SUMMARY
The location, size and number of synapses critically influence the specificity and strength of neural connections. In axons, synaptic vesicle (SV) and active zone (AZ) proteins are transported by molecular motors and accumulate at discrete presynaptic loci. Little is known about the mechanisms coordinating presynaptic protein transport and deposition to achieve proper distribution of synaptic material. Here we show that SV and AZ proteins exhibit extensive co-transport and undergo frequent pauses. At the axonal and synaptic pause sites, the balance between the capture and dissociation of mobile transport packets determines the extent of presynaptic assembly. The small G protein ARL-8 inhibits assembly by promoting dissociation, while a JNK kinase pathway and AZ assembly proteins inhibit dissociation. Furthermore, ARL-8 directly binds to the UNC-104/KIF1A motor to limit the capture efficiency. Together, molecular regulation of the dichotomy between axonal trafficking and local assembly controls vital aspects of synapse formation and maintenance.
INTRODUCTION
Neurotransmission relies on the fusion of synaptic vesicles (SVs) with the plasma membrane at the presynaptic terminals, where SVs are clustered near the active zones (AZs). AZs are specialized regions of the plasma membrane defined by a protein meshwork which contain the molecular machinery necessary for SV recruitment and recycling (Jin and Garner, 2008; Owald and Sigrist, 2009; Südhof, 2012). The number, size and location of synapses vary among different types of neurons and critically impact the efficacy of neurotransmission (Atwood and Karunanithi, 2002; Holderith et al., 2012). For example, in both vertebrate and invertebrate nervous systems, some neurons specify a single synaptic connection at the axon terminal, while others elaborate sequential release sites called en passant synapses. Although many extrinsic cues and cell surface molecules have been shown to shape synaptic connectivity (Shen and Scheiffele, 2010), our understanding of the intracellular mechanisms involved in synaptic patterning remains incomplete.
The targeting of SVs and AZ proteins to specific sites depends on their directed axonal delivery by molecular motors (Goldstein et al., 2008; Hirokawa et al., 2010). Electron and light micrographic studies have demonstrated that many SV components are trafficked in SV protein transport vesicles (STVs) (Matteoli et al., 1992; Ahmari et al., 2000; Tao-Cheng, 2007). Live imaging has revealed that STV packets travel along axons bi-directionally and intermittently, occasionally splitting into smaller packets or merging into larger clusters (Kraszewski et al., 1995; Dai and Peng, 1996; Ahmari et al., 2000; Sabo et al., 2006). In addition, they can rapidly accumulate at new axodendritic contact sites and become capable of stimulation-evoked SV recycling (Ahmari et al., 2000; Washbourne et al., 2002; Sabo et al., 2006). On the other hand, the 80-nanometer dense core Piccolo-Bassoon transport vesicles (PTVs) are proposed to represent modular packets that assemble the AZ cytomatrix in vertebrate neurons (Zhai et al., 2001; Shapira et al., 2003; Maas et al., 2012). Interestingly, recent electron micrographic (EM) and live imaging studies reported that AZ and SV proteins may be pre-assembled into multi-vesicle transport complexes and co-trafficked in cultured neurons (Tao-Cheng, 2007; Bury and Sabo, 2011).
We previously identified a conserved Arf-like small G protein, ARL-8, as a critical regulator of presynaptic patterning and axonal transport in C. elegans (Klassen et al., 2010). Loss-of-function mutations in arl-8 caused ectopic accumulation of presynaptic specializations in the proximal axon and a loss of presynapses in distal segments, leading to deficits in neurotransmission. Time-lapse imaging revealed that arl-8 mutant STVs prematurely associate into immotile clusters en route, suggesting that ARL-8 facilitates the trafficking of presynaptic cargo complexes by repressing excessive self-assembly during axonal transport.
To further understand the molecular mechanisms coordinating presynaptic protein transport with assembly, we performed forward genetic screens to identify molecules that functionally interact with arl-8. Here we report that loss-of-function mutations in a JNK MAP kinase pathway partially and strongly suppress the abnormal distribution of presynaptic proteins in arl-8 mutants. We show that the JNK pathway is required for excessive STV aggregation during transport in arl-8 mutants and promotes the clustering of SVs and AZ proteins at the presynaptic terminals. Time-lapse imaging further reveals that transiting AZ proteins are in extensive association with STVs and promote STV aggregation during transport, with ARL-8 and the JNK pathway antagonistically controlling STV/AZ association en route. In addition, the anterograde motor UNC-104/KIF1A functions as an effector of ARL-8 and acts in parallel to the JNK pathway to control STV capture at the presynaptic terminals and during transport. Collectively, these findings uncover novel mechanisms that modulate the balance between presynaptic protein transport and self-assembly and highlight the close link between transport regulation and the spatial patterning of synapses.
RESULTS
Loss of JNK Pathway Suppresses Abnormal Distribution of SV Proteins in arl-8 Mutants
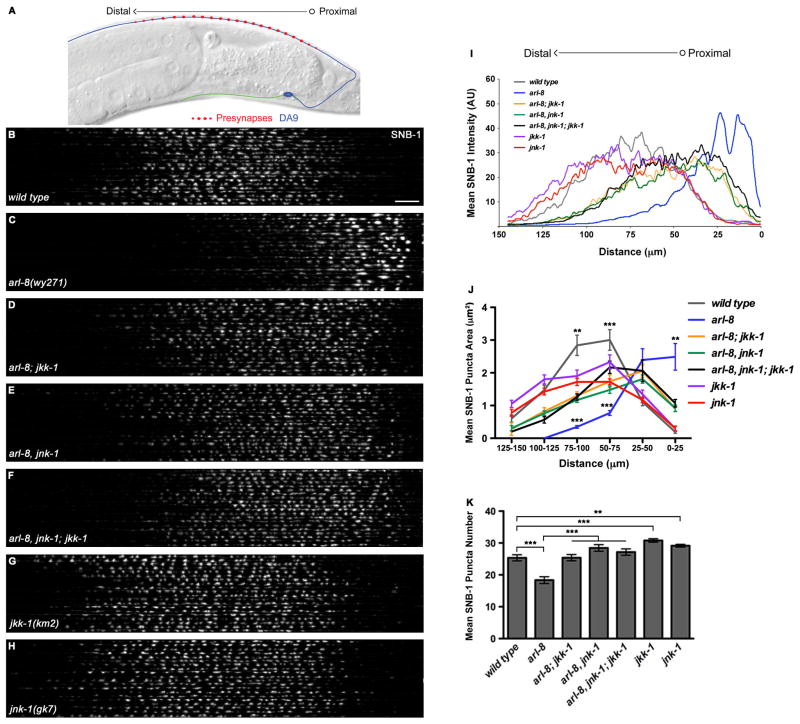
The C. elegans cholinergic motoneuron DA9 provides an in vivo model to investigate the molecular mechanisms regulating presynaptic patterning. DA9 is born embryonically. During development, its axon elaborates a series of en passant synapses with the dorsal body wall muscles within a discrete and stereotyped domain, as visualized with YFP-tagged SV protein synaptobrevin (SNB-1::YFP) (Figures 1A–1B; White et al., 1976; Klassen and Shen, 2007). This synaptic pattern is already present at hatching, but the synapses continue to grow in size and number during post-embryonic development. Loss of function in arl-8 results in ectopic accumulation of SNB-1::YFP in the proximal axon and the appearance of abnormally large clusters in this region, accompanied by a loss of distal puncta (Figure 1C; Klassen et al., 2010). To identify additional molecules regulating presynaptic patterning, we performed forward genetic screens for suppressors of the arl-8 phenotype and isolated two recessive mutations, wy733 and wy735, which strongly and partially suppressed the abnormal distribution of SV proteins in arl-8(wy271) loss-of-function mutants (Figure S1A–S1D).
Figure 1. Loss of JNK Pathway Function Suppresses Abnormal SV Protein Distribution in arl-8 Mutants.
(A) Diagram of synapse distribution in wild-type DA9. Proximodistal polarity is indicated by an open circle (proximal) transitioning to an arrowhead (distal). (B–-H) Confocal images of 20 wild type (B), arl-8(wy271) (C), arl-8(wy271); jkk-1(km2) (D), arl-8(wy271), jnk-1(gk7) (E), arl-8(wy271), jnk-1(gk7); jkk-1(km2) (F), jkk-1(km2) (G) and jnk-1(gk7) (H) DA9 dorsal axons expressing SNB-1::YFP, straightened and aligned along the proximodistal axis (wyIs92). Scale bar, 10 μm. (I) Mean SNB-1::YFP fluorescence intensity as a function of distance from the commissure. (J) Quantification of puncta size along DA9 dorsal axons, binned into 25 μm segments. Statistical significance is between arl-8 and arl-8; jkk-1, arl-8; jnk-1, or arl-8, jnk-1; jkk-1, or between wild type and jnk-1. n=40–154 puncta. (K) Quantification of puncta number in DA9 dorsal axons. Statistical tests for all panels are t tests. ***p<0.0001, **p<0.001. Error bars, SEM. See also Figures S1 and S2.
Using SNP-SNIP mapping, whole-genome sequencing and transgenic rescue, we identified these two suppressors as missense mutations at conserved amino acid residues in JKK-1, a homolog of the mammalian MAP kinase kinase MKK7 (Figure S1E). A null allele jkk-1(km2) (Kawasaki et al., 1999) suppressed the arl-8 phenotype to the same degree as wy733 and wy735 (Figure 1D), indicating that loss of JKK-1 activity suppresses the arl-8 phenotype. We used the jkk-1(km2) allele for our subsequent analyses.
We quantified the suppression of arl-8 by jkk-1. Compared to the arl-8 single mutants, SNB-1::YFP is redistributed into more distal axonal regions in arl-8; jkk-1 double mutants (Figures 1I and 1J). The size of the proximal SNB-1::YFP puncta (0–25 μm from commissure) is also significantly reduced in the double mutants (Figure 1J). Furthermore, while the arl-8 mutants exhibited reduced number of presynaptic SNB-1::YFP puncta, this defect is abolished in the arl-8; jkk-1 double mutants (Figure 1K). Similar results were obtained with two additional SV proteins, RAB-3 (Figures S2A–S2D) and SNG-1/synaptogyrin (data not shown). Therefore, jkk-1 mutations partially and strongly suppressed multiple aspects of the arl-8 mutant phenotype in DA9.
MAP kinases (MAPKs) act in cascades in which each MAPK is activated via phosphorylation by a MAPK kinase (MAPKK), which is in turn activated by a MAPKK kinase (MAPKKK) (Davis, 2000). JKK-1 was shown to be a specific upstream activator of JNK (c-Jun N-terminal kinase) −1, a homolog of mammalian JNK3 (Kawasaki et al., 1999). A null mutation in jnk-1, gk7, caused the same degree of suppression of the arl-8 phenotype as jkk-1(km2) (Figures 1E and 1I–1K). Moreover, the degree of suppression in arl-8, jnk-1; jkk-1 triple mutants is indistinguishable from that in either double mutants (Figures 1F and 1I–1K), indicating that jkk-1 and jnk-1 function in the same pathway. jkk-1 and jnk-1 mutants were previously shown to partially mislocalize SNB-1::GFP to the dendrite in the DD motoneurons (Byrd et al., 2001). We found that the jkk-1 and jnk-1 single mutants appeared grossly normal in SV protein localization in DA9 (Figures 1G and 1H) and did not show mislocalization of SV proteins to the DA9 dendrite (data not shown). However, these mutants did exhibit subtle but significant decreases in SNB-1::YFP puncta size (Figure 1J) and increases in puncta number (Figure 1K), suggesting that JNK also promotes SV clustering in wild-type animals.
To determine whether JNK functionally interacts with arl-8 broadly in the C. elegans nervous system, we examined several other neuron types, including the cholinergic motoneuron DB7, the GABAergic DD motoneurons and the thermosensory neuron AFD, all of which have a proximal axonal region devoid of presynapses and form en passant presynapses in the distal axon (Hallam and Jin, 1998; Klassen and Shen, 2007; Hellman and Shen, 2011). As in DA9, these neurons accumulate abnormally large presynaptic puncta within their proximal axon at the expense of distal puncta in arl-8 mutants (Klassen et al., 2010; data not shown). jkk-1(km2) strongly suppressed these phenotypes in all three types of neurons (Figures S2E–2H and data not shown), indicating that arl-8 genetically interacts with the JNK pathway to regulate presynaptic protein distribution in many neuron types.
Loss of JNK Pathway Suppresses Defects in AZ Protein Distribution and Synaptic Function in arl-8 Mutants
We previously showed that the proximally mislocalized STV accumulations in arl-8 mutants also contain AZ proteins and ultrastructurally resemble bona fide presynapses (Klassen et al., 2010). To address whether JNK also regulates AZ localization, we used an integrated UNC-10::GFP transgene to characterize changes in AZ protein distribution. UNC-10 encodes the C. elegans homolog of mammalian RIM1, an integral AZ protein which interacts with several presynaptic proteins to regulate SV docking, priming and synaptic plasticity (Südhof, 2012). In wild-type DA9, UNC-10::GFP forms discrete puncta juxtaposed with mCherry::RAB-3 puncta at presynaptic terminals (Figures 2A and S3A). In arl-8 mutants, similar to SV proteins, UNC-10::GFP forms large puncta ectopically in the proximal axon and is reduced in the distal axon (Figures 2B and S3B). jkk-1(km2) partially and robustly suppressed this phenotype, reducing proximal puncta size and increasing distal puncta (Figures 2C, 2F, 2G and S3C). Other AZ proteins, including the scaffold protein SYD-2/Liprin-α and the calcium channel β-subunit CCB-1, showed similar mislocalization in arl-8 mutants and these phenotypes were also strongly and partially suppressed by jkk-1(km2) (Figures S3D–S3I), suggesting that ARL-8 and JKK-1 systematically regulate presynaptic differentiation rather than select markers. arl-8 mutants also displayed proximal shift and distal loss of AZ proteins in other types of neurons, including UNC-10::tdTomato in DDs (Figures 2I and 2J) and SAD-1::YFP in AFD (data not shown). Again, these defects were largely suppressed in arl-8; jkk-1 double mutants (Figure 2K and data not shown). jkk-1(km2) single mutants displayed a reduction in presynaptic UNC-10::GFP puncta size in DA9 (Figures 2D, 2F and 2G), indicating that jkk-1 also supports AZ assembly in wild-type animals. These results suggest an antagonistic relationship between ARL-8 and the JNK pathway in regulating the clustering of presynaptic components. While the JNK pathway promotes presynaptic assembly, ARL-8 limits the extent of clustering. Consistent with this model, we found that overexpression of arl-8 in DA9 led to decreases in presynaptic RAB-3 and UNC-10 puncta size (Klassen et al., 2010; Figures 2E and 2H). The observation that JNK pathway inactivation did not completely suppress the arl-8 mutant phenotype suggests that other pathways function in parallel to JNK to antagonize arl-8 in regulating presynaptic protein clustering.
Figure 2. Inactivation of the JNK Pathway Suppresses Defects in AZ Protein Distribution and Synaptic Function in arl-8 Mutants.
(A–E) Confocal images of 20 straightened and aligned wild type (A), arl-8(wy271) (B), arl-8(wy271); jkk-1(km2) (C), jkk-1(km2) (D) and Ex[itr-1::arl-8] (arl-8 over-expression) (E) DA9 dorsal axons expressing UNC-10::GFP (wyIs301). Scale bar, 10 μm. (F) Mean UNC-10::GFP fluorescence intensity as a function of distance from the commissure. (G) Quantification of puncta size along DA9 dorsal axons, binned into 25 μm segments. ***p<0.0001, **p<0.001, *p<0.005. Statistical significance is between arl-8 and arl-8; jkk-1 or between wild type and jkk-1. n=26–164 puncta. (H) Quantification of UNC-10::GFP puncta size in wild type and Ex[itr-1::arl-8] DA9 dorsal axons. ***p<0.0001, n=493–609 puncta. (I–K) Distribution of UNC-10::tdTomato in DD neurons in wild type (I), arl-8(wy271) (J) and arl-8(wy271); jkk-1(km2) (K) animals (wyIs292). (L–N) Distribution of the GABA receptor UNC-49::YFP in postsynaptic muscles in wild type (L), arl-8(wy271) (M) and arl-8(wy271); jkk-1(km2) (N) animals (krIs1). Proximodistal polarity in DD neurons reflects a bifurcation of the dorsal axon and its bilateral extension, with proximal defined by the branch point. (O) Time course of paralysis for wild type, arl-8(wy271), arl-8(wy271); jkk-1(km2) and jkk-1(km2) animals on aldicarb plates. **p<0.005, *p<0.05. Statistical significance is between arl-8 and arl-8; jkk-1 or between jkk-1 and wild type. Statistical tests for all panels are t tests. Error bars, SEM. See also Figures S3 and S4.
To address whether the suppression of the presynaptic morphological defects in arl-8 by jkk-1 also ameliorates synaptic function, we first visualized the distribution of the postsynaptic GABA receptor UNC-49 in the dorsal body wall muscles, which receive presynaptic inputs from the DD neurons. In wild-type animals, UNC-49::YFP forms evenly distributed clusters apposed to presynapses in DDs (Gally and Bessereau, 2003; Figure 2L). In arl-8 mutants, DDs accumulate large UNC-10::tdTomato puncta in the proximal axon with a loss of distal puncta (Figures 2I and 2J). Interestingly, the distribution of UNC-49::YFP is similarly shifted (Figures 2L and 2M) and this phenotype can be suppressed by expressing arl-8 solely in the presynaptic neurons (wyEx3666; strong rescue in 47/50 animals), suggesting that trans-synaptic communication is preserved in the arl-8 mutants. In arl-8; jkk-1 double mutants, the uniform distribution patterns of both UNC-10 and UNC-49 were largely restored (Figures 2K and 2N), indicating that the jkk-1 mutation suppressed both the pre- and postsynaptic defects of the arl-8 mutants. Secondly, we assessed the efficacy of cholinergic neurotransmission using the aldicarb sensitivity assay (Mahoney et al., 2006). The arl-8 mutants exhibited resistance to the acetylcholinesterase inhibitor, aldicarb, indicating impaired cholinergic transmission (Klassen et al., 2010; Figure 2O). This phenotype was robustly suppressed by jkk-1(km2) (Figure 2O), reflecting improvements in cholinergic synaptic function in the arl-8; jkk-1 double mutants. The jkk-1 single mutants also displayed some degree of aldicarb resistance (Figure 2O), consistent with reduced AZ and SV assembly in these mutants. We conclude that loss of the JNK pathway affects not only synapse morphology but also synapse function.
JKK-1 and JNK-1 Function Cell-Autonomously in Neurons to Regulate Presynaptic Patterning and Are Enriched at the Presynaptic Terminals
Both JKK-1 and JNK-1 are expressed in the C. elegans nervous system throughout development (Kawasaki et al., 1999). To determine whether they function cell-autonomously in neurons to suppress the arl-8 phenotype, we expressed jkk-1 or jnk-1 cDNA under the Pitr-1 pB or Pmig-13 promoter, which we use to label DA9, in arl-8; jkk-1 or arl-8, jnk-1 double mutants. These manipulations robustly rescued the suppression of arl-8 by the kinase mutations, whereas expression in the postsynaptic muscles or of a mutant JNK-1 lacking kinase activity (Hanks et al., 1988) failed to rescue (Figure S4A and data not shown). Together, these data suggest that jkk-1 and jnk-1 interact with arl-8 cell-autonomously in the presynaptic neuron to shape synaptic organization in a kinase-dependent manner.
The arl-8 mutant phenotypes and the jkk-1/jnk-1 suppression are already present at hatching. To test whether JNK also functions in the maintenance of synapse distribution, we induced jkk-1 expression driven by a heat-shock promoter (Stringham et al., 1992) at the L4 larval stage in the arl-8; jkk-1 double mutants and examined SV protein distribution at the young adult stage. This manipulation strongly rescued the suppression of the arl-8 phenotype by the jkk-1 mutation, whereas transgenic animals not receiving the heat-shock treatment or heat-shocked animals without the transgene showed no rescue (Figure S4A and data not shown). These results indicate that JNK continues to impact presynaptic pattern after its initial establishment.
To understand where JNK might function within the neuron, we further examined the subcellular localization of JKK-1 and JNK-1 in DA9 using functional GFP fusion proteins. Consistent with a presynaptic function, both JKK-1::GFP and JNK-1::GFP are highly enriched at the presynaptic terminals and colocalize with mCherry::RAB-3 (Figures S4B–S4J and S4K–S4S). This presynaptic enrichment requires the anterograde motor for STVs, UNC-104/KIF1A (Hall and Hedgecock, 1991); JKK-1::GFP and JNK-1::GFP are absent from the presynaptic terminals and accumulate in the cell body and dendrite in unc-104 mutants (data not shown).
ARL-8 and the JNK Pathway Antagonistically Regulate STV Dissociation during Axonal Transport
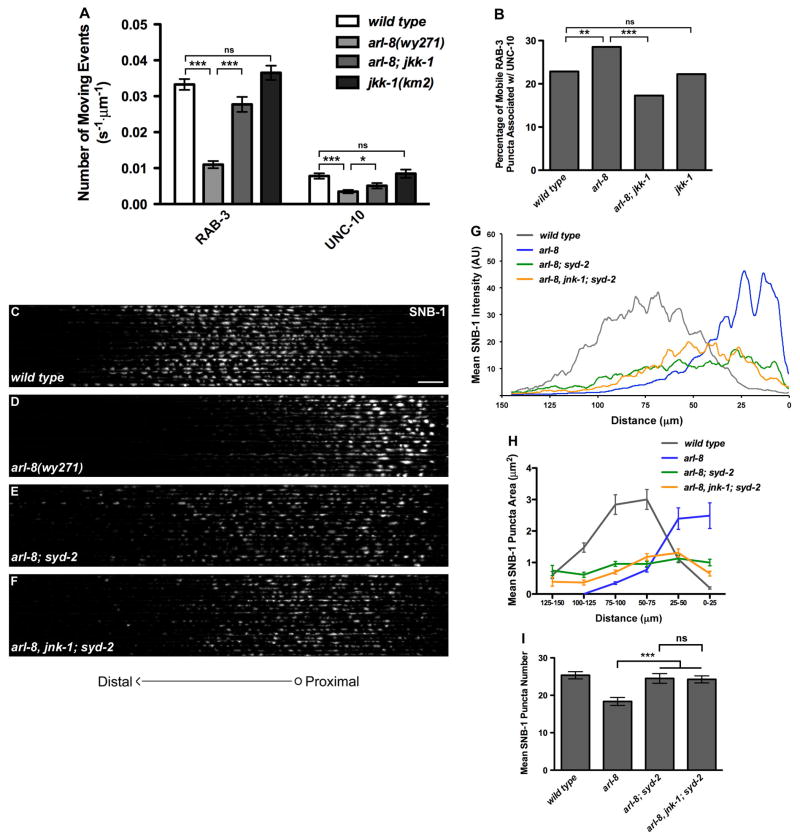
Axonal transport is critical for the establishment and maintenance of presynapses (Hirokawa et al., 2010). Alterations in the cell-wide distribution of presynaptic components could originate from changes in their axonal transport dynamics. We previously showed that presynaptic protein mislocalization in arl-8 mutants likely results from premature clustering of STVs during trafficking (Klassen et al., 2010). To further investigate the role of the JNK pathway in regulating STV clustering during transport, we performed time-lapse imaging of STVs labeled with GFP::RAB-3 in vivo. Within the proximal axon of wild-type DA9, small mobile and stationary GFP::RAB-3 puncta can be visualized with a high-sensitivity CCD camera, evident as diagonal and vertical lines in the kymographs, respectively, with the mobile puncta representing trafficking STVs, which pause frequently en route and form stationary puncta. (Klassen et al., 2010; Figures 3A and 3B). arl-8 mutants did not exhibit changes in the directionality or velocity of STV movements (Klassen et al., 2010). However, we observed significant increases in the number and fluorescence intensity of stationary puncta (Figures 3C, 3F and 4G). Meanwhile, there was a decrease in the number of moving events (Figure 3H). The jkk-1 mutation strongly alleviated the STV trafficking abnormalities in arl-8 mutants; the number of stationary puncta en route and their fluorescence intensity were significantly reduced in the arl-8; jkk-1 double mutants (Figures 3D, 3F and 3G). Accordingly, there was a significant increase in the number of moving events (Figure 3H). The jkk-1 single mutants showed no significant difference from wild-type animals (Figures 3E–3H).
Figure 3. Inactivation of the JNK Pathway Represses Excessive STV aggregation in arl-8 Mutants.
(A) Diagram of the imaging area in the ventral axon (green box). (B–E) Representative GFP::RAB-3 kymographs in the ventral axon of wild type (B), arl-8(wy271) (C), arl-8(wy271); jkk-1(km2) (D) and jkk-1(km2) (E) animals (wyIs251). (F) Quantification of the number of stable GFP::RAB-3 puncta in the ventral axon. Normalized to the length of the imaged axonal segment. n=29–31 animals. (G) Quantification of the fluorescence intensity of stable puncta in the ventral axon. n=68–142 puncta. (H) Quantification of the number of moving events in the ventral axon, normalized to imaging duration and length of the imaged axon. n=32–44 animals. (I) Diagram and kymograph showing a dissoication event (green arrow) and quantification of the dissociation rate at stable puncta in the ventral axon. n=39–106 stable puncta. (J) Diagram and kymograph showing a mobile packet bypassing (green arrowhead) or captured (green arrow) by a stable punctum and quantification of the capture probability in the ventral axon. n=39–65 stable puncta. “wt intensity”, puncta of fluorescence intensity within the wild-type range; “bright”, puncta with intensities greater than that of the brightest wild type punctum. (K) Diagram of the imaging area in the dorsal synaptic region (blue box). (L) Quantification of the dissociation rate at mature synapses. n=65–131 stable puncta. (M) Quantification of the capture probability at mature synapses. n=64–99 stable puncta. Statistical tests for all panels are t tests. *** p<0.0001, **p<0.001. Error bars, SEM.
Figure 4. STVs and AZ Proteins Are Co-transported.
(A) Diagram of the imaging area in the dorsal axon (green box). (B–D) Representative kymographs of mCherry::RAB-3 (B), UNC-10::GFP (C) and double labeling (D) in wild type DA9. Arrows and arrowheads point to examples of moving mCherry::RAB-3 puncta associated or not associated with UNC-10::GFP, respectively (wyIs301). (E) Quantification of normalized number of mCherry::RAB-3 and UNC-10::GFP moving events. *** p<0.0001, t test, n=37 animals. Error bars, SEM. (F) Percentage of anterograde and retrograde movements of mCherry::RAB-3 and UNC-10::GFP. Chi-square test, n=214–936 moving events. (G–H) Percentage of mobile (G) or stationary (H) mCherry::RAB-3 particles associated with UNC-10::GFP and percentage of mobile (G) or stationary (H) UNC-10::GFP particles associated with mCherry::RAB-3. See also Figures S5.
The coexistence of stable and motile puncta is consistent with previous findings that STV undergo intermittent moving and stationary phases en route to the presynaptic terminals (Ahmari et al., 2000; Sabo et al., 2006). The transitions between these two states might serve as regulated switches to control the trafficking and aggregation of STVs. We observed two types of interactions between the motile and stable puncta: some motile puncta coalesce with existing stable puncta, whereas stable puncta can shed a fraction of their content to generate motile packets (Figures 3I and 3J). To better understand how these transitions are regulated, we quantified the frequency that moving particles dissociate from a stable punctum (dissociation rate) and the probability that mobile particles stop moving and cluster with a stable punctum (capture probability). We separated the arl-8 puncta into two groups: arl-8 puncta of fluorescence intensity within the wild-type range (wt intensity) and those with intensity values greater than the wild-type maximum (bright). The normal sized arl-8 stable puncta showed a significantly lower dissociation rate compared to wild type stable puncta, whereas the abnormally bright arl-8 stable puncta exhibited a significant increase in capture probability (Klassen et al., 2010; Figures 3I and 3J). The initial reduction in STV dissociation from small stable puncta and subsequent increase in STV capture at large stable puncta likely underlie the strong perturbation in presynaptic protein distribution in arl-8 mutants. In contrast, the stable puncta in arl-8; jkk-1 double mutants exhibited a significantly higher dissociation rate compared to arl-8 mutant puncta with similar intensity (Figure 3I). In addition, the capture probability of arl-8; jkk-1 stable puncta is comparable to that of the wild-type stable puncta and significantly lower than that of the abnormally bright arl-8 puncta (Figure 3J).
The above measurements were performed in the axon shaft where no mature synapses are found in wild-type animals. To investigate whether the same molecular regulation of trafficking and local assembly also takes place at mature synapses, we analyzed RAB-3 clusters in the dorsal presynaptic region (Figure 3K). Compared to wild-type clusters of similar intensity, the arl-8 mutant clusters exhibited a significantly lower dissociation rate (Figure 3L) and a significantly higher capture probability (Figure 3M), suggesting that arl-8 also promotes STV dissociation and inhibits STV capture at mature synapses. The jkk-1 mutation strongly suppressed the decrease in dissociation rate in arl-8 mutants (Figure 3L), without significantly affecting the capture probability (Figure 3M). Together, these findings suggest that loss of JKK-1 prevents excessive STV clustering in arl-8 mutants, likely by promoting dissociation of STVs from stationary clusters. This model whereby arl-8 and jkk-1 antagonistically regulate the dissociation of STVs is consistent with the changes in the cell-wide distribution of SV proteins in arl-8, jkk-1 and arl-8; jkk-1 mutants.
AZ Proteins and STVs Are Co-Transported
AZ proteins are thought to be transported in dense core vesicles, a vesicle population distinct from STVs (Zhai et al., 2001; Shapira et al., 2003; Maas et al., 2012), raising the question of how the transport of SV and AZ proteins can both be regulated by ARL-8 and JNK. To address this question, we performed two-color time-lapse imaging for UNC-10/RIM1::GFP and mCherry::RAB-3 in DA9. In wild-type animals, UNC-10::GFP movements are much less frequent than those of mCherry::RAB-3 (Figures 4B–4E), possibly reflecting a lower turnover rate of UNC-10 compared to SVs. However, we observed extensive association of the two markers during transport; 95.5% mobile UNC-10::GFP puncta also contained mCherry::RAB-3 and 22.9% mobile mCherry::RAB-3 puncta also contained UNC-10::GFP (Figure 4G). To determine whether this feature is unique to UNC-10::GFP, we also examined other AZ proteins, including GFP-tagged SYD-2/Liprin-α and the serine-threonine kinase SAD-1. The movements of these markers are even rarer than those of UNC-10::GFP, possibly due to their lower copy number on the trafficking packets and/or lower turnover rates. Nonetheless, when SYD-2/Liprin-α or SAD-1 movements were detected, association with mCherry::RAB-3 was also observed (Figures S5A–S5F). Furthermore, we also observed association of trafficking UNC-10::GFP with an integral SV protein, synaptogyrin (Figures S5G–S5I). These observations are consistent with previous Immuno EM and live imaging studies in cultured neurons (Tao-Cheng, 2007; Bury and Sabo, 2011).
Consistent with the model of STV/AZ co-transport, the ratio between anterograde and retrograde movements is similar for UNC-10::GFP and mCherry::RAB-3 (Figure 4F). In addition, the anterograde transport of STVs and several AZ proteins in DA9 are both dependent on UNC-104/KIF1A (Klassen et al., 2010). Together, our dynamic imaging analyses provide direct in vivo evidence that AZ proteins and STVs can be pre-assembled into transport complexes, providing a mechanism for the co-regulation of their axonal delivery. Therefore, the excessive aggregation of STVs in arl-8 mutants is likely accompanied by premature clustering of associated AZ proteins, resulting in defects in both STV and AZ protein distribution, which in turn can be simultaneously suppressed by JNK inactivation.
AZ Assembly Proteins Promote STV Aggregation during Axonal Transport
Several AZ molecules are critical for SV recruitment at presynaptic terminals (Jin and Garner, 2008; Owald and Sigrist, 2009). Loss-of-function mutations in the AZ molecules syd-2/liprin-α, syd-1 and sad-1 lead to a dramatic reduction in presynaptic SV cluster size and dispersal of SV clusters throughout the DA9 axon, and strongly suppress the enlarged size of SV clusters in arl-8 mutants (Klassen et al., 2010; Figures 6C–6I). Furthermore, we noticed that the stationary UNC-10::GFP and mCherry::RAB-3 puncta in the proximal axon showed almost complete co-localization, as evident by the double labeled vertical stripes in the kymographs (Figures 4B–4D and 4H), indicating that sites of AZ protein pause correspond to locations of STV aggregation during transport. These results, together with the observation that AZ proteins are co-trafficked with STVs, raise the possibility that AZ molecules may also promote STV clustering during trafficking, thus impacting STV axonal transport. To directly test this possibility, we performed time-lapse imaging of GFP::RAB-3 in syd-2 null mutants. Consistent with our hypothesis, we found the dissociation rate for stable GFP::RAB-3 puncta in the axon shaft in syd-2 mutants is significantly higher compared to wild-type stable puncta with similar intensity (Figure 5E). Accordingly, the number of moving events in syd-2 mutants is increased, reflecting an increase in STV motility (Figure 5G). In contrast, the capture probability was not affected in syd-2 mutants (Figure 5F). Similarly, for RAB-3 clusters at the mature synapses in the dorsal presynaptic region, the syd-2 mutation significantly increased the dissociation rate (Figure 5I), without significantly affecting the capture probability (Figure 5J). These results indicate that SYD-2 prevents the dispersion of mobile STV packets from anchored STV/AZ complexes. SYD-2 is also known to promote the clustering of several other AZ proteins at the presynaptic terminals (Patel et al., 2006), we therefore asked whether SYD-2 also promotes the association between other AZ proteins and STVs during transport. Indeed, in syd-2 mutants we found a moderate but significant decrease in the ratio of moving RAB-3 clusters associated with UNC-10 (17.8% in syd-2(wy5) vs. 22.9% in wild type, p<0.05, chi-square test, n=936–1212 moving events).
Figure 6. STV/AZ Association during Transport Is Antagonistically Regulated by ARL-8 and JNK.
(A) Quantification of normalized number of mCherry::RAB-3 and UNC-10::GFP moving events in different mutants. wyIs301. n=32–45 animals. (B) Percentage of mobile mCherry::RAB-3 particles associated with UNC-10::GFP in different mutants. n=340–936 mCherry::RAB-3 moving events. (C–F) Confocal images of 20 straightened and aligned wild type (C), arl-8(wy271) (D), arl-8(wy271); syd-2(wy5) (E) and arl-8(wy271), jnk-1(gk7); syd-2(wy5) (F) DA9 dorsal axons expressing SNB-1::YFP (wyIs92). Scale bar, 10 μm. (G) Mean SNB-1::YFP fluorescence intensity as a function of distance from the commissure. (H) Quantification of SNB-1::YFP puncta size along DA9 dorsal axons, binned into 25 μm segments. (I) Quantification of SNB-1::YFP puncta number in DA9 dorsal axons. Statistical tests for all panels are t tests. For panels A and B, ***p<0.0001, **p<0.05, *p<0.1. For panel I, ***p<0.001. Error bars, SEM. See also Figures S6.
Figure 5. AZ Proteins Promote STV Clustering during Transport.
(A–D) Representative GFP::RAB-3 kymographs in wild type (A), syd-2(wy5) (B), arl-8(wy271) (C) and arl-8(wy271); syd-2(wy5) (D) DA9 ventral axons (wyIs251). (E–F) Quantification of the dissociation rate (E) and the capture probability (F) in the ventral axon. n=35–106 stable puncta. (G) Quantification of normalized number of GFP::RAB-3 moving events. n=30–44 animals. (H) Quantification of the fluorescence intensity of stable GFP::RAB-3 puncta. n=78–142 puncta. (I–J) Quantification of the dissociation rate (I) and the capture probability (J) at mature synapses. n=67–76 stable puncta. Statistical tests for all panels are t tests. ***p<0.0001, **p<0.01, *p<0.05. Error bars, SEM.
We further carried out time-lapse imaging in arl-8; syd-2 double mutants to better understand how the syd-2 mutation suppresses the arl-8 STV aggregation phenotype. As in syd-2 single mutants, stable puncta in arl-8; syd-2 double mutants exhibited an increased dissociation rate compared to those in arl-8 mutants (Figure 5E). Accordingly, there is a significant increase in the number of moving events and a decrease in the fluorescence intensity of stable puncta en route (Figures 5D, 5G and 5H). On the other hand, syd-2 did not affect the STV capture probability in arl-8 mutants (Figure 5F). Together, our data provide direct evidence that AZ proteins promote STV clustering during axonal transport and raise the possibility that ARL-8 and JNK might control STV aggregation via regulation of STV/AZ interaction during transport.
ARL-8 and the JNK Pathway Antagonistically Control STV/AZ Association during Axonal Transport
To determine whether ARL-8 and the JNK pathway regulate STV/AZ association during axonal transport, we performed two-color time-lapse imaging for UNC-10::GFP and mCherry::RAB-3 in the arl-8 and jkk-1 single and double mutants. As in wild-type animals, mobile UNC-10 clusters exhibited a high degree of association with RAB-3 during trafficking in these mutants (97/107, 106/114 and 196/206 mobile UNC-10 clusters associate with RAB-3 in arl-8, arl-8; jkk-1 and jkk-1 mutants, respectively) and the stable clusters also almost completely colocalized (data not shown). However, compared to wild-type animals, we found an increase in the proportion of moving RAB-3 puncta that carry UNC-10 in arl-8 mutants (Figure 6B), suggestive of increased STV/AZ association during transport. Since AZ proteins promote STV clustering during trafficking, the enhanced STV/AZ association may contribute to excessive STV aggregation in arl-8 mutants. This increased STV/AZ association was suppressed in arl-8; jkk-1 double mutants (Figure 6B), consistent with the hypothesis that ARL-8 and the JNK pathway may control STV aggregation during transport in part by regulating STV/AZ association. Further supporting this hypothesis, although the jnk-1 mutation led to strong suppression in arl-8 single mutants, in arl-8; syd-2 double mutants, in which AZ function is already severely defective, the same mutation only produced a subtle effect (Figures 6C–6I).
In light of these findings, we further examined whether ARL-8 and JNK-1 associate with STVs and/or AZ proteins during transport. Previous studies suggested that ARL-8 associates with SVs (Takamori et al., 2006; Klassen et al., 2010). Indeed, we observed that moving GFP::RAB-3 and UNC-10::GFP particles frequently associate with ARL-8::mCherry (Figures S6A–S6F) in the axon shaft. Notably, the stationary ARL-8 puncta also colocalized extensively with RAB-3 or UNC-10 (Figure S6A–S6F, vertical lines in the kymographs).
Dynamic imaging analyses showed that JNK-1 was also actively transported in the axon with pauses en route (Figures S6H and S6K). Interestingly, the majority of moving RAB-3 or UNC-10 packets were not associated with mobile JNK-1 puncta (Figures S6G–S6L). However, the stationary JNK-1 puncta still largely colocalized with the stationary RAB-3 and UNC-10 puncta (Figure S6G–S6L, vertical lines in the kymographs). Therefore, although JNK-1 and the STVs do not move together, they do pause at the same loci. Taken together, the colocalization of STVs, AZ proteins, ARL-8 and JNK-1 at common pause sites along the axon supports the notion that these sites represent regulatory points where ARL-8 and the JNK pathway control the switch between STV trafficking and aggregation.
Regulation of UNC-104/KIF1A Motor Activity Controls STV Capture Efficiency and Synapse Distribution
Presynaptic proteins are transported to the synapses by molecular motors (Goldstein et al., 2008; Hirokawa et al., 2010). Regulation of motor activity may determine where presynaptic cargoes are deposited, thereby impacting synapse distribution. We previously found that overexpression of the kinesin motor UNC-104/KIF1A in DA9 strongly suppressed the arl-8 phenotype (Klassen et al., 2010). In our arl-8 suppressor screen, we further isolated a putative gain-of-function (gf) allele of unc-104, which suppressed the STV and AZ localization defects in arl-8 mutants (Figures 7A–7C and data not shown). We identified the molecular lesion as a G-to-R missense mutation at a highly conserved amino acid (Figure S7A). A mutation in the corresponding residue in human KIF1A (G631) disrupts inhibitory intramolecular interactions between the FHA and CC domains, resulting in increased KIF1A activity (Lee et al., 2004). These results raised the possibility that fine-tuning of motor activity may also serve as a mechanism to regulate STV clustering. Indeed, time-lapse imaging revealed that unc-104(gf) significantly reduced the capture probability in the axon shaft in both wild type and arl-8 mutant animals (Figure 7K). Interestingly, unlike the jkk-1 and syd-2 mutations, unc-104(gf) did not affect the dissociation rate (Figure 7L). Similarly, for RAB-3 clusters at the mature synapses, unc-104(gf) significantly reduced the capture probability (Figure 7M), with no significant effect on the dissociation rate (Figure 7N). Thus, control of UNC-104/KIF1A motor activity likely represents a mechanism that regulates STV capture. Consistent with this hypothesis, unc-104(gf) single mutants exhibited decreased presynaptic SNB-1::YFP puncta size (Figures 7D and 7O). Together, these findings suggest that both STV exit from and entry into stable clusters are subject to molecular regulation. While the exit process is regulated by ARL-8, the JNK-1 pathway and SYD-2, the entry process is controlled by ARL-8 and UNC-104/KIF1A. The fact that the abnormal distribution of presynaptic components in arl-8 mutants can be partially suppressed by either an increase in dissociation caused by the jkk-1 and syd-2 mutations or an decrease in capture efficiency caused by the unc-104(gf) mutation argues that it is the balance between the entry and exit processes that controls the position and size of presynaptic protein clusters. This model predicts that perturbation of both STV entry and exit may produce a stronger phenotype compared to manipulation of a single factor. Indeed, unc-104(gf); jkk-1 double mutants, in which capture is decreased and dissociation increased, showed enhanced reduction in the size of presynaptic SNB-1::YFP puncta compared to either single mutants (Figures 7A, 7D, 7E and 7O), supporting the notion that unc-104 and jkk-1 function in parallel to modulate STV clustering. Conversely, we built double mutants between two partial loss-of-function alleles, arl-8(tm2388) and unc-104(lf) (Figure S7A), in which capture is increased and dissociation decreased. While the single mutants showed much weaker phenotypes than their respective strong loss-of-function mutants (Figures 7G, 7H and 7P), the double mutants showed a strongly enhanced phenotype, with large RAB-3 puncta forming in the ventral axon and commissural regions (Figures 7I and 7P).
Figure 7. UNC-104/KIF1A Activity Regulates STV Capture Probability and Synapse Distribution.
(A–E) Confocal images of 20 straightened and aligned wild type (A), arl-8(wy271) (B), unc-104(gf); arl-8(wy271) (C), unc-104(gf) (D) and unc-104(gf); jkk-1(km2) (E) DA9 dorsal axons expressing SNB-1::YFP (wyIs92). (F–J) Confocal images of 20 wild type (F), arl-8(tm2388) (G), unc-104(lf) (H), unc-104(lf); arl-8(tm2388) (I) and arl-8(tm2388); Ex[mig-13::CC3-PH] (J) DA9 axons expressing GFP::RAB-3. The axons are straightened and aligned starting from cell body to show ectopic puncta in the ventral axon and commissure (wyIs85). (K–L) Quantification of the capture probability (K) and the dissociation rate (L) in the ventral axon in wild type, arl-8(wy271), unc-104(gf) and unc-104(gf); arl-8(wy271) animals. n=25–106 stable puncta. (M–N) Quantification of the capture probability (M) and the dissociation rate (N) at mature synapses in wild type and unc-104(gf) animals. (O) Quantification of presynaptic SNB-1::YFP puncta size in DA9 dorsal axons of indicated genotypes. n=463–616 puncta. (P) Quantification of GFP::RAB-3 fluorescence intensity in the micrographs shown in (F–J) as a function of distance. Statistical tests for all panels are t tests. ****p<0.0001, ***p<0.01, **p<0.05, *p<0.1. Error bars, SEM. See also Figure S7 and S8.
UNC-104/KIF1A Functions as an Effector of ARL-8 in Regulating Synapse Distribution
The downstream functions of small G proteins are mediated by effector proteins that bind specifically to the GTP-bound form of the G proteins (Donaldson and Jackson, 2011). The strong genetic interaction between arl-8 and unc-104 led us to test whether UNC-104/KIF1A is an ARL-8 effector. We first performed affinity chromatography with glutathione S-transferase (GST)-tagged human ARL8A and various GFP-tagged human KIF1A fragments (Figure 8A; CC1-FHA-CC2, 430–694 aa; CC3-UDR, 694–1209 aa; CC3, 694–775 aa; UDR, 776–1209 aa). We observed specific binding of the KIF1A CC3 domain to GST-ARL8A Q75L (GTP-locked, constitutively active) and to a less extent to wild-type ARL8A, and little binding to ARL8A T34N (GDP-locked, inactive) or GST alone (Figure 8B). Although the function of the CC3 domain is unknown, it is highly conserved between C. elegans and human (Figure S7B). We further confirmed that the binding of wild-type ARL8A to the KIF1A CC3 domain is dependent on GTP binding (Figure 8C). Similar results were also obtained for C. elegans ARL-8 and the UNC-104 CC3 domain (Figure 8D). Furthermore, in yeast two-hybrid assays, we detected interaction of the CC3 domain with ARL8A Q75L but not with ARL8A T34N (Figure 8E). Together, these results suggest that ARL-8/ARL8A physically interacts with the CC3 domain of UNC-104/KIF1A in a GTP-dependent manner.
Figure 8. UNC-104/KIF1A is an ARL-8 Effector in Regulating Synapse Distribution.
(A) Diagram of the domain structure of UNC-104/KIF1A. MD, motor domain; NC, neck coil; CC, coiled-coil; FHA, forkhead-associated domain; UDR, undefined region; PH, pleckstrin homology domain. (B) Left panel, GST affinity chromatography for interactions between GST, GST-ARL8A, GST-ARL8A Q75L, or GST-ARL8A T34N with various KIF1A fragments tagged with GFP. Right panel, Coomassie-blue stained gel indicating the level of GST and GST fusion proteins on the beads. (C–D) Left panels, GST affinity chromatography for the interaction between GST-ARL8A and the KIF1A CC3 domain (C), or between GST-ARL-8 and the UNC-104 CC3 domain (D), in the presence of GTP or GDP. Right panels, Coomassie-blue stained gels indicating the level of GST and GST fusion proteins on the beads. (E) Yeast two-hybrid assays for interactions between ARL8A Q75L or ARL8A T34N with the KIF1A CC3 domain. See also Figure S7 and S8.
If the interaction between ARL-8 and the CC3 domain is of functional importance, one prediction would be that overexpression of the UNC-104 CC3 domain may cause a dominant-negative effect and phenocopy the arl-8 mutants by competing with endogenous UNC-104/KIF1A for ARL-8 binding. We therefore overexpressed a C-terminal fragment of UNC-104 containing the CC3, UDR and PH domains in DA9. We included the PH domain as it is known to be required for SV binding (Klopfenstein et al., 2002). Indeed, when over-expressed in the wild-type background, this fragment caused an arl-8-like phenotype (Figures S7C–S7E), leading to a proximal shift of SNB-1::YFP signal. In addition, when over-expressed in the arl-8(tm2388) weak loss-of-function mutants, this fragment caused a strong enhancement of the phenotype (Figures 7J and 7P). The CC3 domain is required for generating this dominant-negative effect as a fragment containing only the UDR and PH domains did not cause any effect (data not shown). Together, these results suggest that UNC-104 functions as a downstream effector of ARL-8 in regulating synapse distribution.
Collectively, our findings provided new insights into how the axonal transport and local assembly of STVs and AZ proteins are coordinated to achieve the proper size, number and location of synapses (Figure S8): In wild-type animals, AZ proteins are associated with STVs during motor-driven axonal transport. STVs undergo frequent stops en route and form immotile STV/AZ clusters due to their intrinsic propensity of aggregation. Trafficking STV packets can cluster with the existing stable puncta, while the stable puncta can also shed a fraction of their content to generate mobile packets. At the pause sites, AZ assembly molecules and JNK promote STV aggregation by limiting dissociation of mobile STVs from the stable clusters, whereas ARL-8 inhibits excessive STV aggregation by promoting STV dissociation. On the other hand, ARL-8 and UNC-104/KIF1A negatively regulate the coalescence of mobile STVs with stable aggregates. In addition, UNC-104/KIF1A functions as an effector of ARL-8 by binding to the GTP-bound form of ARL-8. Together, these findings reveal a molecular network that regulates the balance between presynaptic protein transport and assembly to control the spatial distribution of presynaptic components.
DISCUSSION
Precise control of the size, number and location of synapses requires regulated distribution of SVs and AZ proteins, a process that is achieved through coordinated transport and assembly of presynaptic material. We identify novel molecular mechanisms that control the balance between presynaptic protein transport and assembly. We show that a JNK MAP kinase pathway and the small G protein ARL-8 antagonistically control a switch between aggregation and trafficking for STVs and AZ proteins, thereby determining their distribution. Interestingly, AZ proteins extensively associate with STVs and promote their aggregation at pause sites during transport. In addition, the anterograde motor UNC-104/KIF1A functions as an effector of ARL-8 and acts in parallel to JNK to control STV capture and synapse distribution. It is conceivable that the trafficking state of presynaptic proteins is favored in the proximal axon to facilitate efficient axonal transport, whereas aggregation prevails at sites of synaptogenesis due to the enhancement in pro-assembly forces and/or inhibition of anti-assembly mechanisms.
The JNK Pathway Represents a Novel Mechanism for Regulating Presynaptic Patterning
The evolutionarily conserved JNKs have been implicated in many critical processes including immunity, stress responses and tumorigenesis (Davis, 2000). In the vertebrate nervous system, JNKs have been involved in stress-induced cell death (Bozyczko-Coyne et al., 2002), regulation of motor binding to microtubules and cargoes (Morfini et al., 2006; Stagi et al., 2006; Horiuchi et al., 2007; Morfini et al., 2009), microtubule dynamics, commissure tract formation and optic fissure closure (Chang et al., 2003; Weston et al., 2003). In C. elegans, JKK-1 and JNK-1, homologs of mammalian MKK7 and JNK3, respectively, are required in the nervous system for coordinated locomotion (Kawasaki et al., 1999). JKK-1 and JNK-1 interact with the scaffold protein UNC-16/JIP3, an adaptor for the UNC-116/KIF5 motor (Byrd et al., 2001). Here we report a novel function of the JNK pathway in promoting presynaptic protein assembly. Inactivation of this pathway strongly suppressed the abnormal synapse distribution in arl-8 mutants. Live imaging revealed that a jkk-1 mutation promotes a trafficking identity for STVs by increasing their dissociation from immotile clusters during transport in arl-8 mutants. In addition, jkk-1 and jnk-1 single mutants also exhibited reduced SV and AZ protein clustering at presynaptic sites.
The C. elegans genome encodes a number of MAP kinases (Sakaguchi, 2004). Of note, the ubiquitin ligase RPM-1 was previously shown to inhibit a DLK-1/p38 MAP kinase pathway to regulate presynaptic development in the DD neurons (Schaefer et al., 2000; Zhen et al., 2000; Nakata et al., 2005). The Drosophila homolog of RPM-1, Highwire, restricts synapse number and size by attenuating a DLK/JNK MAP kinase pathway (Collins et al., 2006). We detected no suppression of arl-8 by dlk-1 mutations (data not shown). In addition, while mutations in the rpm-1 pathway produced dramatic effects on presynaptic organization in the DD motoneurons, they did not cause obvious presynaptic abnormalities in DA9 (data not shown). In contrast, arl-8/jkk-1 interaction strongly impacts synapse distribution in DDs, DA9, as well as multiple other neuronal types. Therefore, there is so far no data supporting a genetic interaction between the arl-8/jkk-1 and the rpm-1/dlk-1 pathway.
Two effectors of ARL8 in regulating lysosomal trafficking were recently identified, including the HOPS complex and the SKIP protein that links lysosomes to the KIF5 motor complex (Garg et al., 2011; Rosa-Ferreira and Munro, 2011). We have investigated their potential involvement in presynaptic development. Firstly, human ARL8B was reported to recruit the HOPS complex to direct lysosomal trafficking (Garg et al., 2011). We examined the phenotypes of deletion mutants in two of the core subunits of the HOPS complex in C. elegans, VPS-16 (ok719) and VPS-18 (tm1125), and an accessory subunit, VPS-39 (ok2442). Despite their lysosomal trafficking and/or lethality phenotypes (Hermann et al., 2005; Kinchen et al., 2008; Xiao et al., 2009), all three mutants appear normal in presynaptic development in DA9 (data not shown). Secondly, the human SKIP protein was proposed to bind to lysosome-localized ARL8B and the kinesin light chain, thus linking lysosomes to the KIF5 motor complex (Rosa-Ferreira and Munro, 2011). Knockdown of ARL8B, SKIP, or KIF5B generated similar changes in lysosome distribution in cultured cells. In the C. elegans genome, the gene Y51H1A.2 encodes the only protein sharing limited sequence homology with SKIP. However, a deletion mutation in this gene (K. Kontani, personal communication) did not cause abnormal SV protein localization (data not shown). In addition, loss-of-function mutations in klc-1 and klc-2, which encode the only two kinesin light chains in C. elegans, did not phenocopy arl-8 in DA9, nor did they enhance or suppress the arl-8 phenotype (data not shown). Furthermore, loss of function in UNC-116/KIF5 did not cause an arl-8-like presynaptic phenotype in DA9 either (data not shown). Collectively, these findings suggest that the JNK pathway represents a novel mechanism that strongly interacts with arl-8 in regulating presynaptic patterning.
AZ Proteins Are Co-trafficked with STVs and Promote STV Clustering during Axonal Transport
It has been suggested that SV and AZ proteins are sorted into different vesicular cargoes at the Golgi. While SV proteins are transported in STVs (Matteoli et al., 1992; Ahmari et al., 2000; Tao-Cheng, 2007), the PTVs are thought to carry AZ material in vertebrate neurons (Zhai et al., 2001; Shapira et al., 2003; Maas et al., 2012). Interestingly, live imaging combined with retrospective EM analysis revealed that STVs are in proximity to dense core vesicles in the axon of cultured neurons (Ahmari, 2000). Immuno EM analyses using antibodies against the AZ proteins Piccolo and Basson and a number of SV proteins further provided ultrastructure evidence that various AZ and SV proteins may be preassembled into multi-vesicle transport aggregates (Tao-Cheng, 2007). In addition, two-color live imaging in cultured neurons also revealed that a proportion of STVs and PTVs are co-transported (Bury and Sabo, 2011). Consistent with these findings, we found extensive association between AZ proteins and STVs during transport in vivo. The association of various presynaptic components prior to synapse formation provides a mechanism for the co-regulation of their axonal transport and assembly, explaining the high degree of colocalization even in the absence of synaptic patterning cues and how the same molecular pathways regulate the distribution of both AZ and SV proteins.
Dynamic imaging analyses of STVs, AZ markers, ARL-8 and JNK-1 showed that all of them exhibited saltatory movements and largely shared identical pause sites during transport. These pause sites appear to represent regulatory points where the switch between the trafficking and aggregation states for STVs is controlled. Trafficking STV packets can stop moving and cluster with the existing stable puncta, potentially through interaction between presynaptic cargoes. The stable puncta can also shed motile packets. The balance between trafficking and aggregation is critically dependent on arl-8 and the JNK pathway. Interestingly, the AZ assembly proteins not only promote SV clustering at the presynaptic terminals, but also prevent STV dissociation from stable clusters en route. Furthermore, AZ/STV association during transport is antagonistically regulated by arl-8 and JNK. Together, these data are consistent with a model in which arl-8 and the JNK pathway control STV aggregation and trafficking by modulating STV/AZ interaction during transport. Interestingly, it has been shown that in cultured vertebrate neurons, synapses form preferentially at predefined STV pause sites upon axodendritic contacts (Sabo et al., 2006). Therefore, regulation of the balance between STV capture and dissociation at the pause sites may represent a general mechanism to control the distribution of presynaptic components.
UNC-104/KIF1A is an ARL-8 Effector in Regulating Synapse Distribution
SV and AZ components are delivered to the presynapses by motor proteins (Goldstein et al., 2008; Hirokawa et al., 2010). At the synapses, the motors may need to be inactivated in order to unload their cargoes. Therefore, regulation of motor activity may dictate where presynaptic cargoes are deposited, thereby determining the spatial pattern of synapses. As a critical motor for the axonal transport of presynaptic proteins, UNC-104/KIF1A is under several levels of intricate regulation. It is activated by phospholipid binding and dimerization (Hall and Hedgecock, 1991; Klopfenstein et al., 2002; Tomishige et al., 2002). In addition, intramolecular interactions between the NC and CC1 domains, FHA and CC2 domains, or FHA and CC1 domains have been shown to modulate UNC-104/KIF1A activity (Al-Bassam et al., 2003; Lee et al., 2004; Huo et al., 2012). Besides intramolecular regulation, the protein DENN/MADD has been identified as an adaptor between SVs and KIF1A (Niwa et al., 2008). SYD-2/Liprin-α has also been suggested to promote the clustering of monomeric UNC-104/KIF1A, thus enhancing its activity (Wagner et al., 2009). Here we show that ARL-8 likely represents a novel mechanism for UNC-104/KIF1A regulation. The GTP-bound form of ARL-8/ARL8A, but not the GDP-bound form, binds specifically to the CC3 domain of UNC-104/KIF1A. Overexpression of the UNC-104 CC3 domain phenocopies the arl-8 mutant in a wild-type background, and enhances the phenotype in a weak loss-of-function arl-8 mutant. Furthermore, overexpression of wild-type UNC-104 or a gain-of-function mutation in unc-104 partially and strongly suppressed the phenotype in arl-8 mutants. Dynamic imaging revealed that this gain-of-function mutation decreases the capture of mobile STV packets by stable clusters whereas the arl-8 mutation leads to increased capture. Conversely, a weak loss-of-function mutation in unc-104 strongly enhances the phenotype in weak loss-of-function arl-8 mutants. Together, these findings identify UNC-104/KIF1A as the first ARL-8 effector in regulating synapse distribution. The conformational changes in small G proteins triggered by GTP/GDP binding might serve as switches to control motor-cargo association, motor processivity, and/or motor binding to microtubules.
Collectively, our findings underlie an intimate link between transport regulation and the spatial patterning of synapses. We also uncovered molecular players that control the stop-go transitions for presynaptic cargoes to achieve appropriate synapse distribution. Interestingly, a recent study suggests that the even distribution of dense core vesicles among synaptic boutons at the Drosophila neuromuscular junction is also achieved by coordinating cargo transport and capture (Wong et al., 2012). Similar cellular strategies might also be utilized to achieve proper distribution of other cargoes, such as lysosomes, mitochondria and neurotransmitter receptors.
EXPERIMENTAL PROCEDURES
Strains and Genetics
Worms were raised on OP50 E. coli seeded NGM plates at 20°C, excepting for the dynamic imaging experiments as detailed below. The mutant strains CZ5730 dlk-1(ju476)I, VC548 vps-16(ok719)III/hT2[bli-4(e937) let-?(q782) qIs48](I;III), VC8 jnk-1(gk7)IV, RB1975 klc-1(ok2609)IV, VC2542 vps-39(ok2442)V/nT1[qIs51](IV;V), and KU2 jkk-1(km2)X were obtained through the Caenorhabditis Genetics Center. wyIs292III (Punc-47::unc-10::tdTomato, Punc-129dorsal muscle::nlg-1::yfp) was kindly provided by G. Maro, klc-2(km11)V by K. Matsumoto, and krIs1V (Punc-47::snb-1::cfp, unc-49::YFP) by J. Bessereau. N2 Bristol was utilized as the wild-type reference strain.
Cloning and Constructs
Expression clones were made in the pSM vector, a derivative of pPD49.26 (A. Fire) with extra cloning sites (S. McCarroll and C. I. Bargmann, personal communication). The following plasmids and strains were generated as previously described: wyIs85 (Pitr-1 pB::gfp::rab-3), wyIs92 (Pmig-13::snb-1::yfp), wyIs109 (Pmig-13::cfp::rab-3, Pmig-13::snb-1::yfp, Pmig-13::sng-1::mcherry), wyEx771 (Pmig-13::gfp::ccb-1, Pmig-13::mCherry::rab-3), wyEx1199 (Punc-129DB neuron::gfp::rab-3). Other transgenic strains were generated using standard techniques as detailed in Supplemental Experimental Procedures.
Other methods are detailed in Supplemental Experimental Procedures.
Supplementary Material
Acknowledgments
This work was supported by the Howard Hughes Medical Institute, the National Institutes of Health NIH 5R01 NS048392, the National Major Basic Research Program of China (2011CB910503), and the National Natural Science Foundation of China (31070657 and 31190062). We thank the Caenorhabditis Genetics Center, the National Bioresource Project (Japan), G. Maro, P. Kurshan, and the labs of J. Bessereau, K. Kontani, S. Mitani, Y. Jin, and S. Munro for strains and reagents. We also thank S. Shaham for help with the analysis of the whole-genome sequencing data, C. Gao and Y. Fu for technical assistance, and A. McAllister, M. Klassen, and P. Kushan for thoughtful comments on the manuscript. Y. W. and K. S. designed experiments and wrote the paper. C. M. built some transgenes for dynamic imaging. L. H. and W. F. performed the experiments in Figure 8. Y. W. performed the other experiments and analyzed the data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmari SE, Buchanan J, Smith SJ. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat Neurosci. 2000;3:445–451. doi: 10.1038/74814. [DOI] [PubMed] [Google Scholar]
- Al-Bassam J, Cui Y, Klopfenstein D, Carragher BO, Vale RD, Milligan RA. Distinct conformations of the kinesin Unc104 neck regulate a monomer to dimer motor transition. The Journal of Cell Biology. 2003;163:743–753. doi: 10.1083/jcb.200308020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood HL, Karunanithi S. Diversification of synaptic strength: presynaptic elements. Nat Rev Neurosci. 2002;3:497–516. doi: 10.1038/nrn876. [DOI] [PubMed] [Google Scholar]
- Bozyczko-Coyne D, Saporito MS, Hudkins RL. Targeting the JNK pathway for therapeutic benefit in CNS disease. Curr Drug Targets CNS Neurol Disord. 2002;1:31–49. doi: 10.2174/1568007023339472. [DOI] [PubMed] [Google Scholar]
- Bury LAD, Sabo SL. Coordinated trafficking of synaptic vesicle and active zone proteins prior to synapse formation. Neural Dev. 2011;6:24. doi: 10.1186/1749-8104-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd DT, Kawasaki M, Walcoff M, Hisamoto N, Matsumoto K, Jin Y. UNC-16, a JNK-signaling scaffold protein, regulates vesicle transport in C. elegans. Neuron. 2001;32:787–800. doi: 10.1016/s0896-6273(01)00532-3. [DOI] [PubMed] [Google Scholar]
- Chang L, Jones Y, Ellisman MH, Goldstein LSB, Karin M. JNK1 is required for maintenance of neuronal microtubules and controls phosphorylation of microtubule-associated proteins. Developmental Cell. 2003;4:521–533. doi: 10.1016/s1534-5807(03)00094-7. [DOI] [PubMed] [Google Scholar]
- Collins CA, Wairkar YP, Johnson SL, DiAntonio A. Highwire restrains synaptic growth by attenuating a MAP kinase signal. Neuron. 2006;51:57–69. doi: 10.1016/j.neuron.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Dai Z, Peng HB. Dynamics of synaptic vesicles in cultured spinal cord neurons in relationship to synaptogenesis. Mol Cell Neurosci. 1996;7:443–452. doi: 10.1006/mcne.1996.0032. [DOI] [PubMed] [Google Scholar]
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Donaldson JG, Jackson CL. ARF family G proteins and their regulators: roles in membrane transport, development and disease. Nature Publishing Group. 2011;12:362–375. doi: 10.1038/nrm3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gally C, Bessereau JL. GABA is dispensable for the formation of junctional GABA receptor clusters in Caenorhabditis elegans. Journal of Neuroscience. 2003;23:2591–2599. doi: 10.1523/JNEUROSCI.23-07-02591.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg S, Sharma M, Ung C, Tuli A, Barral DC, Hava DL, Veerapen N, Besra GS, Hacohen N, Brenner MB. Lysosomal trafficking, antigen presentation, and microbial killing are controlled by the Arf-like GTPase Arl8b. Immunity. 2011;35:182–193. doi: 10.1016/j.immuni.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein AY, Wang X, Schwarz TL. Axonal transport and the delivery of pre-synaptic components. Curr Opin Neurobiol. 2008;18:495–503. doi: 10.1016/j.conb.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DH, Hedgecock EM. Kinesin-related gene unc-104 is required for axonal transport of synaptic vesicles in C. elegans. Cell. 1991;65:837–847. doi: 10.1016/0092-8674(91)90391-b. [DOI] [PubMed] [Google Scholar]
- Hallam SJ, Jin Y. lin-14 regulates the timing of synaptic remodelling in Caenorhabditis elegans. Nature. 1998;395:78–82. doi: 10.1038/25757. [DOI] [PubMed] [Google Scholar]
- Hanks SK, Quinn AM, Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988;241:42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hellman AB, Shen K. Sensory transduction channel subunits, tax-4 and tax-2, modify presynaptic molecular architecture in C. elegans. PLoS ONE. 2011;6:e24562. doi: 10.1371/journal.pone.0024562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann GJ, Schroeder LK, Hieb CA, Kershner AM, Rabbitts BM, Fonarev P, Grant BD, Priess JR. Genetic analysis of lysosomal trafficking in Caenorhabditis elegans. Mol Biol Cell. 2005;16:3273–3288. doi: 10.1091/mbc.E05-01-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Niwa S, Tanaka Y. Molecular Motors in Neurons: Transport Mechanisms and Roles in Brain Function, Development, and Disease. Neuron. 2010;68:610–638. doi: 10.1016/j.neuron.2010.09.039. [DOI] [PubMed] [Google Scholar]
- Holderith N, Lorincz A, Katona G, Rózsa B, Kulik A, Watanabe M, Nusser Z. Release probability of hippocampal glutamatergic terminals scales with the size of the active zone. Nat Neurosci. 2012;15:988–997. doi: 10.1038/nn.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi D, Collins CA, Bhat P, Barkus RV, DiAntonio A, Saxton WM. Control of a kinesin-cargo linkage mechanism by JNK pathway kinases. Curr Biol. 2007;17:1313–1317. doi: 10.1016/j.cub.2007.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo L, Yue Y, Ren J, Yu J, Liu J, Yu Y, Ye F, Xu T, Zhang M, Feng W. The CC1-FHA Tandem as a Central Hub for Controlling the Dimerization and Activation of Kinesin-3 KIF1A. Structure. 2012 doi: 10.1016/j.str.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Jin Y, Garner CC. Molecular mechanisms of presynaptic differentiation. Annu Rev Cell Dev Biol. 2008;24:237–262. doi: 10.1146/annurev.cellbio.23.090506.123417. [DOI] [PubMed] [Google Scholar]
- Kawasaki M, Hisamoto N, Iino Y, Yamamoto M, Ninomiya-Tsuji J, Matsumoto K. A Caenorhabditis elegans JNK signal transduction pathway regulates coordinated movement via type-D GABAergic motor neurons. Embo J. 1999;18:3604–3615. doi: 10.1093/emboj/18.13.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinchen JM, Doukoumetzidis K, Almendinger J, Stergiou L, Tosello-Trampont A, Sifri CD, Hengartner MO, Ravichandran KS. A pathway for phagosome maturation during engulfment of apoptotic cells. Nat Cell Biol. 2008;10:556–566. doi: 10.1038/ncb1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen MP, Shen K. Wnt Signaling Positions Neuromuscular Connectivity by Inhibiting Synapse Formation in C. elegans. Cell. 2007;130:704–716. doi: 10.1016/j.cell.2007.06.046. [DOI] [PubMed] [Google Scholar]
- Klassen MP, Wu YE, Maeder CI, Nakae I, Cueva JG, Lehrman EK, Tada M, Gengyo-Ando K, Wang GJ, Goodman M, et al. An Arf-like small G protein, ARL-8, promotes the axonal transport of presynaptic cargoes by suppressing vesicle aggregation. Neuron. 2010;66:710–723. doi: 10.1016/j.neuron.2010.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfenstein DR, Tomishige M, Stuurman N, Vale RD. Role of phosphatidylinositol(4,5)bisphosphate organization in membrane transport by the Unc104 kinesin motor. Cell. 2002;109:347–358. doi: 10.1016/s0092-8674(02)00708-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraszewski K, Mundigl O, Daniell L, Verderio C, Matteoli M, De Camilli P. Synaptic vesicle dynamics in living cultured hippocampal neurons visualized with CY3-conjugated antibodies directed against the lumenal domain of synaptotagmin. J Neurosci. 1995;15:4328–4342. doi: 10.1523/JNEUROSCI.15-06-04328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-R, Shin H, Choi J, Ko J, Kim S, Lee HW, Kim K, Rho S-H, Lee JH, Song H-E, et al. An intramolecular interaction between the FHA domain and a coiled coil negatively regulates the kinesin motor KIF1A. Embo J. 2004;23:1506–1515. doi: 10.1038/sj.emboj.7600164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas C, Torres VI, Altrock WD, Leal-Ortiz S, Wagh D, Terry-Lorenzo RT, Fejtová A, Gundelfinger ED, Ziv NE, Garner CC. Formation of Golgi-derived active zone precursor vesicles. Journal of Neuroscience. 2012;32:11095–11108. doi: 10.1523/JNEUROSCI.0195-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney TR, Luo S, Nonet ML. Analysis of synaptic transmission in Caenorhabditis elegans using an aldicarb-sensitivity assay. Nat Protoc. 2006;1:1772–1777. doi: 10.1038/nprot.2006.281. [DOI] [PubMed] [Google Scholar]
- Matteoli M, Takei K, Perin MS, Südhof TC, De Camilli P. Exo-endocytotic recycling of synaptic vesicles in developing processes of cultured hippocampal neurons. The Journal of Cell Biology. 1992;117:849–861. doi: 10.1083/jcb.117.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini G, Pigino G, Szebenyi G, You Y, Pollema S, Brady ST. JNK mediates pathogenic effects of polyglutamine-expanded androgen receptor on fast axonal transport. Nat Neurosci. 2006;9:907–916. doi: 10.1038/nn1717. [DOI] [PubMed] [Google Scholar]
- Morfini GA, You YM, Pollema SL, Kaminska A, Liu K, Yoshioka K, Björkblom B, Coffey ET, Bagnato C, Han D, et al. Pathogenic huntingtin inhibits fast axonal transport by activating JNK3 and phosphorylating kinesin. Nat Neurosci. 2009;12:864–871. doi: 10.1038/nn.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata K, Abrams B, Grill B, Goncharov A, Huang X, Chisholm AD, Jin Y. Regulation of a DLK-1 and p38 MAP Kinase Pathway by the Ubiquitin Ligase RPM-1 Is Required for Presynaptic Development. Cell. 2005;120:407–420. doi: 10.1016/j.cell.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Niwa S, Tanaka Y, Hirokawa N. KIF1Bbeta- and KIF1A-mediated axonal transport of presynaptic regulator Rab3 occurs in a GTP-dependent manner through DENN/MADD. Nat Cell Biol. 2008;10:1269–1279. doi: 10.1038/ncb1785. [DOI] [PubMed] [Google Scholar]
- Owald D, Sigrist SJ. Assembling the presynaptic active zone. Curr Opin Neurobiol. 2009;19:311–318. doi: 10.1016/j.conb.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Patel MR, Lehrman EK, Poon VY, Crump JG, Zhen M, Bargmann CI, Shen K. Hierarchical assembly of presynaptic components in defined C. elegans synapses. Nat Neurosci. 2006;9:1488–1498. doi: 10.1038/nn1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa-Ferreira C, Munro S. Arl8 and SKIP Act Together to Link Lysosomes to Kinesin-1. Developmental Cell. 2011;21:1171–1178. doi: 10.1016/j.devcel.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabo SL, Gomes RA, McAllister AK. Formation of presynaptic terminals at predefined sites along axons. Journal of Neuroscience. 2006;26:10813–10825. doi: 10.1523/JNEUROSCI.2052-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi A. Roles of MAP Kinase Cascades in Caenorhabditis elegans. Journal of Biochemistry. 2004;136:7–11. doi: 10.1093/jb/mvh097. [DOI] [PubMed] [Google Scholar]
- Schaefer AM, Hadwiger GD, Nonet ML. rpm-1, a conserved neuronal gene that regulates targeting and synaptogenesis in C. elegans. Neuron. 2000;26:345–356. doi: 10.1016/s0896-6273(00)81168-x. [DOI] [PubMed] [Google Scholar]
- Shapira M, Zhai RG, Dresbach T, Bresler T, Torres VI, Gundelfinger ED, Ziv NE, Garner CC. Unitary assembly of presynaptic active zones from Piccolo-Bassoon transport vesicles. Neuron. 2003;38:237–252. doi: 10.1016/s0896-6273(03)00207-1. [DOI] [PubMed] [Google Scholar]
- Shen K, Scheiffele P. Genetics and cell biology of building specific synaptic connectivity. Annu Rev Neurosci. 2010;33:473–507. doi: 10.1146/annurev.neuro.051508.135302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagi M, Gorlovoy P, Larionov S, Takahashi K, Neumann H. Unloading kinesin transported cargoes from the tubulin track via the inflammatory c-Jun N-terminal kinase pathway. Faseb J. 2006;20:2573–2575. doi: 10.1096/fj.06-6679fje. [DOI] [PubMed] [Google Scholar]
- Stringham EG, Dixon DK, Jones D, Candido EP. Temporal and spatial expression patterns of the small heat shock (hsp16) genes in transgenic Caenorhabditis elegans. Mol Biol Cell. 1992;3:221–233. doi: 10.1091/mbc.3.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC. The presynaptic active zone. Neuron. 2012;75:11–25. doi: 10.1016/j.neuron.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori S, Holt M, Stenius K, Lemke EA, Grønborg M, Riedel D, Urlaub H, Schenck S, Brügger B, Ringler P, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Tao-Cheng JH. Ultrastructural localization of active zone and synaptic vesicle proteins in a preassembled multi-vesicle transport aggregate. Neuroscience. 2007;150:575–584. doi: 10.1016/j.neuroscience.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomishige M, Klopfenstein DR, Vale RD. Conversion of Unc104/KIF1A kinesin into a processive motor after dimerization. Science. 2002;297:2263–2267. doi: 10.1126/science.1073386. [DOI] [PubMed] [Google Scholar]
- Wagner OI, Esposito A, Köhler B, Chen CW, Shen CP, Wu GH, Butkevich E, Mandalapu S, Wenzel D, Wouters FS, et al. Synaptic scaffolding protein SYD-2 clusters and activates kinesin-3 UNC-104 in C. elegans. Proc Natl Acad Sci USa. 2009;106:19605–19610. doi: 10.1073/pnas.0902949106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washbourne P, Bennett JE, McAllister AK. Rapid recruitment of NMDA receptor transport packets to nascent synapses. Nat Neurosci. 2002;5:751–759. doi: 10.1038/nn883. [DOI] [PubMed] [Google Scholar]
- Weston CR, Wong A, Hall JP, Goad MEP, Flavell RA, Davis RJ. JNK initiates a cytokine cascade that causes Pax2 expression and closure of the optic fissure. Genes Dev. 2003;17:1271–1280. doi: 10.1101/gad.1087303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the ventral nerve cord of Caenorhabditis elegans. Philos Trans R Soc Lond, B, Biol Sci. 1976;275:327–348. doi: 10.1098/rstb.1976.0086. [DOI] [PubMed] [Google Scholar]
- Wong MY, Zhou C, Shakiryanova D, Lloyd TE, Deitcher DL, Levitan ES. Neuropeptide delivery to synapses by long-range vesicle circulation and sporadic capture. Cell. 2012;148:1029–1038. doi: 10.1016/j.cell.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H, Chen D, Fang Z, Xu J, Sun X, Song S, Liu J, Yang C. Lysosome biogenesis mediated by vps-18 affects apoptotic cell degradation in Caenorhabditis elegans. Mol Biol Cell. 2009;20:21–32. doi: 10.1091/mbc.E08-04-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai RG, Vardinon-Friedman H, Cases-Langhoff C, Becker B, Gundelfinger ED, Ziv NE, Garner CC. Assembling the presynaptic active zone: a characterization of an active one precursor vesicle. Neuron. 2001;29:131–143. doi: 10.1016/s0896-6273(01)00185-4. [DOI] [PubMed] [Google Scholar]
- Zhen M, Huang X, Bamber B, Jin Y. Regulation of presynaptic terminal organization by C. elegans RPM-1, a putative guanine nucleotide exchanger with a RING-H2 finger domain. Neuron. 2000;26:331–343. doi: 10.1016/s0896-6273(00)81167-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.