Highlights
-
•
We monitor HPV infection in sexually active young women in England.
-
•
The prevalence of HPV 16/18 has reduced within 3 years of HPV immunisation.
-
•
Reductions in HPV 16/18 were greatest at ages with highest immunisation coverage.
-
•
The data suggest reductions in HPV 16/18 amongst unvaccinated young women and men.
Abbreviations: CI, Confidence Interval; GP, General Practice; HC2, Hybrid Capture 2; HPV, Human Papillomavirus; HR, High-risk; LA, Linear Array; LR, Low-risk; LSOA, Lower Super Output Area; NCSP, National Chlamydia Screening Programme; OR, Odds ratio; PCR, Polymerase Chain Reaction; PCT, Primary Care Trust; PDH, Pyruvate dehydrogenase; PHE, Public Health England; REC, Research Ethics Committee; VVS, Vulva-vaginal Swab
Keywords: HPV prevalence, HPV immunisation, Cervarix, Surveillance
Abstract
Background
Reduction in the prevalence of vaccine type HPV infection in young women is an early indication of the impact of the HPV immunisation programme and a necessary outcome if the subsequent impact on cervical cancer is to be realised.
Methods
Residual vulva-vaginal swab (VVS) specimens from young women aged 16–24 years undergoing chlamydia screening in community sexual health services (formerly known as family planning clinics), general practice (GP), and youth clinics in 2010–2012 were submitted from 10 laboratories in seven regions around England. These specimens were linked to demographic and sexual behaviour data reported with the chlamydia test, anonymised, and tested for type-specific HPV DNA using a multiplex PCR and Luminex-based genotyping test. Estimated immunisation coverage was calculated and findings were compared to a baseline survey conducted prior to the introduction of HPV immunisation in 2008.
Results
A total of 4664 eligible specimens were collected and 4178 had a valid test result. The post-immunisation prevalence of HPV 16/18 infection was lowest in this youngest age group (16–18 years) and increased with age. This increase with age was a reversal of the pattern seen prior to immunisation and was inversely associated with estimates of age-specific immunisation coverage (65% for 16–18 year olds). The prevalence of HPV 16/18 infection in the post-immunisation survey was 6.5% amongst 16–18 year olds, compared to 19.1% in the similar survey conducted prior to the introduction of HPV immunisation.
Conclusions
These findings are the first indication that the national HPV immunisation programme is successfully preventing HPV 16/18 infection in sexually active young women in England. The reductions seen suggest, for the estimated coverage, high vaccine effectiveness and some herd-protection benefits. Continued surveillance is needed to determine the effects of immunisation on non-vaccine HPV types.
1. Introduction
Clinical trials have shown human papillomavirus (HPV) prophylactic vaccines to have high efficacy against cervical HPV infection and HPV-related cervical disease associated with the vaccine HPV types [1–3] and HPV immunisation programmes have been introduced in many countries [4]. In England, the national HPV immunisation programme began in September 2008, using the bivalent HPV 16/18 vaccine (Cervarix®). Routine vaccination is offered, in schools (with few exceptions), to girls aged 12 years at the start of each academic year (September). Catch-up immunisation was provided, in schools and by general practitioners (mostly for the oldest cohorts), to girls who were aged 13–17 years when the programme began (September 2008). Vaccine uptake has been high with coverage of over 80% of 12 year olds for all three vaccine doses. Coverage amongst the catch-up cohorts was lower and varied by age at vaccination (overall 56% for three doses; range 39% to 76%) [5]. The programme changed to use the quadrivalent HPV 6/11/16/18 vaccine (Gardasil®) for routine immunisation of 12 year olds in September 2012.
In England, women are invited for cervical screening from 25 years of age: hence the earliest we expect to see any effect of vaccination on the incidence of cervical abnormalities is 2015, and girls immunised aged 12 years will not be invited for screening until 2020. To monitor the impact of the immunisation programme prior to impact on disease, we are conducting surveillance of vaccine and non-vaccine HPV type infections amongst specimens obtained from sexually active young (16–24 years) females undergoing opportunistic screening for Chlamydia trachomatis as part of the English National Chlamydia Screening Programme (NCSP) [6]. Chlamydia screening is recommended for all sexually active young women, annually and on partner change, and is offered opportunistically when they attend a range of services [6]. An HPV survey was first done in 2008. This analysis explores the prevalence of HPV16/18 within 3 years of starting wide-spread immunisation and compares this to pre-immunisation findings from 2008 [7]. The prevalence of other HR types is also reported.
2. Methods
2.1. Sample and data collection
Residual vulva-vaginal swab (VVS) specimens submitted for chlamydia screening from community sexual health services (formerly known as family planning clinics), general practice (GP), and youth clinics were collected from 10 laboratories (six serving largely urban populations and four serving more rural areas) in seven regions around England. These laboratories were recruited based on their throughput of eligible specimens (at least 700 during a 6 month period), and distribution throughout England. Specimens collected between October 2010 and end of June 2012 and tested by September 2012 were included in this analysis.
Procedures for specimen and data collection have been described previously for the pre-immunisation survey conducted in 2008 [7]. In brief, residual chlamydia screening specimens were sent to Public Health England (PHE) labelled with a unique study number. A temporary list of identifiers enabled matching to data reported separately to PHE for the chlamydia screen (age, date specimen collection, lower layer super output area (LSOA) of residence, screening venue of specimen collection, ethnicity, two or more sexual partners in the previous 12 months, new sexual partner in past 3 months, chlamydia screen result). All personal identifiers were then irreversibly deleted prior to release for HPV testing. Specimens that could not be linked to reported data were excluded, as were any specimens matched to data indicating that they did not meet the inclusion criteria. HPV immunisation status for each subject was not available for this analysis: coverage within each age-group was estimated by combining published data for each birth-cohort by year [5]. Coverage estimates generated using the national coverage data and using coverage data only from the relevant local areas (i.e. the PCTs of our subjects’ places of residence) were similar: the national data were used.
This unlinked anonymous survey methodology, conducting HPV testing without seeking specific consent from subjects, was given a favourable ethical opinion by South East Research Ethics Committee (REC reference number 10/H1102/7).
2.2. HPV testing
The collected, eligible, VVS specimens were tested for type-specific HPV DNA using an in-house multiplex PCR and Luminex-based genotyping test [8]. This test detects the 13 high-risk types (HR) classified by the International Agency for Research on Cancer 2009 as at least ‘probably’ carcinogenic in the human cervix (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68), five possible HR types (HPV 26, 53, 70, 73 and 82), and two low-risk (LR) types (HPV 6 and 11) [9]. Specimens were deemed inadequate if they were negative for both HPV and the housekeeping gene, pyruvate dehydrogenase (PDH).
Specimens collected in 2008 had been tested using the Hybrid Capture 2 HPV DNA test (HC2; originally developed by the Digene Corporation, now marketed by Qiagen) using the Combined Probe Cocktail Method to detect probable HR types (as above) and five LR types (6, 11, 42, 43 and 44). HC2 positive specimens were genotyped using the Linear Array HPV Genotyping (LA) test (Roche Molecular Systems). Although all HR HPV types detectable by the HC2/LA algorithm were also detectable using our in-house test, detection rates may be expected to differ between tests. This potential source of bias in our findings on comparison with the pre-immunisation data was informed by the re-testing of a panel (N = 428) of HC2 positive and negative specimens from the pre-immunisation (2008) survey with the in-house Luminex-based test. This showed the post-immunisation test generated more HR HPV positives than the HC2/LA testing algorithm, likely due to the reduced sensitivity of the HC2 test compared to a PCR amplification based system [10]. However, there was close agreement between the two approaches for detection of HPV 16/18 (positivity of 23.8% by the in house genotyping test vs. 22.2% by HC2/LA, kappa 0.809), and HPV 31/33/45 (11.2% vs. 11.4%, kappa 0.756). Difference in detection of non-vaccine HR HPV was greater (27.8% vs. 23.6%, kappa 0.768) and may be important for interpretation of prevalence differences.
2.3. Data analysis
We compared reported characteristics of subjects in the post-immunisation period to those of subjects in the pre-immunisation period to investigate any differences associated with HPV prevalence. Several sub-analyses were conducted to check that key findings were not sensitive to potential biases due to differences in the selection of specimens collected pre- and post-immunisation. Data were weighted so that each laboratory contributed equally to the analysis, rather than in proportion to the number of specimens submitted (as in the pre-immunisation survey).
Prevalence estimates were calculated for the following outcomes: (i) vaccine-type HPV (16/18) (ii) non-vaccine HR HPV, (iii) any HR HPV and (iv) HR types for which cross-protection has been reported. Confidence intervals (95% CI) were calculated using a logit transformation.
Logistic regression was used to explore the association of HPV prevalence with the period of collection (i.e. a binary variable classified as pre or post the start of the HPV immunisation programme), adjusting for age, submitting laboratory, chlamydia screening venue, ethnicity, sexual behaviour and chlamydia infection. The association was expressed as odds ratios (ORs) and confidence intervals (95% CI) calculated using linearised standard errors to show statistical significance.
Data analyses were conducted using Stata v12.
3. Results
3.1. Study population
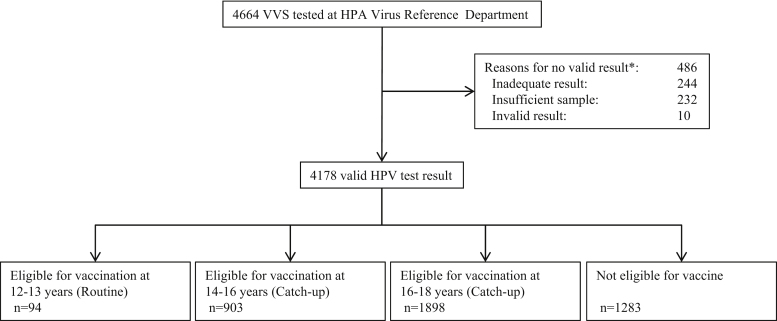
Of 4664 VVS specimens tested for type-specific HPV DNA, 4178 (90%) had a valid result and were included in the analysis: 234 from 2010, 2691 from 2011 and 1253 from 2012 (Fig. 1).
Fig. 1.
Flow chart of specimen collection and testing and immunisation categories.
* Inadequate result: the sample is HPV and housekeeping gene (PDH) negative; Insufficient result: not sufficient sample to be tested; Invalid result: other technical reason.
The source and reported demographic and sexual behaviour data for these specimens are shown in Table 1, alongside the data for the pre-immunisation (baseline) specimens. The mean age of subjects in the post-immunisation survey was 19.3 years (Standard deviation (SD) 2.1 years), similar to the pre-immunisation survey (19.2 years, SD 2.4 years). There were fewer specimens from community sexual health services in the post-immunisation period (3.1% vs. 24.0% pre-immunisation), which was the venue with the highest HR HPV prevalence in 2008 (with relatively more from youth clinics post-immunisation). The proportion of women with missing information on sexual behaviour increased between the two surveys but there was no change in the reported data with around half of respondents reporting two or more sexual partners in the previous year and a new sexual partner in the previous 3 months. The specimens were broadly representative, in terms of reported sexual behaviour data, of all chlamydia screens reported to PHE for females at the selected venues. Relatively high chlamydia positivity was seen amongst specimens from two laboratories (Leeds 26.4%, Lewisham 7.2%, vs. 4.7% at all other laboratories combined) but no reason could be identified for systematic selection bias.
Table 1.
Characteristics of women included in the pre- and post-immunisation surveys.
| Pre-immunisation (2008) |
Post-immunisation (2010–2011) |
|||
|---|---|---|---|---|
| Data completeness (%) | N (%) | Data completeness (%) | N (%) | |
| Region of specimen collection (laboratory) | 100 | 100 | ||
| North West (Aintreea) | 472 (19.9) | 225 (5.4) | ||
| Yorkshire and The Humber (Leedsb) | – | 941 (22.5) | ||
| West Midlands (Stokec) | 261 (11.0) | 311 (7.4) | ||
| East of England (Norfolk and Norwichd) | 767 (32.4) | 271 (6.5) | ||
| East of England (Cambridgee) | – | 312 (7.5) | ||
| South East (East Kentf) | – | 693 (16.6) | ||
| South East (Portsmouthg) | – | 56 (1.3) | ||
| South West (Cornwallh) | 478 (20.2) | 491 (11.8) | ||
| London (University College Londoni) | 391 (16.5) | 475 (11.4) | ||
| London (Lewishamj) | – | 403 (9.6) | ||
| Age–years | 100 | 100 | ||
| 16–18 years | 1054 (44.5) | 1540 (36.9) | ||
| 19–21 years | 809 (34.1) | 1974 (47.2) | ||
| 22–24 years | 506 (21.4) | 664 (15.9) | ||
| Ethnicity | 88 | 76 | ||
| White | 1936 (92.6) | 2552 (80.1) | ||
| Black | 95 (4.5) | 405 (12.7) | ||
| Asian | 25 (1.2) | 86 (2.7) | ||
| Other | 34 (1.6) | 145 (4.5) | ||
| Chlamydia screening venue | 100 | 100 | ||
| General practice | 611 (25.8) | 1102 (26 4) | ||
| Youth clinic | 1190 (50.2) | 2948 (70.6) | ||
| Family planning (Community Sexual Health Services) | 568 (24.0) | 128 (3.1%) | ||
| Two or more sexual partners in the previous 12 months | 81 | 1030 (53.5) | 42 | 881 (50.1) |
| New sexual partner in the previous 3 months | 81 | 926 (48.2) | 44 | 853 (46.9) |
| Chlamydia positivity | 99 | 210 (9.0) | 99.9 | 410 (9.8) |
| Chlamydia positivity (excluding Leeds & Lewisham) | NA | NA | 99.8 | 133 (4.7) |
Microbiology Laboratory, University Hospital Aintree.
Microbiology Department, Leeds Teaching Hospitals.
Central Pathology Laboratory, University Hospital of North Staffordshire.
Department of Microbiology, Norfolk and Norwich University Hospital.
Clinical Microbiology and Public Health laboratory, Addenbrooke's Hospital.
Virology Department, East Kent Hospitals University.
Department of Clinical Microbiology, Queen Alexandra Hospital.
Department of Microbiology, Royal Cornwall Hospital.
University College London Hospital.
Department of Microbiology, University Hospital Lewisham.
The estimated HPV vaccine coverage was 65% for subjects aged 16–18 years, 30% for those 19–21 years and 0% for those 22–24 years.
3.2. HPV 16/18 prevalence
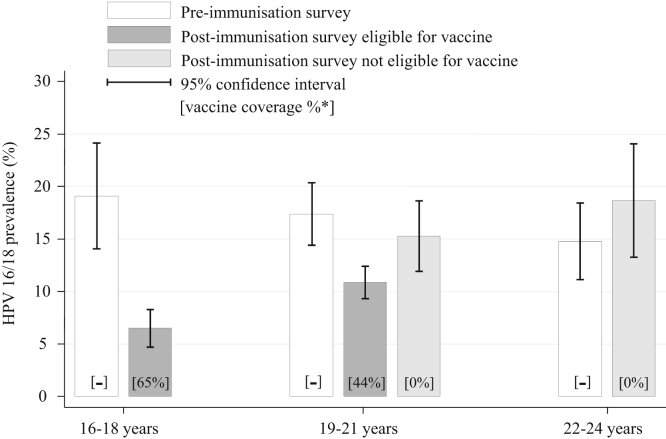
The prevalence of HPV 16 and/or 18 in the post-immunisation survey was lowest in 16–18 year olds, at 6.5% (95% CI: 5.2–8.0%) (Fig. 2). Prevalence increased with age to 12.5% in 19–21 year olds and 18.6% in 22–24 year olds (p-value for trend <0.0001). In contrast in 2008, the prevalence was highest in 16–18 year olds (19.1%, 95% CI: 16.6–21.8%) and lower at older ages (14.8%, 95% CI: 11.9–18.3% in 22–24 year olds).
Fig. 2.
Prevalence of vaccine HPV types (16/18) by age-group and survey period.
* Vaccine coverage estimated using published data on national HPV coverage for each birth-cohort by year. In the 19–21 year old age-group this is calculated separately for those women who would have been offered the vaccine as part of the national HPV immunisation programme.
The 19–21 year olds in the post-immunisation survey (2010–2012) included females eligible and not eligible for immunisation: both these groups had lower HPV prevalence than found pre-immunisation. Females who were in birth-cohorts eligible for vaccination had a lower prevalence of HPV 16/18 (10.9% [95% CI: 9.2–12.9%]) than those who were not eligible for vaccination (15.3% [95% CI: 11.7–19.7%]), p-value = 0.036. There was no sign of any reduction amongst females aged 22–24 years.
There were significant differences in the reduction of prevalence for different ethnic groups; among white women the prevalence of HPV 16/18 infection in 16–18 year olds reduced from 19.7% to 6.7% (66%) in pre- vs. post-immunisation surveys whereas for black women this reduction was less marked (and not significant) from 14.9% to 9.4% (37%). There were too few individuals of Asian and other ethnic origin for formal comparison.
The adjusted odds ratio for HPV 16/18 infection comparing the post-immunisation period with the pre-immunisation was 0.3 (95%CI: 0.2–0.5) for 16–18 year olds and increased with age (Table 2) as would be expected as a reflection of vaccine coverage and age of immunisation (p-value for heterogeneity <0.0001).
Table 2.
Prevalence and odds ratio of HPV infection in the post-immunisation period compared to pre-immunisation, by age group.
| Pre-immunisation: N (%)* | Post-immunisation: N (%)* | OR (95% CI) | Adjusted OR** (95% CI) | ||
|---|---|---|---|---|---|
| 16–18 years | |||||
| HPV 16/18 with or without other HR types | 184 (19.1) | 102 (6.5) | 0.3 (0.2–0.4) | 0.3 (0.2–0.5) | |
| HPV 16/18 alone | 80 (8.3) | 42 (2.7) | 0.3 (0.2–0.5) | 0.2 (0.1–0.5) | |
| Non-vaccine HR type(s) with or without HPV 16/18 | 261 (34.1) | 515 (34.1) | 1.3 (1.1–1.6) | 1.4 (1.0–1.8) | |
| HPV 31/33/45 | 88 (8.6) | 99 (6.4) | 0.7 (0.5–1.0) | 0.5 (0.3–0.8) | |
| HPV 6/11 | 61 (6.3) | 133 (8.1) | 1.3 (0.9–1.8) | 1.6 (1.0–2.8) | |
| 19–21 years | |||||
| HPV 16/18 with or without other HR types | 136 (17.4) | 247 (12.5) | 0.7 (0.5–0.9) | 0.6 (0.4–0.9) | |
| HPV 16/18 alone | 60 (8.0) | 113 (5.8) | 0.7 (0.5–1.0) | 0.5 (0.3–0.9) | |
| Non-vaccine HR type(s) with or without HPV 16/18 | 216 (35.8) | 796 (46.2) | 1.8 (1.4–2.2) | 1.9 (1.4–2.5) | |
| HPV 31/33/45 | 67 (8.7) | 178 (9.9) | 1.2 (0.8–1.6) | 0.9 (0.6–1.4) | |
| HPV 6/11 | 47 (6.0) | 157 (8.9) | 1.5 (1.0–2.3) | 1.7 (0.9–3.0) | |
| 22–24 years | |||||
| HPV 16/18 with or without other HR types | 77 (14.8) | 112 (18.6) | 1.3 (0.9–2.0) | 2.0 (1.0–3.8) | |
| HPV 16/18 alone | 32 (6.4) | 50 (10.2) | 1.7 (0.9–3.0) | 3.0 (1.2–7.6) | |
| Non-vaccine HR type(s) with or without HPV 16/18 | 133 (33.8) | 230 (41.7) | 1.2 (0.9–1.7) | 1.1 (0.6–1.9) | |
| HPV 31/33/45 | 45 (9.3) | 59 (8.2) | 0 9 (0.5–1.5) | 0.8 (0.3–2. 0) | |
| HPV 6/11 | 22 (4.2) | 24 (2.9) | 0 7 (0.4–1.3) | 0.4 (0.1–1.3) | |
Unweighted number given with weighted prevalence (%) so that each laboratory contributes equally to the analysis, rather than in proportion to the number of specimens submitted.
Adjusted for sexual history, age, venue type, ethnicity and chlamydia positivity.
3.3. Sub-analysis
Four sub-analyses were conducted: (i) excluding the Leeds and Lewisham specimens, (ii) restricted to laboratories participating in both periods, (iii) by chlamydia status and (iv) by venue of chlamydia screening.
HPV16/18 prevalence pre- and post-immunisation among 16–18 year olds was (i) 19.1% vs. 6.2% (68% reduction) (ii) 19.1% vs. 7.4% (61% reduction), (iii) 38.6% vs. 13.8% in chlamydia positives (64% reduction) and 16.7% vs. 5.9% in chlamydia negatives (65% reduction), and (iv) 19.7% vs. 4.8% in the GP clinics (76% reduction), 18.4% vs. 6.7% in community sexual health services (64% reduction) and 19.6% vs. 8.9% in Youth clinics (55% reduction), respectively.
3.4. HR HPV prevalence
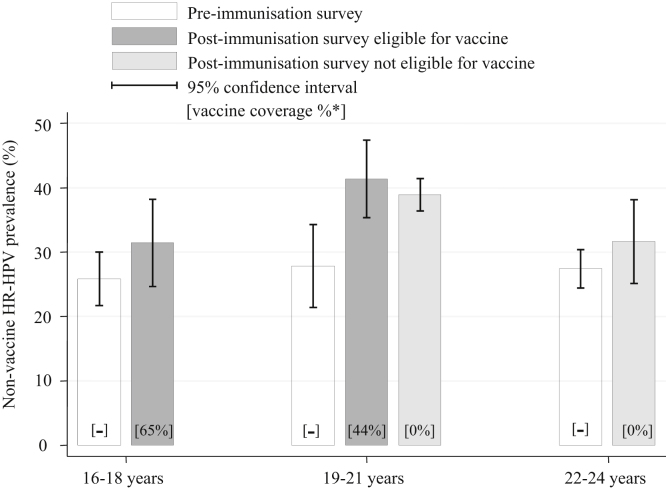
The detected prevalence of non-vaccine HR HPV types was slightly higher in the post-immunisation period than pre-immunisation for each age group (Fig. 3). There was no clear change in the pattern of age-specific prevalence, nor trend in the adjusted odds ratio by age group (Table 2). These increases combined with the decreases in HPV 16/18 resulted in similar prevalence of all HR HPV (i.e. vaccine and non-vaccine types) among 16–18 year olds in both periods (post-immunisation 34.1% (95% CI 31.4–36.9): pre-immunisation 34.1% (95% CI 31.1–37.3) p-value = 0.998).
Fig. 3.
Prevalence of non-vaccine high-risk HPV types by age-group and survey period.
* Vaccine coverage estimated using published data on national HPV coverage for each birth-cohort by year. In the 19–21 year old age-group this is calculated separately for those women who would have been offered the vaccine as part of the national HPV immunisation programme.
The detected prevalence of three HR HPV types against which cross-protection has been reported from clinical trials, HPV 31, 33 and 45 [11,12] was slightly lower overall post-immunisation, but with no clear change in the pattern of age-specific prevalence (data not shown), nor trend in the adjusted odds ratio by age group (Table 2).
Multiple infections remained common in this age group, albeit somewhat reduced in the immunised ages in line with reduced prevalence of HPV 16/18 (36.8% of HR HPV positive 16–18 year olds with more than one HR HPV vs. 52 7% in 2008). As in 2008, non-vaccine HR HPV types were found in over half of the HPV 16/18 positives.
4. Discussion
These findings are an early indication that the national HPV immunisation programme is successfully preventing HPV 16/18 infection in sexually active young women in England. There was a clear change in the pattern of age-specific HPV 16/18 prevalence and the prevalence amongst females eligible for immunisation was considerably lower than previously measured in 2008 prior to immunisation. Lower HPV16/18 prevalence was associated with higher immunisation coverage.
These surveillance data show the impact of a high coverage immunisation programme within the targeted, and slightly older, population. Without vaccination status, we could not report the effectiveness amongst those immunised, however that would likely be heavily influenced by biases in vaccine uptake in these catch-up cohorts. The finding of no fall in HPV 16/18 prevalence between time periods among females above the age of HPV immunisation, and no change in the age-specific pattern of non-vaccine HR prevalence argues against the HPV 16/18 changes being solely due to selection biases or time trends and supports their attribution to the impact of the immunisation programme. In fact, the known changes in selection of subjects (e.g. larger proportion of black women and women attending at Youth clinics) and the change in assay tend more towards an expectation of higher HPV16/18 prevalence in the post-immunisation data, if all else was equal.
That we see reductions in VVS-based HPV 16/18 prevalence estimates is encouraging for expectations that HPV immunisation will reduce not only cervical infection but also transmission of infections that may be only transiently present in the lower genital tract [13]. This therefore favours optimistic assumptions about herd-protection of unvaccinated males and females. The reductions we find in HPV 16/18 are even greater than those predicted by the mathematical modelling that informed the HPV immunisation programme [14,15]. This is possibly because the surveillance sampled sexually active young women, who have a higher risk of infection and hence more to gain from vaccination. However, if there were no selection biases in play, the falls in HPV 16/18 are consistent with close to 100% efficacy among those immunised, or with lower efficacy (perhaps to be expected in these vaccinated at an older age) plus some herd-protection effect amongst the unimmunised, and/or higher immunisation coverage than estimated from the estimated from national data. Conversely, the lower reductions in some sub-groups (e.g. black women and women attending Youth clinics) may reflect lower uptake of vaccine amongst these sub-groups than the national average. Among 19–21 year olds in the post-immunisation survey, even those too old to have been eligible for immunisation had lower prevalence than 19–21 year olds in 2008 and lower than contemporary 22–24 year olds which further strengthens the evidence for a herd-protection effect, although more data are needed to confirm the size of this benefit. Given the levels of coverage and of pre-existing infection in young women of ages eligible for catch-up immunisation [7], we expect to see larger reductions in future as herd-protection effects develop and surveillance includes more girls who have received routine immunisation at 12 years.
The higher prevalence of non-vaccine HR HPV types in our post-immunisation survey can be interpreted in several ways. Any immunisation-associated type-replacement, either due to non-vaccine types filling the ecological niches created by removal of the vaccine types [16,17], or by loss of cross-immunity acquired through natural infection with HPV 16/18 [18] would likely manifest in this way, at least in the younger vaccinated age-groups. However, comparison of our pre- and post-immunisation findings has some important limitations. The change in assay between the pre- and post-immunisation surveys was advantageous in terms of affordability and sustainability of testing for our surveillance. Cuschieri et al. have shown the potential importance of the effect of changing assays [19] and the higher detection rate of non-vaccine HR types found for our post-immunisation assay suggests this has contributed to the higher prevalence of non-vaccine types post-immunisation across all ages. In addition, such broad-spectrum assays, can potentially miss types present in much lower concentrations than others, when multiple HPV types are present, as they commonly are in sexually active young women [7,20–23] hence non-vaccine type HPV infection may have been underestimated in the pre-immunisation survey due to “masking” by co-infection with HPV 16/18 [24,21]. There may also have been temporal changes in the prevalence of some or all non-vaccine types (unrelated to immunisation) between 2008 and 2010–2012. The reduction in the prevalence of HPV 31, 33 and 45, against the backdrop of increased non-vaccine HR-HPV is consistent with some cross-protective efficacy against these types. It will be interesting to see whether the change in age-specific pattern that we have seen for HPV16/18 emerges for these types in subsequent analyses.
The use of a convenience source of residual genital specimens from young women undergoing chlamydia screening around England allows a large sample to assess the early impact of the HPV immunisation programme. Women screened for chlamydia tend to be at higher risk of chlamydia infection than the general population [25] and may therefore be at increased risk of HPV infection, which likely increases power to detect changes, but limits representativeness of the general population with regard to risk of HPV and uptake of HPV immunisation. In 2011, an estimated 41% of females aged 16–24 years were screened for chlamydia (assuming one test per person). This was an increase from approximately 15% in 2008/09. It is possible, therefore, that the population from which our specimens were drawn had changed somewhat between 2008 and 2010–2012. There was no evidence of a change in reported sexual behaviour. However, missing data on sexual behaviour increased, likely associated with the large increase in testing in venues where this was not asked, and this limited our ability to track shifts in the risk profile of this specimen source.
Studies from other countries have shown similar findings since have introduction of HPV immunisation programmes using the quadrivalent vaccine. Tabrizi et al. [26] compared a survey of 202 women aged 18–24 years old in 2005–2007 to a similar survey of 404 women from 2010 to 2011 in Australia, with estimated coverage 86%, and showed a substantial decrease (28.7% to 6.7%) in the vaccine-targeted genotypes (16/18/6/11) as well as a slightly lower prevalence of non-vaccine oncogenic types. Markowitz et al. [27] have analysed data from the National Health and Nutrition Examination Surveys in the United States. Amongst women aged 14–19 years, the prevalence of the HPV vaccine-types (16/18/6/11) decreased from 11.5% in 1363 unvaccinated women in 2003–2006 to 5.1% in 740 women in 2007–2010 with an estimated vaccination coverage of 34% for one dose or more.
This surveillance considers the impact of the immunisation programme in England. Surveillance subjects and methods elsewhere in the UK are different and will offer complementary evidence regarding the impact and effectiveness of the UK immunisation programme. In England, this surveillance will continue in order to determine the extent of herd- protection and of cross-protection and any type-replacement. To address these remaining questions future analysis will include larger numbers of surveillance specimens, more time since immunisation, more sampling from the birth-cohorts with high coverage of routine immunisation and vaccine effectiveness will be estimated once immunisation status has been obtained for some subjects.
Funding
This work was supported by Public Health England.
Details of Contributors
KS and ONG initiated and designed the surveillance. RHJ, DM and KS conducted the sample collection and data management. SB, KP and PM performed the HPV testing. MJ contributed to data analysis and interpretation, particularly relating to mathematical modelling. DM conducted the statistical analysis. All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. DM and KS wrote the first draft of the manuscript. All authors contributed to and approved the final analysis and manuscript.
Conflict of interest statement
None declared.
Acknowledgements
We thank staff at participating laboratories who have provided NCSP specimens for testing: Bridget Reed, Ian Robinson and Mike Rothburn at University Hospital Aintree; Heather Etherington, Amanda Ronson-Binns and Susan Smith at Leeds Teaching Hospital; Nick Doorbar and David Frodsham at University Hospital of North Staffordshire; Gail Carr and Laura Ryall at Public Health Laboratory, Cambridge, Addenbrooke's Hospital; Samir Dervisevic and Emma Meader at Norfolk and Norwich University Hospital; Roberta Bourlet and Marie Payne at East Kent Hospitals University; Allyson Lloyd and Colin Walker at Queen Alexandra Hospital; Vic Ellis at Royal Cornwall Hospital; Caroline Carder at University College London Hospital; Ruth Hardwick, Tacim Karadag and Paul Michalczyk at University Hospital Lewisham.
We thank the National Chlamydia Screening Programme (NCSP), particularly Alireza Talebi and Bersebeh Sile and the Chlamydia Screening Offices, for supporting the collection of NCSP specimens, assistance recruiting laboratories and conducting data linking. Thanks also to Heather Northend, Tracey Cairns and Krishna Gupta for help with data-processing, Sarah Woodhall for helpful discussions about changing chlamydia screening trends, Sarika Desai for developing the protocol for the post-immunisation surveillance, Natasha de Silva, Sara Bissett, and John Parry for helping to establish and maintain the HPV assay, and Tom Nichols for advice on data analysis.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.De Carvalho N., Teixeira J., Roteli-Martins C.M. Sustained efficacy and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine up to 7.3 years in young adult women. Vaccine. 2010;28:6247–6255. doi: 10.1016/j.vaccine.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Lehtinen M., Paavonen J., Wheeler C.M. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2011 doi: 10.1016/S1470-2045(11)70286-8. [DOI] [PubMed] [Google Scholar]
- 3.Dillner J., Kjaer S.K., Wheeler C.M. Four year efficacy of prophylactic human papillomavirus quadrivalent vaccine against low grade cervical, vulvar, and vaginal intraepithelial neoplasia and anogenital warts: randomised controlled trial. BMJ. 2010;341:c3493. doi: 10.1136/bmj.c3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization, Number of countries having introduced HPV vaccines to date, Available from http://www.who.int/nuvi/hpv/decision_implementation/en/index.html (accessed September 2012).
- 5.Department of Health, Annual HPV vaccine uptake in England: 2010/11, Available from URL: https://www.wp.dh.gov.uk/immunisation/files/2012/03/120319_HPV_UptakeReport2010-11-revised_acc.pdf (accessed October 2012).
- 6.National Chlamydia Screening Programme, National Chlamydia Screening Programme Standards, sixth ed., Available from URL: http://www.chlamydiascreening.nhs.uk/ps/resources/core-requirements/NCSP%20Standards%20Edition%206_Accompanying%20Information%20Document_October%202012.pdf (accessed June 2013).
- 7.Howell-Jones R., de Silva N., Akpan M. Prevalence of human papillomavirus (HPV) infections in sexually active adolescents and young women in England, prior to widespread HPV immunisation. Vaccine. 2012;30:3867–3875. doi: 10.1016/j.vaccine.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Bissett S.L., Howell-Jones R., Swift C. Human papillomavirus genotype detection and viral load in paired genital and urine samples from both females and males. J Med Virol. 2011;83:1744–1751. doi: 10.1002/jmv.22167. [DOI] [PubMed] [Google Scholar]
- 9.Bouvard V., Baan R., Straif K. A review of human carcinogens—Part B: Biological agents. Lancet Oncol. 2009;10:321–322. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 10.Castle P.E., Solomon D., Wheeler C.M., Gravitt P.E., Wacholder S., Schiffman M. Human papillomavirus genotype specificity of hybrid capture 2. J Clin Microbiol. 2008;46:2595–2604. doi: 10.1128/JCM.00824-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harper D.M., Franco E.L., Wheeler C.M. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006:367. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 12.Paavonen J., Naud P., Salmeron J. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–314. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 13.Castle P.E., Rodriguez A.C., Porras C. A comparison of cervical and vaginal human papillomavirus. Sex Transm Dis. 2007;34:849–855. doi: 10.1097/OLQ.0b013e318064c8c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jit M., Choi Y.H., Edmunds W.J. Economic evaluation of human papillomavirus vaccination in the United Kingdom. BMJ. 2008;337:a769. doi: 10.1136/bmj.a769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jit M., Chapman R., Hughes O., Choi Y.H. Comparing bivalent and quadrivalent human papillomavirus vaccines: economic evaluation based on transmission model. BMJ. 2011;343:d5775. doi: 10.1136/bmj.d5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byington C.L., Samore M.H., Stoddard G.J. Temporal trends of invasive disease due to Streptococcus pneumoniae among children in the intermountain west: emergence of nonvaccine serogroups. Clin Infect Dis. 2005;41:21–29. doi: 10.1086/430604. [DOI] [PubMed] [Google Scholar]
- 17.Lipsitch M. Vaccination against colonizing bacteria with multiple serotypes. PNAS. 1997;94:6571–6576. doi: 10.1073/pnas.94.12.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durham D.P., Poolman E.M., Ibuka Y., Townsend J.P., Galvani A.P. Reevaluation of epidemiological data demonstrates that it is consistent with cross-immunity among human papillomavirus types. J Infect Dis. 2012;206:1291–1298. doi: 10.1093/infdis/jis494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuschieri K., Kavanagh K., Sinka K. Effect of HPV assay choice on perceived prevalence in a population-based sample. Diagn Mol Pathol. 2013;22:85–90. doi: 10.1097/PDM.0b013e31827f3f7e. [DOI] [PubMed] [Google Scholar]
- 20.van Alewijk D., Kleter B., Vent M. An HPV testing algorithm comprising a combination of the L1 broad-spectrum SPF10 PCR and a novel E6 high-risk multiplex type-specific genotyping PCR. J Clin Microbiol. 2013 doi: 10.1128/JCM.02831-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mori S., Nakao S., Kukimoto I., Kusumoto-Matsuo R., Kondo K., Kanda T. Biased amplification of human papillomavirus DNA in specimens containing multiple human papillomavirus types by PCR with consensus primers. Cancer Sci. 2011;102:1223–1227. doi: 10.1111/j.1349-7006.2011.01922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Doorn L.J., Molijn A., Kleter B., Quint W., Colau B. Highly effective detection of human papillomavirus 16 and 18 DNA by a testing algorithm combining broad-spectrum and type-specific PCR. J Clin Microbiol. 2006;44:3292–3298. doi: 10.1128/JCM.00539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmitt M., Dondog B., Waterboer T., Pawlita M., Tommasino M., Gheit T. Abundance of multiple high-risk human papillomavirus (HPV) infections found in cervical cells analyzed by use of an ultrasensitive HPV genotyping assay. J Clin Microbiol. 2010;48:143–149. doi: 10.1128/JCM.00991-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eklund C., Zhou T., Dillner J. Global proficiency study of human papillomavirus genotyping. J Clin Microbiol. 2010;48:4147–4155. doi: 10.1128/JCM.00918-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riha J., Mercer C.H., Soldan K., French C.E., Macintosh M. Who is being tested by the English National Chlamydia Screening Programme? A comparison with national probability survey data. Sex Transm Infect. 2011;87:306–311. doi: 10.1136/sti.2010.047027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tabrizi S.N., Brotherton J.M., Kaldor J.M. Fall in human papillomavirus prevalence following a national vaccination program. J Infect Dis. 2012;206:1645–1651. doi: 10.1093/infdis/jis590. [DOI] [PubMed] [Google Scholar]
- 27.Markowitz L.E., Hariri S., Lin C. Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, national health and nutrition examination surveys, 2003–2010. J Infect Dis. 2013;208:385–393. doi: 10.1093/infdis/jit192. [DOI] [PubMed] [Google Scholar]