Abstract
Autobiographical memory (AM) defines the memory systems that encode, consolidate, and retrieve personal events and facts, AM is strongly related to self-perception and self representation. We review here the neural correlates of AM retrieval. AM retrieval encompasses a large neural network including the prefrontal, temporal, and parietal cortex, and limbic structures. All these regions subserve the cognitive processes (episodic remembering, cognitive control, self-processing, and scene construction) at play during memory retrieval. We emphasize the specific role of medial prefrontal cortex and precuneus in self-processing during autobiographical memory retrieval. Overall, these data call for further studies in psychiatric patients, to investigate the neural underpinnings of autobiographical memory and self-representation in mental disorders.
Keywords: autobiographical memory, medial prefrontal cortex, precuneus, retrieval, self
Abstract
La memoria autobiográfica (MA) se refiere a los sistemas de memoria que codifican, consolidan y recuperan los acontecimientos y hechos personales. La MA está muy relacionada con la auto-percepción y la auto-representación. En este artículo se revisan los correlatos neurales de la recuperación de la MA. La recuperación de la MA comprende una gran red neural que incluye cortezas prefrontales, temporales y parietales, y estructuras límbícas. Todas estas regiones colaboran con los procesos cognitivos (recuerdo episódico, control cognítivo, auto-procesamíento y construcción de escena) que están en juego durante la recuperación de la memoria. Se hace hincapié en el papel específico de la corteza prefrontal medial y del precúneo en el autoprocesamíento durante la recuperación de la memoria autobiográfica. En general estos datos requieren de más estudios en pacientes psiquiátricos para investigar los fundamentos neurales de la memoria autobiográfica y la auto-representación en los trastornos mentales.
Abstract
La mémoire autobiographique définit les systèmes mnésiques qui codent, consolident et récupèrent les informations et les événements personnels. Elle est fortement liée à la perception de soi et la représentation de soi. Nous analysons dans cet article les correspondances neuronales de la récupération de la mémoire autobiographique qui englobe un vaste réseau neuronal dont les cortex préfrontal, temporal et pariétal et les structures limbiques. Toutes ces régions favorisent le processus cognitif (remémoration épisodique, contrôle cognitif, référence à soi, et construction de scène) en jeu lors de la récupération de la mémoire. Nous soulignons le rôle spécifique du cortex préfrontal médian et du precuneus dans les phénomènes de référence à soi lors de la récupération de la mémoire autobiographique. Dans l'ensemble, ces données appellent d'autres études chez les patients psychiatriques pour rechercher les bases neuronales de la mémoire autobiographique et de l'auto-représentation dans les troubles mentaux.
Introduction
Autobiographical memory (AM) defines the memory systems that encode, consolidate, and retrieve personal events and facts. Behavioral and brain imaging studies of AM have become increasingly popular as an example of ecological experimental paradigms. Indeed, AM allows the investigation of real-life memories, which are different from laboratory memories, using stimuli such as lists of words.
In this paper we will briefly review the brain regions associated with AM retrieval, with a special emphasis on the medial prefrontal cortex and precuneus, their role in self-processing, and their relationships to autobiographical memory processes.
Neural correlates of autobiographical memory retrieval
Several brain imaging studies using PET or functional magnetic resonance imaging (MRI) have investigated the neural correlates of AM retrieval. Usually in these studies participants are required to retrieve and/or re-experience events from their own history in response to personally or experimentally generated retrieval cues. According to results of meta-analyses and reviews,1-3 AMR retrieval entails the activation of an extensive brain network encompassing cortical midline structures (ventral and dorsal medial prefrontal cortex, and posterior cingulate), ventral and dorsal lateral prefrontal cortex, medial (ie, hippocampus) and lateral temporal lobes, temporoparietal junction, and cerebellum. Components of this network reflect the different cognitive processes engaged during AM retrieval, such as executive control and retrieval monitoring (ie, dorsal lateral prefrontal cortex), emotion-related processes (ie, ventral medial prefrontal cortex and amygdala), episodic remembering (ie, hippocampus), self-processing (ie, dorsal medial and ventral medial prefrontal cortex, posterior cingulate), and visuospatial processing (ie, retrosplenial cortex, precuneus, and parietal regions).
Martinelli et al4 conducted a new meta-analysis of positron emission tomography (PET) and functional MRI studies to disentangle brain regions associated respectively with episodic AM and semantic AM. Episodic AM relates to the recall of personally relevant events acquired in a specific spatiotemporal context and characterized by an autonoetic state of consciousness.5 This latter component enables conscious recollection of a personal event in its original encoding context and implies mental time travel involving a vivid experience of remembering. Semantic AM relates to the recall of personal general events (ie, repeated events and/or events extended in time) and personal information (ie, birth date). The authors describe a rostrocaudal gradient in brain activation with more posterior regions involved in episodic AM and recollection of sensory-perceptual details and semantic AM retrieval associated with left anterior frontotemporal regions reflecting strategic and semantic retrieval processes. More importantly, although results from this meta-analysis suggested that semantic AM and episodic AM are independent, both memory systems activated common regions, especially the medial prefrontal cortex and cortical midline structures.
Autobiographical memory and self
As emphasized by Conway,6 AM is strongly related to the self. AM grounds the self, and self-related processes influences both the content and the organization of AM. It is now well admitted that the medial prefrontal cortex and the cortical midline structures, including the posterior cingulate and medial part of the parietal, are involved in self-processing and self-representation.7-9 In their meta-analysis, Martinelli et al4 found activation of the ventral and dorsal medial prefrontal cortex during the engagement of self-referential processes in AM retrieval and association of stimuli to one's own person. The distinct role of the ventral and dorsal medial prefrontal cortex are still a matter of debate in the literature on the self. However, it has been suggested that the ventral medial prefrontal cortex may encode the personal relevance and significance of external and internal stimuli10 whereas the dorsal medial prefrontal cortex is more involved in self-reflection, evaluation, and mentalizing. Likewise, Moscovitch et al11 suggested that the activity of the ventral medial prefrontal cortex indicates a specific form of monitoring that contributes to the “feeling of rightness” during AM retrieval. It is noteworthy that lesions of this region may facilitate the production of false memories and confabulation.12
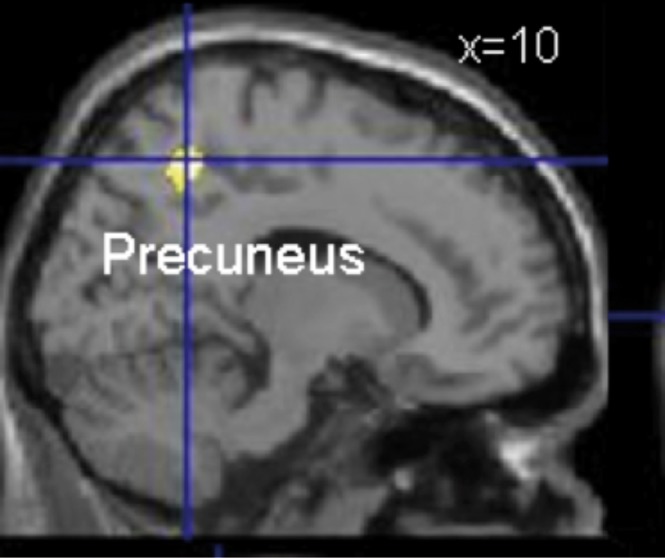
In a recent study by our group, we assessed the neural correlates of visual perspective (ie, first-person versus third-person perspective) adopted during AM retrieval. Autobiographical memories can be retrieved from either the first-person perspective, in which individuals see the event through their own eyes, or from the third-person perspective, in which individuals see themselves and the event from the perspective of an external observer.13 Visual perspective during AM retrieval plays a role in both emotional regulation and self-related processes, and may serve as a device to appraise whether retrieved memories are congruent or incongruent with the current remembering self. We found that the tendency to recall memories from a first-person perspective was positively correlated with the volume of the anterior part of the right precuneus (Figure 1). The precuneus is the medial part of the parietal cortex that plays a critical role in egocentric vs allocentric spatial processing and in updating spatial representations. The precuneus is also a component of the default mode network that supports the ability of subjects to project themselves into worlds that differ mentally, temporally, or physically from their current experience. Our results provide the first evidence on the role of precuneus in integration of both visuospatial information and self in the context of navigation in the personal space.14
Figure 1. Region (precuneus) showing a positive correlation with the first perspective score during autobiographical memory retrieval and gray matter volume.
Conclusion
We reviewed brain imaging studies evaluating brain regions activated by AM retrieval. The AM retrieval networks involve the medial and lateral part of the temporal, frontal, and parietal cortex as well as limbic structures. Among these regions, the medial prefrontal cortex and the precuneus are key players in self-processing during autobiographical memory retrieval. Overall, these data emphasize the need to study AM impairment and its neural underpinnings in mental disorders characterized by abnormal self-representation and impaired self-regulation of emotion.
REFERENCES
- 1.Cabeza R., St Jacques P. Functional neuroimaging of autobiographical memory. Trends Cogn Sci. 2007;11:219–227. doi: 10.1016/j.tics.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Svoboda E., McKinnon MC., Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychology. 2006;44:2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maguire EA. Neuroimaging studies of autobiographical event memory. Philos Trans R Soc Lond B Biol Sci. 2001;356:1441–1451. doi: 10.1098/rstb.2001.0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinelli P., Sperduti M., Piolino P. Neural substrates of the self-memory system: new insights from a meta-analysis. Hum Brain Mapp. 2013;34:1515–1529. doi: 10.1002/hbm.22008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tulving E. Multiple memory systems and consciousness. Hum Neurobiol. 1987;6:67–80. [PubMed] [Google Scholar]
- 6.Conway MA., Pleydell-Pearce CW. The construction of autobiographical memories in the self-memory system. Psychol Rev. 2000;107:261–288. doi: 10.1037/0033-295x.107.2.261. [DOI] [PubMed] [Google Scholar]
- 7.Fossati P., Hevenor SJ., Lepage M., et al Distributed self in episodic memory: neural correlates of successful retrieval of self-encoded positive and negative personality traits. Neuroimage. 2004;22:1596–1604. doi: 10.1016/j.neuroimage.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 8.Fossati P., Hevenor S., Graham S., et al In search of the emotional self. An fMRI study with positive and negative emotional words. Am J Psychiatry. 2003;160:1938–1945. doi: 10.1176/appi.ajp.160.11.1938. [DOI] [PubMed] [Google Scholar]
- 9.Northoff G., Bermpohl F. Cortical midline structures and the self. Trends Cogn Sci. 2004;8:102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 10.D'Argembeau A. On the role of the ventromedial prefrontal cortex in self-processing: the valuation hypothesis. Front Hum Neurosci. 2013;7:372. doi: 10.3389/fnhum.2013.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moscovitch M., Winocur G. The frontal cortex and working with memory. In: Stuss DTE, Knight RTE, eds. Principles of Frontal Lobe Function. New York, NY: Oxford University Press; 2002:188–209. [Google Scholar]
- 12.Turner MS., Cipolotti L., Yousry TA., Shallice T. Confabulation: damage to a specific inferior medial prefrontal system. Cortex. 2008;44:637–648. doi: 10.1016/j.cortex.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Sutin AR., Robins RW. When the “I” looks at the “Me”: autobiographical memory, visual perspective, and the self. Conscious Cogn. 2008;17:1386–1397. doi: 10.1016/j.concog.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freton M., Lemogne C., Bergouignan L., Delaveau P., Lehéricy S., Fossati P. The eye of the self: precuneus volume and visual perspective during autobiographical memory retrieval. Brain Struct Funct. [Epub ahead of print] 2013 doi: 10.1007/s00429-013-0546-2. [DOI] [PubMed] [Google Scholar]


