p53-induced apoptosis requires DNA binding cooperativity. Cell Cycle. 2010;9:4068–4076. doi: 10.4161/cc.9.20.13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murray-Zmijewski F, Slee EA, Lu X. A complex barcode underlies the heterogeneous response of p53 to stress. Nature reviews Molecular cell biology. 2008;9:702–712. doi: 10.1038/nrm2451. [DOI] [PubMed] [Google Scholar]
- 9.Purvis JE, Karhohs KW, Mock C, et al. p53 dynamics control cell fate. Science. 2012;336:1440–1444. doi: 10.1126/science.1218351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fei P, Bernhard EJ, El-Deiry WS. Tissue-specific induction of p53 targets in vivo. Cancer Res. 2002;62:7316–7327. [PubMed] [Google Scholar]
- 11.MacCallum DE, Hupp TR, Midgley CA, et al. The p53 response to ionising radiation in adult and developing murine tissues. Oncogene. 1996;13:2575–2587. [PubMed] [Google Scholar]
- 12.Mohrin M, Bourke E, Alexander D, et al. Hematopoietic stem cell quiescence promotes error-prone DNA repair and mutagenesis. Cell Stem Cell. 2010;7:174–185. doi: 10.1016/j.stem.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Insinga A, Cicalese A, Faretta M, et al. DNA damage in stem cells activates p21, inhibits p53, and induces symmetric self-renewing divisions. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:3931–3936. doi: 10.1073/pnas.1213394110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirsch DG. Using genetically engineered mice for radiation research. Radiation research. 2011;176:275–279. doi: 10.1667/rrxx35.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dainiak N. Hematologic consequences of exposure to ionizing radiation. Exp Hematol. 2002;30:513–528. doi: 10.1016/s0301-472x(02)00802-0. [DOI] [PubMed] [Google Scholar]
- 16.Mauch P, Constine L, Greenberger J, et al. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. International journal of radiation oncology, biology, physics. 1995;31:1319–1339. doi: 10.1016/0360-3016(94)00430-S. [DOI] [PubMed] [Google Scholar]
- 17.Williams JP, Brown SL, Georges GE, et al. Animal models for medical countermeasures to radiation exposure. Radiat Res. 2010;173:557–578. doi: 10.1667/RR1880.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Schulte BA, Zhou D. Hematopoietic stem cell senescence and long-term bone marrow injury. Cell cycle. 2006;5:35–38. doi: 10.4161/cc.5.1.2280. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Schulte BA, LaRue AC, et al. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood. 2006;107:358–366. doi: 10.1182/blood-2005-04-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meng A, Wang Y, Van Zant G, et al. Ionizing radiation and busulfan induce premature senescence in murine bone marrow hematopoietic cells. Cancer research. 2003;63:5414–5419. [PubMed] [Google Scholar]
- 21.Chua HL, Plett PA, Sampson CH, et al. Long-term hematopoietic stem cell damage in a murine model of the hematopoietic syndrome of the acute radiation syndrome. Health physics. 2012;103:356–366. doi: 10.1097/HP.0b013e3182666d6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marusyk A, Casas-Selves M, Henry CJ, et al. Irradiation alters selection for oncogenic mutations in hematopoietic progenitors. Cancer research. 2009;69:7262–7269. doi: 10.1158/0008-5472.CAN-09-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pant V, Quintas-Cardama A, Lozano G. The p53 pathway in hematopoiesis: lessons from mouse models, implications for humans. Blood. 2012;120:5118–5127. doi: 10.1182/blood-2012-05-356014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lotem J, Sachs L. Hematopoietic cells from mice deficient in wild-type p53 are more resistant to induction of apoptosis by some agents. Blood. 1993;82:1092–1096. [PubMed] [Google Scholar]
- 25.Oren M. Relationship of p53 to the control of apoptotic cell death. Semin Cancer Biol. 1994;5:221–227. [PubMed] [Google Scholar]
- 26.Komarova EA, Kondratov RV, Wang K, et al. Dual effect of p53 on radiation sensitivity in vivo: p53 promotes hematopoietic injury, but protects from gastrointestinal syndrome in mice. Oncogene. 2004;23:3265–3271. doi: 10.1038/sj.onc.1207494. [DOI] [PubMed] [Google Scholar]
- 27.Villunger A, Michalak EM, Coultas L, et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 28.Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 29.Yu H, Shen H, Yuan Y, et al. Deletion of Puma protects hematopoietic stem cells and confers long-term survival in response to high-dose gamma-irradiation. Blood. 2010;115:3472–3480. doi: 10.1182/blood-2009-10-248278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shao L, Sun Y, Zhang Z, et al. Deletion of proapoptotic Puma selectively protects hematopoietic stem and progenitor cells against high-dose radiation. Blood. 2010;115:4707–4714. doi: 10.1182/blood-2009-10-248872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harvey M, McArthur MJ, Montgomery CA, Jr, et al. Spontaneous and carcinogen-induced tumorigenesis in p53-deficient mice. Nature genetics. 1993;5:225–229. doi: 10.1038/ng1193-225. [DOI] [PubMed] [Google Scholar]
- 32.Jacks T, Remington L, Williams BO, et al. Tumor spectrum analysis in p53-mutant mice. Current biology: CB. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 33.Marusyk A, Porter CC, Zaberezhnyy V, et al. Irradiation selects for p53-deficient hematopoietic progenitors. PLoS biology. 2010;8:e1000324. doi: 10.1371/journal.pbio.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bondar T, Medzhitov R. p53-mediated hematopoietic stem and progenitor cell competition. Cell Stem Cell. 2010;6:309–322. doi: 10.1016/j.stem.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shao L, Li H, Pazhanisamy SK, et al. Reactive oxygen species and hematopoietic stem cell senescence. Int J Hematol. 2011;94:24–32. doi: 10.1007/s12185-011-0872-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Liu L, Pazhanisamy SK, et al. Total body irradiation causes residual bone marrow injury by induction of persistent oxidative stress in murine hematopoietic stem cells. Free Radic Biol Med. 2010;48:348–356. doi: 10.1016/j.freeradbiomed.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serrano M, Lee H, Chin L, et al. Role of the INK4a locus in tumor suppression and cell mortality. Cell. 1996;85:27–37. doi: 10.1016/s0092-8674(00)81079-x. [DOI] [PubMed] [Google Scholar]
- 38.Brown JP, Wei W, Sedivy JM. Bypass of senescence after disruption of p21CIP1/WAF1 gene in normal diploid human fibroblasts. Science. 1997;277:831–834. doi: 10.1126/science.277.5327.831. [DOI] [PubMed] [Google Scholar]
- 39.van Os R, Kamminga LM, Ausema A, et al. A Limited role for p21Cip1/Waf1 in maintaining normal hematopoietic stem cell functioning. Stem Cells. 2007;25:836–843. doi: 10.1634/stemcells.2006-0631. [DOI] [PubMed] [Google Scholar]
- 40.Kemp CJ, Wheldon T, Balmain A. p53-deficient mice are extremely susceptible to radiation-induced tumorigenesis. Nature genetics. 1994;8:66–69. doi: 10.1038/ng0994-66. [DOI] [PubMed] [Google Scholar]
- 41.Shadad AK, Sullivan FJ, Martin JD, Egan LJ, et al. Gastrointestinal radiation injury: symptoms, risk factors and mechanisms. World J Gastroenterol. 2013 doi: 10.3748/wjg.v19.i2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore JV. The ‘gastrointestinal syndrome’ after chemotherapy: inferences from mouse survival time, and from histologically- and clonogenically-defined cell death in intestinal crypts. The British journal of cancer Supplement. 1986;7:16–19. [PMC free article] [PubMed] [Google Scholar]
- 43.Somosy Z, Horvath G, Telbisz A, et al. Morphological aspects of ionizing radiation response of small intestine. Micron. 2002;33:167–178. doi: 10.1016/s0968-4328(01)00013-0. [DOI] [PubMed] [Google Scholar]
- 44.Potten CS. Extreme sensitivity of some intestinal crypt cells to X and gamma irradiation. Nature. 1977;269:518–521. doi: 10.1038/269518a0. [DOI] [PubMed] [Google Scholar]
- 45.Booth C, Potten CS. Gut instincts: thoughts on intestinal epithelial stem cells. The Journal of clinical investigation. 2000;105:1493–1499. doi: 10.1172/JCI10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Merritt AJ, Potten CS, Kemp CJ, et al. The role of p53 in spontaneous and radiation-induced apoptosis in the gastrointestinal tract of normal and p53-deficient mice. Cancer Res. 1994;54:614–617. [PubMed] [Google Scholar]
- 47.Clarke AR, Gledhill S, Hooper ML, et al. p53 dependence of early apoptotic and proliferative responses within the mouse intestinal epithelium following gamma-irradiation. Oncogene. 1994;9:1767–1773. [PubMed] [Google Scholar]
- 48.Merritt AJ, Allen TD, Potten CS, et al. Apoptosis in small intestinal epithelial from p53-null mice: evidence for a delayed, p53-independent G2/M-associated cell death after gamma-irradiation. Oncogene. 1997;14:2759–2766. doi: 10.1038/sj.onc.1201126. [DOI] [PubMed] [Google Scholar]
- 49.Vakifahmetoglu H, Olsson M, Zhivotovsky B. Death through a tragedy: mitotic catastrophe. Cell Death Differ. 2008;15:1153–1162. doi: 10.1038/cdd.2008.47. [DOI] [PubMed] [Google Scholar]
- 50.Vitale I, Galluzzi L, Castedo M, et al. Mitotic catastrophe: a mechanism for avoiding genomic instability. Nature reviews Molecular cell biology. 2011;12:385–392. doi: 10.1038/nrm3115. [DOI] [PubMed] [Google Scholar]
- 51.Kirsch DG, Santiago PM, di Tomaso E, et al. p53 controls radiation-induced gastrointestinal syndrome in mice independent of apoptosis. Science. 2010;327:593–596. doi: 10.1126/science.1166202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Antignani A, Youle RJ. How do Bax and Bak lead to permeabilization of the outer mitochondrial membrane? Curr Opin Cell Biol. 2006;18:685–689. doi: 10.1016/j.ceb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 53.Dewson G, Kluck RM. Mechanisms by which Bak and Bax permeabilise mitochondria during apoptosis. J Cell Sci. 2009;122:2801–2808. doi: 10.1242/jcs.038166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qiu W, Carson-Walter EB, Liu H, et al. PUMA regulates intestinal progenitor cell radiosensitivity and gastrointestinal syndrome. Cell stem cell. 2008;2:576–583. doi: 10.1016/j.stem.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clarke AR. Puma: mauling the intestinal crypt. Cell stem cell. 2008;2:517–518. doi: 10.1016/j.stem.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 56.Leibowitz BJ, Qiu W, Liu H, et al. Uncoupling p53 functions in radiation-induced intestinal damage via PUMA and p21. Molecular cancer research: MCR. 2011;9:616–625. doi: 10.1158/1541-7786.MCR-11-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bunz F, Dutriaux A, Lengauer C, et al. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 58.Brugarolas J, Chandrasekaran C, Gordon JI, et al. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature. 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- 59.Garcia-Cao I, Garcia-Cao M, Martin-Caballero J, et al. “Super p53” mice exhibit enhanced DNA damage response, are tumor resistant and age normally. The EMBO journal. 2002;21:6225–6235. doi: 10.1093/emboj/cdf595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sullivan JM, Jeffords LB, Lee CL, et al. p21 protects “Super p53” mice from the radiation-induced gastrointestinal syndrome. Radiation research. 2012;177:307–310. doi: 10.1667/rr2545.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barker N, van Oudenaarden A, Clevers H. Identifying the Stem Cell of the Intestinal Crypt: Strategies and Pitfalls. Cell Stem Cell. 2012;11:452–460. doi: 10.1016/j.stem.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 62.Hendry JH, Potten CS. Intestinal cell radiosensitivity: a comparison for cell death assayed by apoptosis or by a loss of clonogenicity. International journal of radiation biology and related studies in physics, chemistry, and medicine. 1982;42:621–628. doi: 10.1080/09553008214551601. [DOI] [PubMed] [Google Scholar]
- 63.Hua G, Thin TH, Feldman R, et al. Crypt base columnar stem cells in small intestines of mice are radioresistant. Gastroenterology. 2012;143:1266–1276. doi: 10.1053/j.gastro.2012.07.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 65.Yan KS, Chia LA, Li X, et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:466–471. doi: 10.1073/pnas.1118857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang J, Boerma M, Fu Q, Hauer-Jensen M, et al. Significance of endothelial dysfunction in the pathogenesis of early and delayed radiation enteropathy. World J Gastroenterol. 2007 doi: 10.3748/wjg.v13.i22.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stewart FA, Hoving S, Russell NS. Vascular damage as an underlying mechanism of cardiac and cerebral toxicity in irradiated cancer patients. Radiat Res. 2010;174:865–869. doi: 10.1667/RR1862.1. [DOI] [PubMed] [Google Scholar]
- 68.Fajardo LF, Stewart JR. Pathogenesis of radiation-induced myocardial fibrosis. Lab Invest. 1973;29:244–257. [PubMed] [Google Scholar]
- 69.Fajardo LF, Stewart JR. Experimental radiation-induced heart disease. I. Light microscopic studies. Am J Pathol. 1970;59:299–316. [PMC free article] [PubMed] [Google Scholar]
- 70.Lauk S, Kiszel Z, Buschmann J, et al. Radiation-induced heart disease in rats. Int J Radiat Oncol Biol Phys. 1985;11:801–808. doi: 10.1016/0360-3016(85)90314-1. [DOI] [PubMed] [Google Scholar]
- 71.Yeung TK, Lauk S, Simmonds RH, et al. Morphological and functional changes in the rat heart after X irradiation: strain differences. Radiat Res. 1989;119:489–499. [PubMed] [Google Scholar]
- 72.Seemann I, Gabriels K, Visser NL, et al. Irradiation induced modest changes in murine cardiac function despite progressive structural damage to the myocardium and microvasculature. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2012;103:143–150. doi: 10.1016/j.radonc.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 73.Paris F, Fuks Z, Kang A, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293–297. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 74.Lee MO, Song SH, Jung S, et al. Effect of Ionizing Radiation Induced Damage of Endothelial Progenitor Cells in Vascular Regeneration. Arteriosclerosis, thrombosis, and vascular biology. 2011 doi: 10.1161/ATVBAHA.111.237651. [DOI] [PubMed] [Google Scholar]
- 75.Mendonca MS, Chin-Sinex H, Dhaemers R, et al. Differential mechanisms of x-ray-induced cell death in human endothelial progenitor cells isolated from cord blood and adults. Radiat Res. 2011;176:208–216. doi: 10.1667/rr2427.1. [DOI] [PubMed] [Google Scholar]
- 76.Nubel T, Damrot J, Roos WP, et al. Lovastatin protects human endothelial cells from killing by ionizing radiation without impairing induction and repair of DNA double-strand breaks. Clin Cancer Res. 2006;12:933–939. doi: 10.1158/1078-0432.CCR-05-1903. [DOI] [PubMed] [Google Scholar]
- 77.Burdelya LG, Komarova EA, Hill JE, et al. Inhibition of p53 response in tumor stroma improves efficacy of anticancer treatment by increasing antiangiogenic effects of chemotherapy and radiotherapy in mice. Cancer Res. 2006;66:9356–9361. doi: 10.1158/0008-5472.CAN-06-1223. [DOI] [PubMed] [Google Scholar]
- 78.Lee CL, Moding EJ, Cuneo KC, et al. p53 functions in endothelial cells to prevent radiation-induced myocardial injury in mice. Science signaling. 2012;5:ra52. doi: 10.1126/scisignal.2002918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chi JT, Chang HY, Haraldsen G, et al. Endothelial cell diversity revealed by global expression profiling. Proc Natl Acad Sci U S A. 2003;100:10623–10628. doi: 10.1073/pnas.1434429100. [DOI] [PMC free article] [PubMed] [Google Scholar]