Abstract
Melanoma is an aggressive cancer that metastasizes rapidly, and is refractory to conventional chemotherapies. Identifying miRNAs that are responsible for this pathogenesis is therefore a promising means of developing new therapies. We identified miR-26a through microarray and qRT-PCR experiments as an miRNA that is strongly down-regulated in melanoma cell lines as compared to primary melanocytes. Treatment of cell lines with miR-26a mimic caused significant and rapid cell death compared to a negative control in most melanoma cell lines tested. In surveying targets of miR-26a, we found that protein levels of SMAD1 and BAG-4/SODD were strongly decreased in sensitive cells treated with miR-26a mimic compared to the control. The luciferase reporter assays further demonstrated that miR-26a can repress gene expression through the binding site in the 3′UTR of SODD. Knockdown of these proteins with siRNA showed that SODD plays an important role in protecting melanoma cells from apoptosis in most cell lines sensitive to miR-26a, while SMAD1 may play a minor role. Furthermore, transfecting cells with a miR-26a inhibitor increased SODD expression. Our findings indicate that miR-26a replacement is a potential therapeutic strategy for metastatic melanoma, and that SODD in particular is a potentially useful therapeutic target.
INTRODUCTION
Metastatic melanoma is a devastating disease that is notoriously resistant to traditional chemotherapies. Recent advances in the use of BRAF inhibitors have marked the first serious progress in treating malignant melanoma in nearly 30 years (Buzaid, 2004; Cummins et al., 2006; Gogas et al., 2007). Yet in spite of the success of these new drugs, they are only appropriate in the roughly one-half of patients with melanomas harboring BRAF V600E mutations, and relapse and resistance are inevitable (Ades and Metzger-Filho, 2012; Flaherty, 2010). Thus there is a still a pressing need for therapies using novel approaches.
Dysregulation of important cell proliferation pathways and tumor suppressors is a necessary step in oncogenesis. Recently, microRNAs (miRNA), short RNAs that target expressed mRNA for degradation, have gained much attention for the role they play in affecting protein expression in cancer tissues (Esteller, 2011; Kasinski and Slack, 2011; Sandhu and Garzon, 2011). Release 18 of miRBase (www.mirbase.org) has nearly 1900 unique mature miRNAs annotated for the human genome (Kozomara and Griffiths-Jones, 2011), and 60% of all genes may be regulated by miRNAs (Friedman et al., 2009). miRNAs thus represent an important regulatory layer that can affect gene expression above and beyond other cellular mechanisms. The small size and natural occurrence of miRNAs have made them attractive for use in therapy. New therapies are currently being developed based primarily on miRNA replacement, since miRNAs are most commonly down-regulated in cancers and are thought to act as tumor suppressors (Kumar et al., 2007). Additionally, miRNA studies can be useful in identifying potential therapeutic targets that may act upstream or downstream of miRNAs.
In the present study, we identified miR-26a in a microarray screen and subsequent qRT-PCR validation as being strongly down-regulated in melanoma cells compared to primary melanocytes. In surveying possible targets of miR-26a, we found two proteins, Mothers Against Decapentaplegic homolog 1 (SMAD1) and BAG family molecular chaperone regulator 4 (BAG4, also known as Silencer of Death Domains (SODD), hereafter referred to only as SODD) that were strongly down-regulated upon miR-26a treatment. SODD, and to a lesser extent SMAD1, appear to be necessary to prevent apoptosis in multiple melanoma lines. We discuss the potential for melanoma therapy through miR-26a replacement or the direct targeting of these proteins.
RESULTS
Microarray analysis and qRT-PCR identified miR-26a as strongly downregulated in melanomas compared to melanocytes
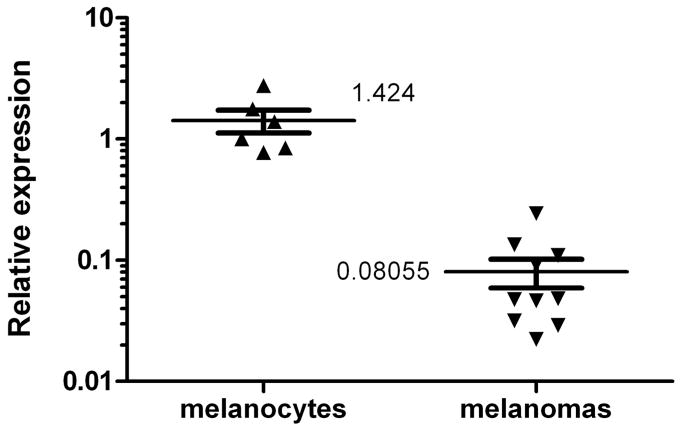
We performed microarray analysis to identify miRNAs that are significantly changed in human melanoma cell lines compared to melanocytes. We found a number of miRNAs that were either down or upregulated (Table 1). We then performed qRT-PCR of samples from numerous melanoma cell lines and two different primary melanocyte cultures at different passages under different culture conditions (Fig.1 and data not shown). Results indicated that miR-26a was strongly downregulated in all of the melanoma cell lines tested compared to the melanocytes. While levels of miR-26a varied considerably among both melanomas and melanocytes, levels were strikingly lower in melanomas, from 4- to as much as 50-fold (average of 17.7-fold) lower than in melanocytes (Fig. 1). These results clearly demonstrate that miR-26a is strongly down-regulated in melanoma cells, validating our microarray results. Since miR-26a showed the strongest and most consistent difference among the miRNAs that we tested (Fig. 1 and data not shown), in the present study, we focused on miR-26a.
Table 1.
MiRNAs identified as down- or up-regulated in melanomas compared to normal melanocytes by Rank Product Statistical Analysis.
| Down-regulated in melanoma | Fold expression (melanoma/melanocyte) |
|---|---|
|
| |
| hsa|let-7e | 0.5 |
| hsa|miR-1234 | 0.2 |
| hsa|miR-125a-5p | 0.3 |
| hsa|miR-130a | 0.5 |
| hsa|miR-20b | 0.7 |
| hsa|miR-26a | 0.6 |
| hsa|miR-30a | 0.6 |
| hsa|miR-361-5p | 0.6 |
| hsa|miR-363 | 0.1 |
| hsa|miR-494 | 0.4 |
| hsa|miR-508-3p | 0.5 |
| hsa|miR-509-3-5p | 0.3 |
| hsa|miR-513a-5p | 0.2 |
| hsa|miR-923 | 0.5 |
| Up-regulated in melanoma | Fold expression (melanoma/melanocyte) |
|
| |
| hsa|miR-100 | 2.0 |
| hsa|miR-222 | 2.7 |
| hsa|miR-23b | 2.1 |
| hsa|miR-27b | 2.4 |
| hsa|miR-31 | 2.5 |
| hsa|miR-99a | 2.4 |
Figure 1.
Scatter plot of qRT-PCR results for miR-26a expression showing melanocytes vs. melanoma cell lines. Melanocytes include two different primary melanocyte lines (HEMNLP and HEMNLP2) at different passages and with different culture conditions (with or without FBS). Melanomas include 10 different established cell lines under standard culture conditions (see Materials and Methods for a list of cell lines). All results were normalized to RNU1A as an internal standard, and denote relative expression compared to one of the melanocyte lines set at 1.0. Melanoma lines had an average of 17.7-fold less miR-26a compared to melanocytes, p < 0.0001.
Transfection with miR-26a mimic decreases viability and increases apoptosis in multiple melanoma cell lines
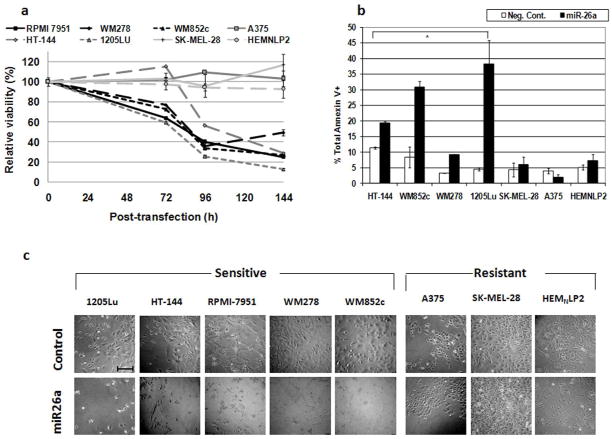
To test whether low levels of miR-26a facilitate melanoma cell viability, we transfected multiple melanoma cell lines with miR-26a mimic. Viability assays as shown in Fig. 2a demonstrate massive reduction in viability starting at 72 h and accelerating by 96 h after transfection with miR-26a mimic compared to transfection with negative control siRNA in most cell lines tested, but not in normal primary melanocytes HEMNLP2. Statistical analyses indicated that at 72 h, the viabilities of miR-26a-transfected cells from RPMI7951, WM278, WM852c, 1205Lu were significantly different from those of control-transfected cells (p < 0.05). At later time points, in addition to the same melanoma cell lines above, the viability of miR-26a-transfected HT-144 was also significantly different from the controls. On the other hand, melanoma cell lines A375, SK-MEL-28, and primary melanocytes HEMNLP2 were resistant to miR-26a.
Figure 2.
Treatment with miR-26a mimic induces cell death in several melanoma cell lines. (a) Time-course MTS assays for cells transfected with 50 nM miR-26a mimic. Results for each cell line at each time point represent the viability of cells transfected with miR-26a mimic as a percentage of those transfected with negative control. Error bars represent SEM of at least 3 replicates. See Results section for statistical significance. (b) Annexin V assays of cells transfected with 50 nM miR-26a or negative control for 96 h. * p-values < 0.05 for comparisons between miR-26a mimic and negative control cells. Error bars represent SEM of at least 2 independent experiments. (c) Visual appearance of cells after 96 h of treatment. Scale bar in top left image = 0.5 mm.
Annexin V assays shown in Fig. 2b corroborate the viability assays and demonstrate that apoptosis is a major mechanism of reduced viability in some cell lines. Statistical analyses indicated that miR-26a significantly increased the percentage of total Annexin V+ cells in HT-144, WM852c, WM278 and 1205Lu (Fig. 2b). The appearance of the cells (Fig. 2c) is consistent with the viability and Annexin V assays, showing reduced proliferation and cell death. Cell lines A375 and SK-MEL-28 were resistant to the effects of the miR-26a mimic, as were primary melanocytes HEMNLP2 (Fig. 2c). Furthermore, immunoblots of lysates from cells transfected with miR-26a mimic showed that miR-26a also increased PARP cleavage in miR-26a-sensitive cells compared to those transfected with negative control siRNA (Fig. 3a), further demonstrating that miR-26a induces apoptosis in these cells.
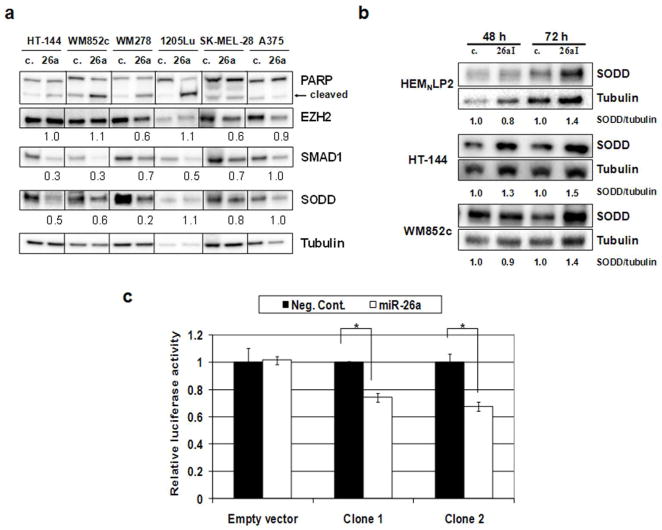
Figure 3.
SODD is a target of miR-26a. (a) Immunoblot. Cells were transfected with miR-26a mimic (26a) or Negative Control (c.) for 72 h. Quantifications are the ratios of the proteins to tubulin with controls set to 1.0. (b) Immunoblot. Cells were transfected with 50 nM miR-26a inhibitor (26aI) or miScript Inhibitor Negative Control (c.). (c) Dual-luciferase reporter assay. Luciferase reporter vectors containing SODD 3′UTR (Clones 1 and 2) or empty vectors were used. Relative luciferase activity was significantly decreased with miR-26a cotransfection (0.74 and 0.68, p = 0.002 and 0.0013, respectively) compared to control mimic for the constructs containing the SODD 3′UTR, but not for the empty vector (1.02, p = 0.82). Error bars represent SD of 3 replicates. *, p-value < 0.05.
To test whether variations in transfection efficiency could account for the differences in sensitivity to miR-26a mimic, we used an identical protocol to transfect multiple melanoma cell lines and melanocytes with Allstars Negative Control siRNA conjugated with a fluorophore. Fluorscence microscopy indicated transfection efficiencies near 100%, even in cells resistant to miR-26a mimic (Supplementary Fig. S1). Additionally, we measured miR-26a in both sensitive and resistant cell lines transfected with miR-26a mimic and negative control by qRT-PCR. miR-26a levels were on the order of 103-fold higher in cells transfected with miR-26a compared to those transfected with negative control at 72 h post-transfection (928- and 3866-fold higher in HT-144 and SK-MEL-28 cells, respectively). We thus concluded that differences in transfection efficiency or miRNA stability were not responsible for differences in sensitivity to miR-26a.
SMAD1 and SODD protein levels are decreased in miR-26a mimic-treated cells, inhibition of miR-26a increases SODD levels, and luciferase reporter assays confirm that SODD is a target of miR-26a
Melanoma cells treated with miR-26a mimic and negative control siRNA were collected after 72 h and lysed for immunoblotting. We tested these lysates for a variety of possible targets to miR-26a, including EZH2, cyclins D2 and E2, metadherin, GSK-3β, SMAD1, and SODD. Each of these proteins, with the exception of SODD, has been previously identified as a target of miR-26a (Kota et al., 2009; Luzi et al., 2008; Mohamed et al., 2010; Sander et al., 2008; Zhang et al., 2011). SODD was identified in the TargetScan database as having a potential 3′UTR binding site for miR-26a close to the stop codon, with 99 percentile context score, in addition to two other binding sites, rendering it a strong candidate target. We found sharp reductions in the levels of SMAD1 and SODD in most sensitive cell lines, but not in the other putative targets (Fig. 3a and data not shown). Resistant cell lines A375 and SK-MEL-28 showed little or no reductions in these proteins. All sensitive cell lines showed reduction in at least one of these two proteins of 50% or more, although 1205Lu showed no noticeable reduction in SODD after miR-26a treatment.
To further test the role of miR-26a in targeting SODD, a putative and previously unreported target, we transfected primary melanocytes HEMNLP2, and two melanoma cell lines that are sensitive to miR-26a, HT-144 and WM852c, with an miRNA inhibitor specific to miR-26a. Lysates of these cells were compared to those transfected with a negative control inhibitor by immunoblot. We found noticeably increased levels of SODD in cells treated with the miR-26a inhibitor at 72 h (Fig. 3b), indicating that endogenous miR-26a has a repressive effect on SODD.
To further confirm the role of miR-26a in regulating SODD, we constructed a dual luciferase reporter system with 479 base pairs (bp) of the 568 bp SODD 3′UTR from the cDNA of a melanoma cell line containing the miR-26a binding site downstream of the Renilla luciferase gene (Fig. 3c). We then cotransfected HEK293 cells with both miRNA mimics (miR-26a or negative control) and reporter constructs (two different clones of the reporter plasmids containing SODD 3′UTR or empty vector without any 3′UTR). The dual luciferase assays showed that at 48 h post-transfection, miR-26a significantly decreased relative luciferase activity compared to the negative control for both clones with the construct containing the SODD 3′UTR (0.74 and 0.68, p = 0.002 and 0.0013, respectively) (Fig. 3c). In contrast, there was no significant difference in relative luciferase activity for the empty vector between miR-26a and control cotransfections (1.02, p = 0.82). In addition, we also constructed reporter plasmids containing a shorter SODD 3′UTR with the same miR-26a binding site, wild-type or mutated (Supplementary Fig. S2). The luciferase assays showed that 48 h post-transfection, miR-26a significantly decreased the relative Renilla luciferase activity when co-transfected with the construct containing the wild-type binding site instead of the mutant binding site (0.52, p < 0.0001) (Supplementary Fig. S2). In contrast, mutations in the miR-26a binding site of the 3′UTR in the reporter plasmid dramatically decreased relative miR-26a repression of luciferase activity (0.88 vs. 0.52, p = 0.0014) (Supplementary Fig. S2)). These results demonstrated that miR-26a can repress gene expression through the miR-26a binding site in the SODD 3′UTR.
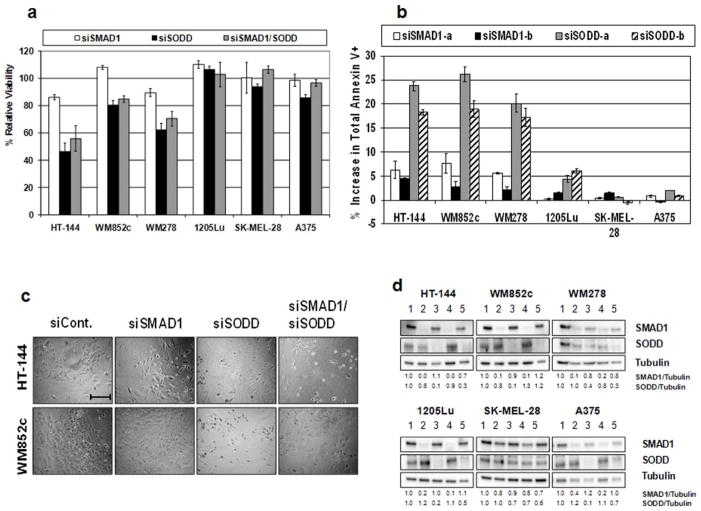
Knockdown of SODD, and to a lesser extent SMAD1, induces cell death in melanoma cell lines sensitive to miR-26a
To test whether miR-26a targeting of SMAD1 and/or SODD was responsible for the cell death observed upon treatment with miR-26a mimic, we knocked-down these transcripts in multiple melanoma cell lines using siRNA. Cell lines were treated with up to 25 nM of total siRNA against SMAD1, SODD, or both together for 120 h and subjected to MTS assays. Cell lines WM278, HT-144, and WM852c all showed significant reduction in viability after treatment with siSODD and for the combination, and HT-144 and WM278 showed a slight reduction in viability with siSMAD1 treatment compared to negative control siRNA (Fig. 4a; for concentration-dependent results, see Supplementary Fig. S3). Cell lines 1205Lu and SK-MEL-28 showed no reduction in viability, while A375 showed only a slight reduction in viability with siSODD treatment compared to the control (Fig. 4a). Similar results were found for the other two siRNAs (not shown). Annexin V assays after 120 h siRNA treatments for cell lines HT-144, WM852c, and WM278 demonstrated a significant apoptotic population ranging from 17 to 26% upon siSODD treatment, and minor apoptosis (~2–8%) for siSMAD1 treatment (Fig. 4b) above the control. 1205Lu showed only slight apoptosis (~5%) with siSODD treatment. Cell lines SK-MEL-28 and A375 were essentially unaffected by siRNA treatments according to Annexin V assays. The appearance of the cells (Fig. 4c) also indicated cell death for lines sensitive to siSODD and was similar to that with miR-26a treatment. Western blotting showed strong knockdown of the respective proteins in all cell lines treated with siSMAD1 and siSODD, except for SK-MEL-28 which had only slight knockdown (Fig. 4d). Results from both MTS and Annexin V assays upon siSODD treatment are broadly consistent with results for miR-26a treatments, although there is the major exception of cell line 1205Lu. Treatment with siSMAD was likewise consistent with miR-26a results, though its effects were very small.
Figure 4.
The effects of knocking-down SODD. (a) MTS assays after 120 h treatment with 25 nM total siRNA normalized to the control. Shown are results using siSMAD1-a and siSODD-a; similar results were found for the other two siRNAs (not shown). Error bars represent SEM of 3 replicates. (b) Annexin V assay results after 120 h treatment with 25 nM siRNA normalized to the control. Y-axis represents the increase in the percentage of total Annexin V positive cells compared to negative control transfected cells. Error bars represent SEM of at least 2 independent experiments. (c) Visual appearance of cells from (a). Scale bar = 0.5 mm. (d) Immunoblots of siRNA-treated-cells from (b). Lane 1, control; lanes 2/4, siSMAD1 (-a and -b); lanes 3/5, siSODD (-a and -b).
DISCUSSION
We identified miR-26a in a microarray screen and subsequent qRT-PCR validation as being strongly down-regulated in melanoma cells compared to primary melanocytes. Prior studies of miRNAs in melanoma have identified a number of miRNAs that are involved in melanoma progression (Bell and Levy, 2011; Bonazzi et al., 2012; Howell et al., 2010; Mueller and Bosserhoff, 2010). These include two miRNAs that our analysis identified as up-regulated, miR-27b and miR-222. However, to the best of our knowledge, none of the miRNAs that we identified as down-regulated have been previously implicated in melanoma, although several, in addition to miR-26a, such as miR-let7e, miR-494, and miR-30a, are known to be down-regulated in other cancers (Kumarswamy et al., 2012; Mitra et al., 2011; Olaru et al., 2011).
Down-regulation or polymorphisms of miR-26a have previously been implicated in a variety of cancers, including nasopharyngeal carcinoma (Lu et al., 2011), breast cancer (Zhang et al., 2011), hepatocellular carcinoma (Kota et al., 2009), oral cancer (Clague et al., 2010), and colon cancer (Boni et al., 2011). Kota and coworkers (Kota et al., 2009) have successfully demonstrated miR-26a replacement therapy in a mouse model of hepatocellular carcinoma. Additionally, down-regulation of miR-26a appears to play roles in other diseases (Leeper et al., 2011).
We found that treatment of melanoma cell lines with a miR-26a mimic induced substantial cell death in multiple melanoma lines, but not in primary melanocytes, indicating that reduction of miR-26a is necessary for the survival of most melanomas. However, we found melanoma cell lines resistant to the mimic, including A375 and SK-MEL-28, showing that in at least some melanomas, compensatory mechanisms must exist and/or there is incomplete degradation of miR-26a targets. Experiments with fluorescently labeled siRNA demonstrated that transfection efficiency with our protocol was extremely high, and qRT-PCR experiments showed high miR-26a levels in cells transfected with miR-26a mimic, including resistant cells, so it is highly unlikely that resistance was the result of insufficient miR-26a exposure. Among the sensitive cell lines was RPMI-7951, which is a p53 null line (Reuland et al., 2011), indicating that the mechanism of cell death is not p53-dependent.
We searched for putative targets of miR-26a by first testing targets previously reported in other studies. EZH2, for example, is commonly cited as an important target of miR-26a (Alajez et al., 2010; Sander et al., 2008; Wong and Tellam, 2008; Zhang et al., 2011). While we found some reduction of EZH2 in some miR-26a-treated cells, the reduction was low and inconsistent (Fig. 3a). Additionally, we treated cell lines with the histone methylation inhibitor DZNep, which depletes EZH2 (Tan et al., 2007), and found that it had little effect even at high doses (Supplementary Fig. S4), with the paradoxical exception of cell line A375, which while sensitive to DZNep is resistant to miR-26a treatment. We therefore concluded that miR-26a was not killing melanoma cells through EZH2 knockdown.
Other previously validated targets of miR-26a include GSK-β (Mohamed et al., 2010), cyclins D2 and E2 (Kota et al., 2009; Lu et al., 2011; Zhu et al., 2011), metadherin (Zhang et al., 2011), and SMAD1 (Luzi et al., 2008; Nigam et al., 2010). Of these, only SMAD1, a transcription factor involved in bone morphogenic protein (BMP) signaling, showed clear and consistent repression by miR-26a in the melanoma cell lines we tested (Fig. 3a and data not shown). We also queried the TargetScan database to identify other potential targets of miR-26a. A binding site in the 3′UTR of SODD was predicted with a 99 percentile context score, and is also close to the stop codon (position 130–137). In addition, SODD encodes a member of the BAG1-family, a potential anti-apoptotic protein (Antoku et al., 2001). As with SMAD1, we found that miR-26a caused strong and consistent down-regulation of SODD protein. We also found that inhibition of endogenous miR-26a in normal melanocytes and two melanoma cell lines leads to increased SODD levels (Fig. 3b), and we further found that miR-26a can repress gene expression through the native SODD 3′UTR using luciferase reporter assays (Fig. 3c, Supplementary Fig. S3). Taken together, the evidence indicates that SODD is a target of miR-26a in melanoma cells.
To test the possible involvement of SMAD1 and/or SODD in melanoma cell death induced by miR-26a replacement, we specifically knocked-down these two proteins with siRNA. The results indicated that SODD expression at least is necessary for preventing cell death in multiple melanoma lines. We found only minor effects by knocking-down SMAD1 alone, although the possibility remains that this protein plays crucial roles under different contexts. Interestingly, one cell line that was highly sensitive to miR-26a mimic, 1205Lu, was completely insensitive to siSODD and siSMAD1 treatments (compare Figs. 2 and 4). This was likely not due to insufficient knock-down (Fig. 4d), but rather implies that there are additional targets of miR-26a that account for the sensitivity of 1205Lu to the mimic. It is well known that miRNAs have many targets and that these vary according to cell type in a context-dependent manner (Didiano and Hobert, 2006). It is not clear to us why this cell line is highly sensitive to the miRNA mimic yet not affected by knockdown of SODD or SMAD1. We hypothesize that one or more targets other than SODD or SMAD1 mediate the effects of miR-26a in the 1205Lu cell line. The identification of these additional targets, and the potentially complex mechanism of their interplay, will require further study. Likewise, the resistance of two melanoma cell lines to both miR-26a and the siRNAs against SMAD1 and SODD would seem to indicate that these proteins are essential only in a subset of melanoma cells. Significant knock-down of both proteins was achieved in siRNA-treated A375 cells (Fig. 4d), and yet this had no effect on viability. Taken together, it appears that while SODD is a potentially critical target of miR-26a in many melanomas, its knockdown by miR-26a treatment can only cause cell death in a subset of melanoma cell lines.
To the best of our knowledge, SODD has not previously reported as a target of miR-26a and as an anti-apoptotic protein in melanoma. SODD is known as an inhibitor of the death domains of the TNF receptor 1 (TNF-R1), preventing trimerization of the receptor subunits in the absence of specific signaling (Jiang et al., 1999; Miki and Eddy, 2002). Upon TNFa binding, the subunits of the receptor trimerize and SODD is quickly released. Activated TNF receptors can then lead to either apoptosis or NF B activation. Additionally, SODD is known to interact with Hsp70, death receptor 3, and the anti-apoptotic protein Bcl-2 (Antoku et al., 2001; Brockmann et al., 2004; Jiang et al., 1999). SODD is overexpressed in pancreatic cancer and leads to resistance of TNFα-induced cell death (Ozawa et al., 2000), and increased SODD is correlated with the severity of acute lymphoblastic leukaemia in children, and the down-regulation of SODD and NFκB induces apoptosis (Tao et al., 2007). Since the balance between pro- and anti-apoptotic signaling in TNF-R1 activation is critical in melanoma (Ivanov et al., 2003), it is likely that SODD acts to tip the balance away from cell death and toward NF B activation, which is constitutively active in melanoma and important in its progression (Madonna et al., 2012; Poser and Bosserhoff, 2004). Additionally, SODD could play a role in the anti-apoptotic functions of Bcl-2 family members, as we and others have found that these proteins are critical mediators of cell survival in melanoma (Reuland et al., 2011; Reuland et al., 2012). Further study is needed to test these and other hypotheses, but regardless, our data indicate that SODD is essential for cell survival of a subgroup of melanoma cells without any other triggers, suggesting that SODD is a potential target for treatment of certain types of melanoma.
In conclusion, we have found that miR-26a is strongly down-regulated in melanoma cells compared to normal melanocytes, and that replacement of miR-26a induces cell death in multiple cell lines. We have identified the anti-apoptotic protein SODD as a target of miR-26a, and found that specific knockdown of SODD produces cell death in some, but not all, cell lines sensitive to miR-26a. While the mechanistic details of its function in melanoma require more study, SODD is a potential target for melanoma therapy. In addition, our data imply that miR-26a must act on additional targets, and that miR-26a replacement therapy is a promising strategy for treating melanoma.
MATERIALS AND METHODS
miRNA microarrays
MicroRNAs from melanoma cell lines SK-MEL-28 and HT-144 were compared to those from two normal melanocyte cultures. Each sample was run in triplicate for the experiment. Total RNAs were extracted using an miRNeasy kit (Qiagen, Valencia, CA), and then enriched for small RNAs using an RNeasy minelute kit (Qiagen) before being sent to the University of Colorado Cancer Center Microarray Core for miRNA microarray analysis. RNA samples were analyzed on a Bioanalyzer Small RNA chip for quality control, then labeled and run on CombiMatrix human miRNA arrays (CombiMatrix Diagnostics, Irvine, CA) by the Core, as directed by the manufacturer. For data normalization, the CombiMatrix recommended method was used: briefly, background signaling was estimated as the lowest 5% of all signaling, including perfect matches and the mismatch controls, and data were then normalized from the arrays using global scale factors to bring all backgrounds to the same value. For filtering, we removed any miRNA with no normalized signals >200, or miRNAs with an intensity less than 1000 in all samples, and only kept miRNAs with signaling on perfect matches (PM) that exceeded background by at least 2x, and PM values that exceeded 1.2x (the intensity of the double mismatch control). We then used Rank Product statistical analysis as reported by Breitling et al. (Breitling et al., 2004) to identify up- or down-regulated miRNAs in melanoma compared to normal melanocytes. The Rank Product analysis is a simple non-parametric statistical method based on ranks of fold changes to detect differentially expressed genes in replicated array experiments (Breitling et al., 2004). The rank products were calculated using the same method and procedure with Excel as described by Breitling et al. (Breitling et al., 2004).
Plasmid constructs, transfections, and luciferase reporter assays
TargetScan was used to identify potential miR-26a binding sites in the SODD 3′UTR (called BAG4 in TargetScan). Total RNA from melanoma cell line WM852c was used to generate cDNAs and to clone the 3′UTR of SODD (NM_001204878). The SODD 3′UTR is predicted to be a 568 bp fragment (see http://www.origene.com/MicroRNA/3-UTR-Clone/SC207066.aspx) or a ~2.8 kb fragment (in TargetScan). We attempted to amplify both the 568 bp and ~2.8 kb 3′UTR from cDNAs of multiple melanoma cells; however, we could only amplify the 568 bp 3′UTR. It is possible that melanoma cells mainly have the SODD transcript with the 568 bp 3′UTR. Thus we cloned 479 bp of this 568 bp 3′UTR into a pSiCheck2 reporter plasmid at Xho1-Not1 sites for luciferase activity assays, using the following PCR rimers: 5′-ACTGCTCTGCTCGAGAGCCTGTTACTAACTTGAC-3′ and 5′AATTAGCGGCCGCCTGCAAATAACAAAACAAAACAGAAGTCC. The constructed psiCHECK2 plasmids were verified by DNA sequencing (Colorado Cancer Center DNA Sequencing Core). This cloned SODD 3′UTR, from cDNAs of melanoma cells, contains one predicted binding site for miR-26a.
HEK293 cells were cotransfected with luciferase reporter plasmids (psiCHECK2 vector with or without indicated SODD 3′UTR, 0.25 μg) and mimics of miR-26a or Allstars Negative Control siRNA (25 nM) in 24-well plates using the Lipofectamine 2000 reagent as directed by the supplier (Invitrogen, Carlsbad, CA). After 48 h, the cells were lysed and assayed for luciferase activity using the Dual Luciferase Reporter Assay kit (Promega) according to the manufacturer’s protocol on a microplate reader (BioTek). The psiCHECK2 reporter construct contains both firefly and Renilla luciferase genes under the control of constitutive promoters, and firefly luciferase activity was used as an internal control. The relative Renilla luciferase activity was calculated as the ratio of Renilla to firefly luciferase activity for each sample, and the relative Renilla luciferase activities were normalized to 1.0 for negative control cotransfected cells.
Statistics
All comparisons were evaluated by two-tailed unpaired t-tests using the program GraphPad Prism (GraphPad Software Inc., La Jolla, CA), with P values below 0.05 considered significant.
Other methods
Further information about other materials and methods used in this work are provided in the Supplementary material.
Supplementary Material
Acknowledgments
This work was supported in part by NIAMS grant R01AR26427-18 to DAN; by a Veterans Administration merit grant from the Department of Veterans Affairs (Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development) to DAN; by NIH training grant 5T32AR007411-29 to SNR (PI: DAN); and by the Intramural Research Program of the NIH, National Cancer Institute for Cancer Research, to VEM. We thank Karen Helm, Christine Childs, and Alistaire S. Acosta at the University of Colorado Cancer Center Flow Cytometry Core (supported by NIH grant P30 CA 046934) and Bifeng Gao at the University of Colorado Microarray Core for their expert technical assistance.
Abbreviations
- miRNA
microRNA
- qRT-PCR
quantitative reverse transcription polymerase chain reaction
- SMAD1
Mothers Against Decapentaplegic homolog 1
- SODD
Silencer of Death Domains
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Ades F, Metzger-Filho O. Targeting the Cellular Signaling: BRAF Inhibition and Beyond for the Treatment of Metastatic Malignant Melanoma. Dermatol Res Pract. 2012;2012:259170. doi: 10.1155/2012/259170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alajez NM, Shi W, Hui AB, Bruce J, Lenarduzzi M, Ito E, et al. Enhancer of Zeste homolog 2 (EZH2) is overexpressed in recurrent nasopharyngeal carcinoma and is regulated by miR-26a, miR-101, and miR-98. Cell Death Dis. 2010;1:e85. doi: 10.1038/cddis.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoku K, Maser RS, Scully WJ, Jr, Delach SM, Johnson DE. Isolation of Bcl-2 binding proteins that exhibit homology with BAG-1 and suppressor of death domains protein. Biochem Biophys Res Commun. 2001;286:1003–10. doi: 10.1006/bbrc.2001.5512. [DOI] [PubMed] [Google Scholar]
- Bell RE, Levy C. The three M’s: melanoma, microphthalmia-associated transcription factor and microRNA. Pigment Cell Melanoma Res. 2011;24:1088–106. doi: 10.1111/j.1755-148X.2011.00931.x. [DOI] [PubMed] [Google Scholar]
- Bonazzi VF, Stark MS, Hayward NK. MicroRNA regulation of melanoma progression. Melanoma Res. 2012;22:101–13. doi: 10.1097/CMR.0b013e32834f6fbb. [DOI] [PubMed] [Google Scholar]
- Boni V, Zarate R, Villa JC, Bandres E, Gomez MA, Maiello E, et al. Role of primary miRNA polymorphic variants in metastatic colon cancer patients treated with 5-fluorouracil and irinotecan. Pharmacogenomics J. 2011;11:429–36. doi: 10.1038/tpj.2010.58. [DOI] [PubMed] [Google Scholar]
- Breitling R, Armengaud P, Amtmann A, Herzyk P. Rank products: a simple, yet powerful, new method to detect differentially regulated genes in replicated microarray experiments. FEBS Lett. 2004;573:83–92. doi: 10.1016/j.febslet.2004.07.055. [DOI] [PubMed] [Google Scholar]
- Brockmann C, Leitner D, Labudde D, Diehl A, Sievert V, Bussow K, et al. The solution structure of the SODD BAG domain reveals additional electrostatic interactions in the HSP70 complexes of SODD subfamily BAG domains. FEBS Lett. 2004;558:101–6. doi: 10.1016/S0014-5793(03)01490-X. [DOI] [PubMed] [Google Scholar]
- Buzaid AC. Management of metastatic cutaneous melanoma. Oncology (Williston Park) 2004;18:1443–50. discussion 57–9. [PubMed] [Google Scholar]
- Clague J, Lippman SM, Yang H, Hildebrandt MA, Ye Y, Lee JJ, et al. Genetic variation in MicroRNA genes and risk of oral premalignant lesions. Mol Carcinog. 2010;49:183–9. doi: 10.1002/mc.20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins DL, Cummins JM, Pantle H, Silverman MA, Leonard AL, Chanmugam A. Cutaneous malignant melanoma. Mayo Clin Proc. 2006;81:500–7. doi: 10.4065/81.4.500. [DOI] [PubMed] [Google Scholar]
- Didiano D, Hobert O. Perfect seed pairing is not a generally reliable predictor for miRNA-target interactions. Nat Struct Mol Biol. 2006;13:849–51. doi: 10.1038/nsmb1138. [DOI] [PubMed] [Google Scholar]
- Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–74. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- Flaherty KT. Narrative review: BRAF opens the door for therapeutic advances in melanoma. Ann Intern Med. 2010;153:587–91. doi: 10.7326/0003-4819-153-9-201011020-00008. [DOI] [PubMed] [Google Scholar]
- Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogas HJ, Kirkwood JM, Sondak VK. Chemotherapy for metastatic melanoma: time for a change? Cancer. 2007;109:455–64. doi: 10.1002/cncr.22427. [DOI] [PubMed] [Google Scholar]
- Howell PM, Jr, Li X, Riker AI, Xi Y. MicroRNA in Melanoma. Ochsner J. 2010;10:83–92. [PMC free article] [PubMed] [Google Scholar]
- Ivanov VN, Bhoumik A, Ronai Z. Death receptors and melanoma resistance to apoptosis. Oncogene. 2003;22:3152–61. doi: 10.1038/sj.onc.1206456. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Woronicz JD, Liu W, Goeddel DV. Prevention of constitutive TNF receptor 1 signaling by silencer of death domains. Science. 1999;283:543–6. doi: 10.1126/science.283.5401.543. [DOI] [PubMed] [Google Scholar]
- Kasinski AL, Slack FJ. Epigenetics and genetics. MicroRNAs en route to the clinic: progress in validating and targeting microRNAs for cancer therapy. Nat Rev Cancer. 2011;11:849–64. doi: 10.1038/nrc3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota J, Chivukula RR, O’Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, et al. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005–17. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozomara A, Griffiths-Jones S. miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011;39:D152–D7. doi: 10.1093/nar/gkq1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet. 2007;39:673–7. doi: 10.1038/ng2003. [DOI] [PubMed] [Google Scholar]
- Kumarswamy R, Mudduluru G, Ceppi P, Muppala S, Kozlowski M, Niklinski J, et al. MicroRNA-30a inhibits epithelial-to-mesenchymal transition by targeting Snai1 and is downregulated in non-small cell lung cancer. Int J Cancer. 2012;130:2044–53. doi: 10.1002/ijc.26218. [DOI] [PubMed] [Google Scholar]
- Leeper NJ, Raiesdana A, Kojima Y, Chun HJ, Azuma J, Maegdefessel L, et al. MicroRNA-26a is a novel regulator of vascular smooth muscle cell function. J Cell Physiol. 2011;226:1035–43. doi: 10.1002/jcp.22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, He ML, Wang L, Chen Y, Liu X, Dong Q, et al. MiR-26a inhibits cell growth and tumorigenesis of nasopharyngeal carcinoma through repression of EZH2. Cancer Res. 2011;71:225–33. doi: 10.1158/0008-5472.CAN-10-1850. [DOI] [PubMed] [Google Scholar]
- Luzi E, Marini F, Sala SC, Tognarini I, Galli G, Brandi ML. Osteogenic differentiation of human adipose tissue-derived stem cells is modulated by the miR-26a targeting of the SMAD1 transcription factor. J Bone Miner Res. 2008;23:287–95. doi: 10.1359/jbmr.071011. [DOI] [PubMed] [Google Scholar]
- Madonna G, Dansky Ullman C, Gentilcore G, Palmieri G, Ascierto PA. NF-kappaB as potential target in the treatment of melanoma. J Transl Med. 2012;10:53. doi: 10.1186/1479-5876-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki K, Eddy EM. Tumor necrosis factor receptor 1 is an ATPase regulated by silencer of death domain. Mol Cell Biol. 2002;22:2536–43. doi: 10.1128/MCB.22.8.2536-2543.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra D, Das PM, Huynh FC, Jones FE. Jumonji/ARID1 B (JARID1B) protein promotes breast tumor cell cycle progression through epigenetic repression of microRNA let-7e. J Biol Chem. 2011;286:40531–5. doi: 10.1074/jbc.M111.304865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed JS, Lopez MA, Boriek AM. Mechanical stretch up-regulates microRNA-26a and induces human airway smooth muscle hypertrophy by suppressing glycogen synthase kinase-3beta. J Biol Chem. 2010;285:29336–47. doi: 10.1074/jbc.M110.101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller DW, Bosserhoff AK. The evolving concept of ‘melano-miRs’-microRNAs in melanomagenesis. Pigment Cell Melanoma Res. 2010;23:620–6. doi: 10.1111/j.1755-148X.2010.00734.x. [DOI] [PubMed] [Google Scholar]
- Nigam V, Sievers HH, Jensen BC, Sier HA, Simpson PC, Srivastava D, et al. Altered microRNAs in bicuspid aortic valve: a comparison between stenotic and insufficient valves. J Heart Valve Dis. 2010;19:459–65. [PMC free article] [PubMed] [Google Scholar]
- Olaru AV, Ghiaur G, Yamanaka S, Luvsanjav D, An F, Popescu I, et al. MicroRNA down-regulated in human cholangiocarcinoma control cell cycle through multiple targets involved in the G1/S checkpoint. Hepatology. 2011;54:2089–98. doi: 10.1002/hep.24591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa F, Friess H, Zimmermann A, Kleeff J, Buchler MW. Enhanced expression of Silencer of death domains (SODD/BAG-4) in pancreatic cancer. Biochem Biophys Res Commun. 2000;271:409–13. doi: 10.1006/bbrc.2000.2610. [DOI] [PubMed] [Google Scholar]
- Poser I, Bosserhoff AK. Transcription factors involved in development and progression of malignant melanoma. Histol Histopathol. 2004;19:173–88. doi: 10.14670/HH-19.173. [DOI] [PubMed] [Google Scholar]
- Reuland SN, Goldstein NB, Partyka KA, Cooper DA, Fujita M, Norris DA, et al. The combination of BH3-mimetic ABT-737 with the alkylating agent temozolomide induces strong synergistic killing of melanoma cells independent of p53. PLoS One. 2011;6:e24294. doi: 10.1371/journal.pone.0024294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuland SN, Goldstein NB, Partyka KA, Smith S, Luo Y, Fujita M, et al. ABT-737 synergizes with Bortezomib to kill melanoma cells. Biology Open. 2012;1:92–100. doi: 10.1242/bio.2011035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruth MC, Xu Y, Maxwell IH, Ahn NG, Norris DA, Shellman YG. RhoC promotes human melanoma invasion in a PI3K/Akt-dependent pathway. J Invest Dermatol. 2006;126:862–8. doi: 10.1038/sj.jid.5700211. [DOI] [PubMed] [Google Scholar]
- Sander S, Bullinger L, Klapproth K, Fiedler K, Kestler HA, Barth TF, et al. MYC stimulates EZH2 expression by repression of its negative regulator miR-26a. Blood. 2008;112:4202–12. doi: 10.1182/blood-2008-03-147645. [DOI] [PubMed] [Google Scholar]
- Sandhu S, Garzon R. Potential applications of microRNAs in cancer diagnosis, prognosis, and treatment. Semin Oncol. 2011;38:781–7. doi: 10.1053/j.seminoncol.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Shellman YG, Ribble D, Miller L, Gendall J, Vanbuskirk K, Kelly D, et al. Lovastatin-induced apoptosis in human melanoma cell lines. Melanoma Res. 2005;15:83–9. doi: 10.1097/00008390-200504000-00001. [DOI] [PubMed] [Google Scholar]
- Tan J, Yang X, Zhuang L, Jiang X, Chen W, Lee PL, et al. Pharmacologic disruption of Polycomb-repressive complex 2-mediated gene repression selectively induces apoptosis in cancer cells. Genes Dev. 2007;21:1050–63. doi: 10.1101/gad.1524107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao H, Hu Q, Fang J, Liu A, Liu S, Zhang L, et al. Expression of SODD and P65 in ALL of children and its relationship with chemotherapeutic drugs. J Huazhong Univ Sci Technolog Med Sci. 2007;27:326–9. doi: 10.1007/s11596-007-0328-2. [DOI] [PubMed] [Google Scholar]
- Wong CF, Tellam RL. MicroRNA-26a targets the histone methyltransferase Enhancer of Zeste homolog 2 during myogenesis. J Biol Chem. 2008;283:9836–43. doi: 10.1074/jbc.M709614200. [DOI] [PubMed] [Google Scholar]
- Zhang B, Liu XX, He JR, Zhou CX, Guo M, He M, et al. Pathologically decreased miR-26a antagonizes apoptosis and facilitates carcinogenesis by targeting MTDH and EZH2 in breast cancer. Carcinogenesis. 2011;32:2–9. doi: 10.1093/carcin/bgq209. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Lu Y, Zhang Q, Liu JJ, Li TJ, Yang JR, et al. MicroRNA-26a/b and their host genes cooperate to inhibit the G1/S transition by activating the pRb protein. Nucleic Acids Res. 2011 doi: 10.1093/nar/gkr1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.